Abstract
This retrieval study documents taper damage at modular interfaces in retrieved MOM THA systems and investigates if increased modularity is associated with increased fretting and corrosion. One hundred thirty-four (134) heads and 60 stems (41 modular necks) of 8 different bearing designs (5 manufacturers) were analyzed. Damage at the shell–liner interface of 18 modular CoCr acetabular liners and the corresponding 11 acetabular shells was also evaluated. The results of this study support the hypothesis that fretting and corrosion damage occurs at a variety of modular component interfaces in contemporary MOM THAs. We also found that modularity of the femoral stem was associated with increased damage at the head. An analysis of component and patient variables revealed that dissimilar alloy pairing, larger head sizes, increased medio-lateral offsets and longer neck moment arms were all associated with increased taper damage at the modular interfaces.
Keywords: total hip arthroplasty, modularity, fretting, corrosion, metal on metal
Contemporary metal on metal (MOM) total hip arthroplasty devices (THAs) may feature several modular metallic components, including modular acetabular liners and modular femoral necks, to more effectively accommodate the anatomical variations among patients, and to allow for more component-targeted revision surgeries. For example, with modular heads and femoral necks, surgeons can adjust medio-lateral offsets and femoral version angle intraoperatively to optimize hip biomechanics and prevent leg-length discrepancies. Also advantageous are modular acetabular components, which permit well-fixed acetabular shells to be retained during bearing surface exchanges, thereby decreasing operation time and patient risk. Despite clinical benefits, increasing the number of modular interfaces may increase the susceptibility of MOM THA devices to metal particulate debris via fretting and corrosion mechanisms [1,2].
Previous investigators have reported on fretting and corrosion at the head–stem interface of retrieved and bench tested MOM THAs [3–5] but damage at other modular junctions is less extensively documented in the literature. We note the predominance of international reports on the subject of modular interface fretting and corrosion that highlight designs primarily used in Europe and Australia [6–9]. Corrosion and metal ion release from the neck–stem junction have recently been reported as a cause of adverse local tissue reactions in metal on polyethylene bearings [10]. Additionally, modular femoral necks have been associated with increased micro-motion [9] and it is unclear what effect this has on fretting and corrosion at the head taper. Similarly, it is not well established which implant, patient, and clinical factors are associated with increased taper corrosion in contemporary MOM designs, or if these factors have similar effects at all modular interfaces. Thus, the occurrence of, and factors influencing, modular taper damage among contemporary MOM THA devices used within the United States remains poorly understood.
In this study, we asked: does taper damage occur at the variety of modular interfaces in contemporary MOM THA devices used within the United States? We also sought to determine whether devices with modular femoral neck components were associated with increased fretting and corrosion at the head–stem taper. To answer these questions, we analyzed a consecutive series of revised components retrieved over a 10-year period by performing a review of the clinical records associated with the devices, combined with semi-quantitative evaluation of all modular interfaces. A secondary goal of our study was to answer the question: which patient and component factors are associated with taper damage of modular components?
Methods
Clinical and Implant Information
One hundred sixty-seven (167) MOM bearing systems were retrieved during revision surgeries at 10 clinical institutions in collaboration with two regional retrieval programs. The retrieved systems were collected under an IRB-approved multi-institutional implant retrieval program. Five devices were returned upon patient request and after excluding resurfacing devices (n = 21), non-modular systems (n = 2) and inseparable components (n = 2), one hundred thirty-seven (137) devices were available for inspection. In addition to the retrieved components, clinical information inclusive of age, gender, reason for implant revision, and implantation time was collected for all devices (Table 1). The average implantation time was 2.9 ± 2.0 years (range, 0 to 11.0 years) and the mean patient age at implantation was 58 ± 11.4 years (range, 30 to 90 years).
Table 1.
Clinical and Device Information Corresponding to the 137 Retrieved MOM THA Systems
| Clinical Information | Device Information | ||
|---|---|---|---|
| Patients | Number of Systems | 137 | |
| Male | 75 | Heads | |
| Female | 62 | CoCrMo | 85 |
| Mean Age at Implantation | 58 (30–90) years | CoCrMo w/ Ti6A1-4 V taper sleeve | 22 |
| Mean Time in situ | 2.9 ± 2.0 (0–11) years | Stems | |
| Reason for Revision | Modular | ||
| Loosening | 98 | Ti6A1-4 V | 41 |
| Infection | 12 | Monolithic | |
| Tissue Reactivity | 7 | Ti6A1-4 V | 14 |
| Instability | 6 | CoCrMo | 5 |
| Other | 14 | ||
| Modular liners | |||
| CoCrMo | 18 | ||
| Modular shells | |||
| Ti6A1-4 V | 11 | ||
The majority of components were revised for loosening (n = 98/ 137, 72%), infection (n = 12/137, 8.8%), adverse local tissue reaction (n = 7/137, 5.1%), and instability (n = 6/137, 4.4%; Table 1). Intraoperative evidence of adverse local tissue reaction (ALTR) was identified in the operative reports of 33/137 (24%) cases. ALTR was not explicitly stated in all 33 cases, but was identified if “masses”, “cysts”, “enlarged bursae”, “pseudotumor”, “hypersensitivity”, and/or “lymphocytic infiltration” (aseptic) were discussed in the operative reports [11].
The retrieved devices were manufactured by 5 different companies: Zimmer, Warsaw, Indiana (n = 84/137, 61%); Biomet, Warsaw, Indiana (n = 28/137, 20%); DePuy, Warsaw, Indiana (n = 20/137, 15%); Wright Medical Technology, Arlington, Tennessee (n = 4/137, 2.9%); Smith and Nephew, Memphis, Tennessee (n = 1/137, 0.7%). For this study, 134 heads, 60 stems (41 modular necks), 18 modular acetabular liners and 11 corresponding acetabular shells were analyzed. All interfacing components in this study were of the same manufacturer. Device information (inclusive of manufacturer, design, size, and constituent material) was obtained from component markings, patient records, or directly from the manufacturer. Alloy composition of retrieved devices was confirmed using X-ray fluorescence (Niton XL3t; Thermo Scientific, Waltham, Massachusetts). In cases where the femoral component was not received, stem designs were determined from radiographs.
Taper Damage Evaluation
Devices were cleaned by two 25 min soaks in a 1:10 ratio of disinfectant (Discide; AliMed, Dedham, Massachusetts) to water, followed by two 30 min ultrasonication periods in de-ionized water. Between these steps, a soft nylon brush was used to help remove biological films and loose debris. After cleaning, modular interfaces were inspected by the naked eye and under a stereomicroscope equipped with a digital camera (Leica DFC490; Leica Microsystems, Wetzlar, Germany), for signs of fretting and corrosion. Fretting, defined by Szolwinski et al. as a contact damage process resulting from micromotions of interfacing metals, was identified as scratching perpendicular to machining lines on the taper, and/or wearing away of the machining lines [12]. Corrosion was identified as white haziness (indicative of intergranular crevice corrosion), discoloration, and/or blackened debris [13]. Damage at the modular interfaces was characterized semi-quantitatively using a previously-published four-point scoring technique [14] with a score of 1 indicating minimal fretting or corrosion, and 4 indicating severe damage. Iatrogenic damage, recognized primarily as irregular, acute artifacts on the surface, was excluded from the wear damage assessment. Components were scored by the same three investigators (G.B.H., J.A.H., and D.W.M.) to ensure a consistent, reproducible procedure.
Biomechanical Analysis
The effect of patient-specific component configuration was assessed using custom software, developed to automate biomechanical calculations using pre-revision surgery radiographs. The software incorporated user point selections to identify component position on the radiograph relative to bony structures. Distance calibration was performed using the size of the acetabular shell component and patient weight provided the basis for bending moment calculations. Output variables included: acetabular shell inclination and anteversion angles, prosthetic medio-lateral offset (perpendicular distance from center of head to central femoral shaft line), neck moment arm (distance from center of femoral head to point where stem enters head taper), and prosthetic neck bending moment.
Statistical Analysis
Due to the non-normal distributions of the data, nonparametric statistical analyses were performed using statistical software (SPSS 19.0, IBM, Chicago, IL). Mann–Whitney U, Kruskal–Wallis and Wilcoxon tests were used to assess differences in taper damage among grouped parameters and Spearman’s rank order correlation was used to identify correlations between variables. The level of significance for the entire statistical analysis was P < 0.05.
Results
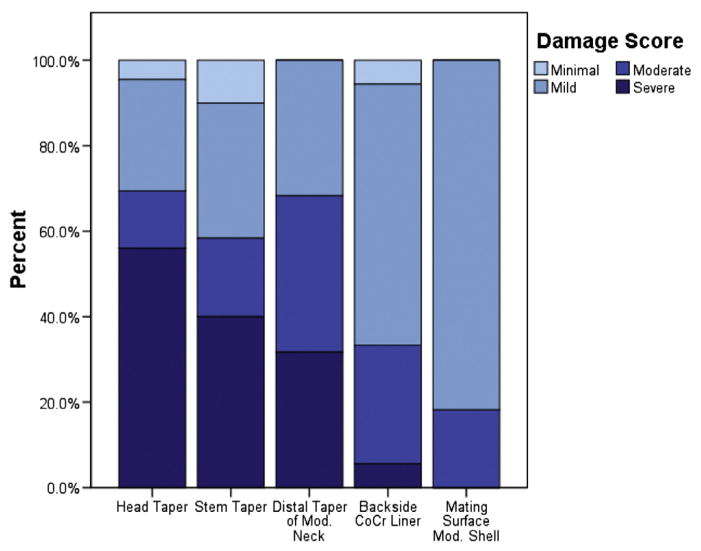
Evidence of taper damage was found on all types of modular interfaces examined in this study (Fig. 1). Mild to severe damage (score ≥ 2) was observed on 128 of 134 (96%) head tapers, 54 of 60 (90%) stem tapers, all 41 (100%) distal tapers of modular necks, 17 of 18 (94%) CoCr acetabular liners, and all 11 modular acetabular shells (Fig. 2). Damage scores tended to increase with implantation time at the head tapers (ρ = 0.46, P < 0.001; Spearman’s rho) and stem tapers (ρ = 0.58, P < 0.001) but not at the distal taper of modular necks (ρ = 0.26, P = 0.1). At the distal taper of modular necks, damage was localized primarily on the curved medial and lateral surfaces of the components. Here, damage was not significantly greater than at the proximal taper of modular necks (P = 0.26; Wilcoxon), but the scores at the two regions were positively correlated (ρ = 0.48, P = 0.001) with each other. Damage on the backside of modular liners ranged from mild to severe, manifested primarily as scratching and discoloration near the rim. Mild to moderate damage, apparent as circular fretting patterns, was noted on the mating surface of all modular shells. Scores on the mating surface of modular shells were correlated with those of the modular liners (ρ = 0.70, P = 0.02).
Fig. 1.
Photographs showing examples of modular interface damage on components in this study. (A) head taper, 4. (B) stem taper–monolithic stem, 3. (C) stem taper–modular neck, 4. (D) distal taper of modular neck, 4. (E) backside of modular liner, 4. (F) mating surface of modular shell, 2.
Fig. 2.
Bar graph showing the distribution of taper damage scores at the head tapers, stem tapers, distal taper of modular necks, backside of CoCr liners, and mating surface of modular shells.
At the head–stem interface, scores were higher within the head tapers than at the stem tapers (mean difference = 0.33, P = 0.04; Mann–Whitney U); these metrics were positively correlated with each other (ρ = 0.64, P < 0.001). When evaluating modular and monolithic stems separately, scores at stem tapers of modular stems were positively correlated with head score (ρ = 0.53, P < 0.001) though this was not the case with monolithic stems (P = 0.53). Femoral heads paired with modular neck stems exhibited higher scores at the head tapers than those paired with monolithic stems (mean difference = 0.94, P < 0.001; Mann–Whitney U). This difference was also significant at the stem tapers (mean difference = 0.96, P = 0.002, Fig. 3).
Fig. 3.
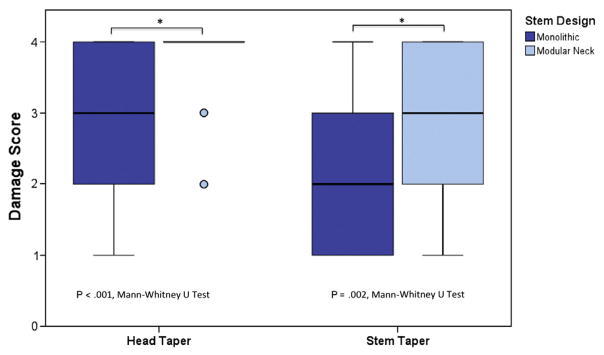
Graph showing the variation in head and stem taper damage across stem design.
From component factor evaluation, significantly lower damage was observed on heads coupled with stems of same alloys as opposed to dissimilar alloys (P < 0.001; Kruskal–Wallis); damage at the ste m tapers was also dependent on this variable (P = 0.007; Kruskal–Wallis). We also noted a positive correlation between head taper damage and head size (ρ = 0.24, P = 0.007). The results of biomechanical analyses failed to elucidate any associations between taper damage and prosthetic joint reaction force, acetabular shell inclination, acetabular anteversion or prosthetic bending moment. Scores at the stem tapers tended to increase with an increase in medio-lateral offset, (ρ = 0.47, P = 0.01), as was the case with scores at the distal taper of modular necks and neck moment arm length (ρ = 0.44, P = 0.04).
Discussion
This retrieval study examined taper damage in contemporary MOM THA implants which may incorporate modular femoral and/or acetabular components. Although taper damage at the head–stem junction has been previously documented [3–5], the extent to which fretting and corrosion occur at modular necks in MOM THAs remains unclear [8]. To the authors’ knowledge, this is the first retrieval study to report on taper damage at the liner–shell interface of modular CoCr liners with acetabular shells. The results of this study support the hypothesis that fretting and corrosion damage occurs at the various modular components in contemporary MOM THAs. We also found that modularity within the femoral stem was associated with increased damage at the head–stem taper. An analysis of component and patient variables revealed that dissimilar alloy pairing and larger head sizes were associated with increased damage at the head taper. From biomechanical analysis, larger medio-lateral offsets and neck moment arm lengths were associated with greater damage at the stem taper and distal taper of modular necks, respectively.
While we highlight the utility of the semi-quantitative evaluation employed in this study as a means by which to categorize the variation in taper damage, we recognize that it is limited by observer subjectivity and may not comprehensively characterize the amount of material loss or corrosion debris at these interfaces. Additionally, the lack of standardization in visual scoring techniques among different retrieval laboratories makes absolute comparison of our damage evaluation results with those of previous studies difficult. Nevertheless, the maintenance of the same three investigators (G.B.H., J.A.H., and D.W.M.) for the examination of all devices enabled us to detect trends within this study that may be compared to previous research.
For example, our findings at the head–stem taper were comparable to those of previous bench-top and retrieval studies involving metal as well as polyethylene bearings [5,13,15,16]. The increase in damage with implantation time was consistent with the theory that longer implantation times provide more time for taper damage via a mechanically-assisted-crevice corrosion mechanism [13]. We also found that devices with similar alloy pairing between the head and stem exhibited less damage at both the head and stem tapers than devices with dissimilar alloy pairing, which was also consistent with the literature [16]. Dissimilar alloy combinations may be susceptible to galvanic corrosion due to the electrical coupling of the metals in the conductive in-vivo environment [15]. It has also been proposed that since cobalt chromium molybdenum (450 Hv) is a harder alloy, it is less susceptible to mechanical damage and galling than is Ti-6-Al-V (330 Hv) [16–18]. However, we observed micro-ridged patterns within these heads resulting from an “imprinting” phenomenon by the Ti-6-Al-V stems (Fig. 1A). The mechanisms by which the harder CoCrMo alloy appears to wear in preference to the softer metal are the subject of further investigation.
Taper damage at other modular interfaces is less extensively reported in the literature. A retrieval study of modular neck prostheses featuring ceramic on polyethylene articulation reported correlations between neck–stem taper damage and implantation time, as well as elevated scores at the neck–stem interface compared to the head–stem interface [6]. Though we noted similar trends in our analysis, neither finding was statistically significant. We did however, observe higher damage scores at the head–stem interface when heads were coupled with modular neck (vs. monolithic) stems, which may be due to micromotion associated with femoral neck modularity [9]. The damage noted at the metallic shell–liner interface of acetabular components proved to be a novel finding and is deserving of further investigation.
As part of this study we sought to quantify patient and component variables such as femoral neck length and prosthetic neck bending moment, that have previously been shown to be correlated with observations of corrosion in retrievals [16]. Biomechanical metrics were analyzed using the pre-revision radiographs that were available for each implanted device. We noted that in some cases, particularly for devices revised for loosening, the pre-revision radiographs may not have been representative of the in-vivo position of the functional prosthesis. We maintain that it may be helpful to obtain post-implantation radiographs of the components, in which their original position is documented. In practice however, these radiographs are often problematic to obtain in a consistent manner by the revising physician, especially when the components were implanted at a different hospital than the revision center.
The results of the current study show that the fretting and corrosion damage that has raised concern at the head–stem interface, is also prevalent at the many additional modular component interfaces in contemporary MOM THAs. The corrosion observed at the shell–liner interface may be further analyzed with electron microscopy to elicit failure mechanisms and ascertain whether the corrosion processes here are similar to what has been reported at the head taper. Additionally, the increased damage at the head taper associated with modularity of the femoral neck warrants further investigation. Recognizing the limitations of semi-quantitative analysis and the rather short implantation times of components in this study, we suggest longer term follow up coupled with quantitative taper wear measurement to better assess the natural progression of taper degradation in modern MOM THAs.
Acknowledgments
This study was supported by the National Institutes of Health (NIAMS) R01 AR47904. Institutional support has been received from Stryker and Zimmer.
Appendix
The writing committee’s affiliations are as follows: Implant Research Center, Hartzband Center for Hip and Knee Replacement, 10 Forest Avenue, Paramus, NJ 07652 (G.R.K.); Rothman Institute, 925 Chestnut Street, Philadelphia, PA 19107 (J.P.); Rubin Institute for Advanced Orthopedics, 2401 West Belvedere Ave, Baltimore, MD 21215 (M.A.M.); Department of Orthopaedic Surgery, University Hospitals Case Medical Center, 11100 Euclid Ave, Cleveland, OH 44106 (M.J.K.); Orthopaedic Research Institute, The University of Chicago Hospitals, 5841S. Maryland Avenue, Chicago, IL 60637 (J.M.M.); Medical Arts & Research Center, University of Texas Health Science Center San Antonio, 8300 Floyd Curl Dr., San Antonio, Texas 78229 (A.D.M.); The Orthopaedic Program at Magee Womens Hospital, 300 Halket Street, Suite 1601-B, Pittsburgh, PA 15213 (B.R.H.); Tennessee Orthopaedic Clinics, 9430 Park West Blvd Suite 130 Knoxville, TN 37923 (H.E.C.); Penn Orthopaedics, Penn Presbyterian Medical Center 51N 39th Street Philadelphia, PA 19104 (G.-C.L.).
Footnotes
The Conflict of Interest statement associated with this article can be found at http://dx.doi.org/10.1016/j.arth.2013.05.040.
References
- 1.Viceconti M, Baleani M, Squarzoni S, et al. Fretting wear in a modular neck hip prosthesis. J Biomed Mater Res. 1997;35(2):207. doi: 10.1002/(sici)1097-4636(199705)35:2<207::aid-jbm9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs JJ, Gilbert JL, Urban RM. Current concepts review — corrosion of metal orthopaedic implants*. J Bone Joint Surg. 1998;80(2):268. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Langton D, Jameson S, Joyce T, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011;93(8):1011. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- 4.Hallab NJ, Messina C, Skipor A, et al. Differences in the fretting corrosion of metal–metal and ceramic–metal modular junctions of total hip replacements. J Orthop Res. 2004;22(2):250. doi: 10.1016/S0736-0266(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 5.Meyer H, Mueller T, Goldau G, et al. Corrosion at the cone/taper interface leads to failure of large-diameter metal-on-metal total hip arthroplasties. Clin Orthop Relat Res. 2012;470(11):3101. doi: 10.1007/s11999-012-2502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009;24(7):1019. doi: 10.1016/j.arth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Kretzer J, Jakubowitz E, Krachler M, et al. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531. doi: 10.1007/s00264-009-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp TM, Weik T, Bloemer W, et al. Modular titanium alloy neck adapter failures in hip replacement–failure mode analysis and influence of implant material. BMC Musculoskelet Disord. 2010;11:3. doi: 10.1186/1471-2474-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauch SY, Huber G, Hoenig E, et al. Influence of material coupling and assembly condition on the magnitude of micromotion at the stem–neck interface of a modular hip endoprosthesis. J Biomech. 2011;44(9):1747. doi: 10.1016/j.jbiomech.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Gill I, Webb J, Sloan K, et al. Corrosion at the neck–stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012;94(7):895. doi: 10.1302/0301-620X.94B7.29122. [DOI] [PubMed] [Google Scholar]
- 11.Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szolwinski MP, Farris TN. Mechanics of fretting fatigue crack formation. Wear. 1996;198(1–2):93. [Google Scholar]
- 13.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27(12):1533. doi: 10.1002/jbm.820271210. [DOI] [PubMed] [Google Scholar]
- 14.Higgs GB, Hanzlik JA, MacDonald DW, et al. Method of characterizing fretting and corrosion at the various taper connections of retrieved modular components from metal-on-metal total hip arthroplasty. STP ASTM Intl In Rev. 2012 [Google Scholar]
- 15.Goldberg JR, Gilbert JL. In vitro corrosion testing of modular hip tapers. J Biomed Mater Res B Appl Biomater. 2003;64(2):78. doi: 10.1002/jbm.b.10526. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg JR, Gilbert JL, Jacobs JJ, et al. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002;401:149. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Neville A, Dowson D. Biotribocorrosion of CoCrMo orthopaedic implant materials—assessing the formation and effect of the biofilm. Tribol Int. 2007;40(10–12):1492. [Google Scholar]
- 18.Wanjara P, Jahazi M. Linear friction welding of Ti-6Al-4 V: processing, microstructure, and mechanical-property inter-relationships. Metall Mat Trans A. 2005;36(8):2149. [Google Scholar]