Abstract
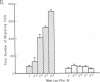
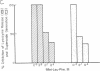
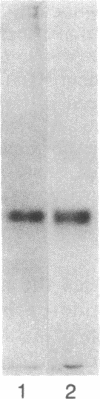
Pertussis toxin inhibits the N-formyl-Met-Leu-Phe (fMet-Leu-Phe) mediated human neutrophil functions of enzyme release, superoxide generation, aggregation, and chemotaxis. As pertussis toxin modifies the GTP binding receptor-regulatory protein "Ni," the association of the fMet-Leu-Phe receptor with such a protein was further examined in purified neutrophil plasma membranes. Both fMet-Leu-Phe-mediated guanine nucleotide exchange and nucleotide-mediated regulation of the fMet-Leu-Phe receptor are inhibited by pertussis toxin. In addition, membrane pretreatment with pertussis toxin abolishes the fMet-Leu-Phe-mediated inhibition of adenylate cyclase. Actions of pertussis toxin are due to the ADP-ribosylation of a single subunit at 41 kDa in the neutrophil plasma membrane, which comigrates on NaDodSO4 gels with the Ni GTP-binding protein in the platelet plasma membrane. Our results suggest that (i) the fMet-Leu-Phe receptor is associated with a Ni GTP regulatory protein, and (ii) a fMet-Leu-Phe-Ni complex is important in the control of several neutrophil functions, probably involving multiple transduction systems, including adenylate cyclase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- Huang C. K., Hill J. M., Jr, Bormann B. J., Mackin W. M., Becker E. L. Chemotactic factors induced vimentin phosphorylation in rabbit peritoneal neutrophil. J Biol Chem. 1984 Feb 10;259(3):1386–1389. [PubMed] [Google Scholar]
- Hyslop P. A., Oades Z. G., Jesaitis A. J., Painter R. G., Cochrane C. G., Sklar L. A. Evidence for N-formyl chemotactic peptide-stimulated GTPase activity in human neutrophil homogenates. FEBS Lett. 1984 Jan 23;166(1):165–169. doi: 10.1016/0014-5793(84)80065-4. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. Guanine nucleotides modulate the binding affinity of the oligopeptide chemoattractant receptor on human polymorphonuclear leukocytes. J Clin Invest. 1983 Sep;72(3):748–753. doi: 10.1172/JCI111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Lad P. M., Glovsky M. M., Richards J. H., Learn D. B., Reisinger D. M., Smiley P. A. Identification of receptor regulatory proteins, membrane glycoproteins, and functional characteristics of adenylate cyclase in vesicles derived from the human neutrophil. Mol Immunol. 1984 Jul;21(7):627–639. doi: 10.1016/0161-5890(84)90048-8. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Glovsky M. M., Smiley P. A., Klempner M., Reisinger D. M., Richards J. H. The beta-adrenergic receptor in the human neutrophil plasma membrane: receptor-cyclase uncoupling is associated with amplified GTP activation. J Immunol. 1984 Mar;132(3):1466–1471. [PubMed] [Google Scholar]
- Lad P. M., Reisinger D. M., Smiley P. A. Ligand requirements for the relaxation of adenylate cyclase from activated and inhibited states. Biochemistry. 1983 Jun 21;22(13):3278–3284. doi: 10.1021/bi00282a037. [DOI] [PubMed] [Google Scholar]
- Michel T., Lefkowitz R. J. Hormonal inhibition of adenylate cyclase. alpha 2 Adrenergic receptors promote release of [3H]guanylylimidodiphosphate from platelet membranes. J Biol Chem. 1982 Nov 25;257(22):13557–13563. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Involvement of membrane calcium in the response of rabbit neutrophils to chemotactic factors as evidenced by the fluorescence of chlorotetracycline. J Cell Biol. 1979 Oct;83(1):179–186. doi: 10.1083/jcb.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I., Atkinson J. P. Evidence that the functional responses of human neutrophils occur independently of transient elevations in cyclic AMP levels. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(1):35–47. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Smith R. J., Bowman B. J., Iden S. S. Effects of trifluoperazine on human neutrophil function. Immunology. 1981 Dec;44(4):677–684. [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H., Yin H. L., Southwick F. S., Zaner K. S. The motor of leukocytes. Fed Proc. 1984 Sep;43(12):2760–2763. [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J., Lauffenburger D. A. Kinetic analysis of chemotactic peptide receptor modulation. J Cell Biol. 1982 Jan;92(1):34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]