Abstract
Proteosome inhibitors such as bortezomib (BTZ) have been used to treat muscle wasting in animal models. However, direct effect of BTZ on skeletal muscle cells has not been reported. In the present study, our data showed that C2C12 cells exhibited a dose - dependent decrease in cell viability in response to increasing concentrations of BTZ. Consistent with the results of cell viability, Annexin V/PI analysis showed a significant increase in apoptosis after exposing the cells to BTZ for 24 h. The detection of cleaved caspase-3 further confirmed apoptosis. The apoptosis induced by BTZ was associated with reduced expression of p-ERK. Cell cycle analysis revealed that C2C12 cells underwent G2/M cell cycle arrest when incubated with BTZ for 24 h. Furthermore, BTZ inhibited formation of multinucleated myotubes. The inhibition of myotube formation was accompanied by decreased expression of Myogenin. Our data suggest that BTZ induces cell death and inhibits differentiation of C2C12 cells at clinically relevant doses.
Keywords: C2C12, bortezomib
Introduction
The ubiquitin-proteasome system is activated in skeletal muscle atrophy under catabolic conditions. Proteasome inhibitors have been developed to prevent muscle atrophy. BTZ is currently the only commercially available drug targeting the 26S proteasome [1,2,3]. BTZ is a dipeptide boronic acid proteasome inhibitor that works by blocking the action of the proteasome [4,5,6]. BTZ was initially approved for treating relapsed multiple myeloma and mantle cell lymphoma [7,8,9]. Subsequently, BTZ has also been used to treat a variety of non-cancer conditions in preclinical studies. Agten et al showed that BTZ partially protected the diaphragm from mechanical ventilation-induced diaphragm contractile dysfunction [10]. BTZ prolongs survival during lethal hemorrhagic shock in rats [11]. Rayavarapu et al showed that BTZ significantly improved muscle function and reduced tumor necrosis factor α expression in the skeletal muscle of mice with myositis [12]. Furthermore, the potential use of BTZ in treating cancer cachexia has also been attempted [13].
BTZ is less toxic compared to other proteasome inhibitors and its therapeutic potential in treated muscle wasting conditions has been explored. Recent studies showed that BTZ reduced muscle loss in various animal models [14,15,16]. However, clinical reports showed that BTZ induced severe congestive cardiac failure in patients who had no prior cardiac history [17,18]. The authors proposed that BTZ may impair cardiac function due to inhibition of proteasome activity which is important for cellular homeostasis. These concerns were addressed by Nowis et al [19] in animal experiments. Rats treated with BTZ led to a significant drop in left ventricle ejection fraction. At the cellular level, BTZ treatment resulted in abnormalities in mitochondria and decreased cardiomyocyte contractility. The direct effect of BTZ on skeletal muscle cells has not been studied. The present study was aimed to investigate BTZ’s effects on the growth and differentiation of a mouse myoblast line C2C12 and explored the signaling pathways involved.
Materials and methods
C2C12 cell culture
The mouse myoblast cell line C2C12 (Typical Culture Preservation Commission Cell Bank, Shanghai) was cultured in DMEM (Bioop, JiangSu) containing 10% fetal calf serum (FCS) (Hyclone) and 100 U/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37°C. To induce differentiation, DMEM with 2% horse serum (Gibco) was added to C2C12 cells when the density of cells reached 70%–80%.
MTT assay
Cell viability was determined by MTT assay. Briefly, C2C12 cells were planted into a 96-well plate and cultured overnight before BTZ was added. The cells were incubated for another 24 h after adding BTZ. After the incubation, 20 µl of MTT reagent (Sigma) was added to each well and incubated for 4 h. After removing the medium, 150 ul DMSO was added and absorbance was detected at 490 nm by a micro-plate reader (Spectra Max M5).
Cell cycle analysis
C2C12 cells were incubated with 10 nM of BTZ for 24 h. The cells were washed once with PBS, and fixed with 70% ethanol and stained with propidium iodide (Sigma, USA). DNA contents were measured by flow cytometry (FACS Calibur, Becton Dickinson, USA) and cell cycle was analyzed using Flowjo software.
Detection of apoptosis
After treating C2C12 cells with BTZ, the cells were washed once with PBS, and stained with Annexin V and propidium iodide (PI) and analyzed by flow cytometry. Cells that are positive for Annexin V but negative for PI are considered undergoing apoptosis.
Immunoblotting
Proteins were extracted from C2C12 cells and separated by SDS- polyacrylamide gel electrophoresis and blotted to PVDF membranes which were subsequently incubated with antibodies. The following primary antibodies were from Cell Signaling Technology: GAPDH (1:2000), cdc2 (1:2000), ERK (1:2000), p-ERK (1:1000), cleaved caspase-3 (1:1000). Myogenin antibody was from Abcam (1:1000). Secondary antibodies were anti-rabbit IgG (1:5000, Cell Signaling) and anti-mouse IgG (1:5000, Sigma). The band density was analyzed by ImageJ.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and incubated with blocking buffer containing 1% BSA and 0.1% Triton X-100. After blocking, cells were incubated overnight in the primary antibody (cleaved caspase-3, 1:500). After washing, cells were incubated in goat anti-rabbit IgG-Alexa Fluor R 568 (Molecular Probes, diluted 1:800). Cells were then rinsed and counterstained with DAPI. Images were taken by confocal microscope (FV1000MPE, Olympus).
Statistical analysis
Data are presented as means ± SD. Student t test was used to determine the significance of the differences between variables. P < 0.05 was considered statistically significant.
Results
BTZ inhibits C2C12 differentiation
C2C12 cells grow as single cells when cultured in DMEM containing 10% FCS (Fig. 1A). However, when cultured in DMEM containing 2% horse serum, the C2C12 cells fused and formed myotubes which becomes evident at day 4 and 5 (Fig. 1A). In the next step, we tested the effect of BTZ on C2C12 differentiation. The cells were incubated with differentiation medium for 36 h before BTZ was added and the incubation continued for another 72 h. The data showed that C2C12 differentiation was inhibited by BTZ in a dose dependent manner (Fig. 1B). At 5 nM, BTZ partially inhibited myotube formation. At higher concentration (10 nM), BTZ completely inhibited C2C12 myotube formation. Myogenin is a muscle specific transcription factor that is required for the fusion of myoblasts to form myotubes. The protein levels of myogenin were examined by immunoblotting. As shown in Fig. 1C, myogenin was not expressed in C2C12 cells cultured in 10% FCS, but was detected when cultured in 2% horse serum. However, when 10 nM BTZ was added to the differentiation medium, the expression of myogenin was completely abolished (Fig. 1C).
Fig. 1.
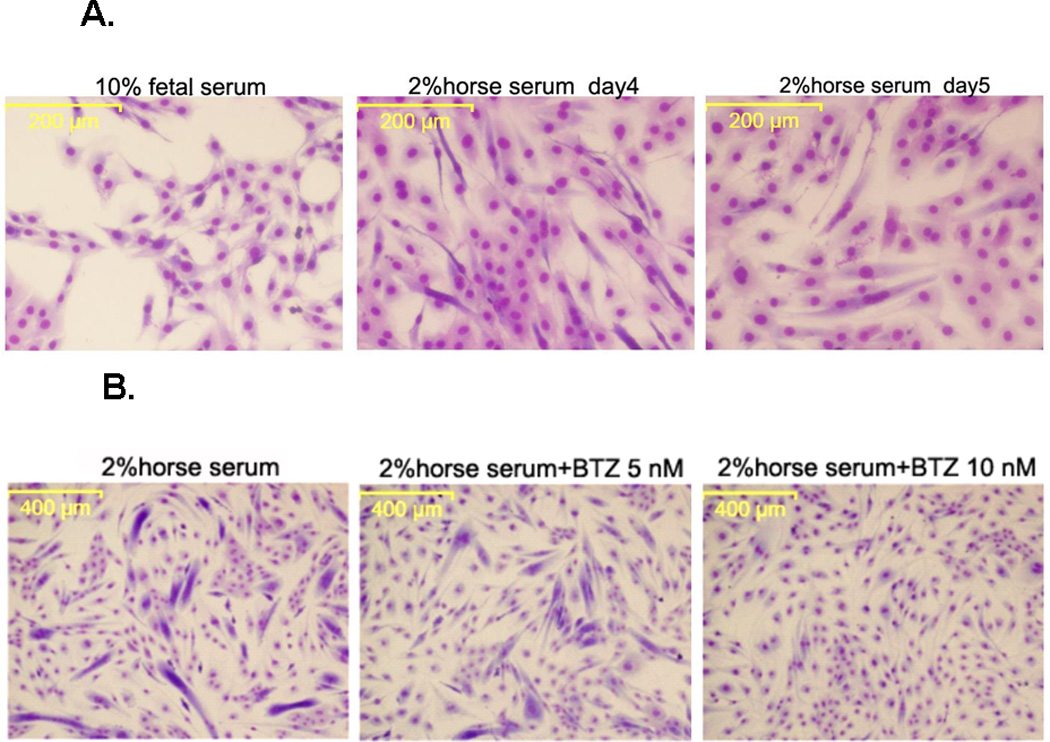
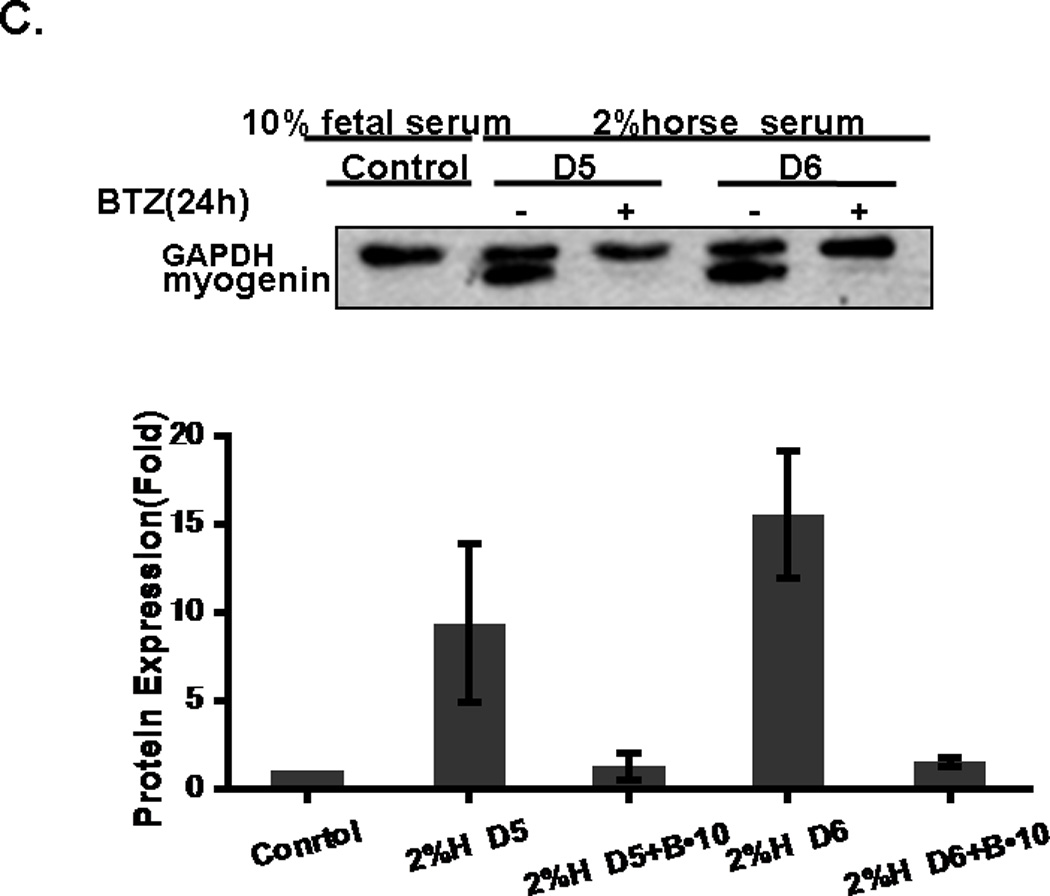
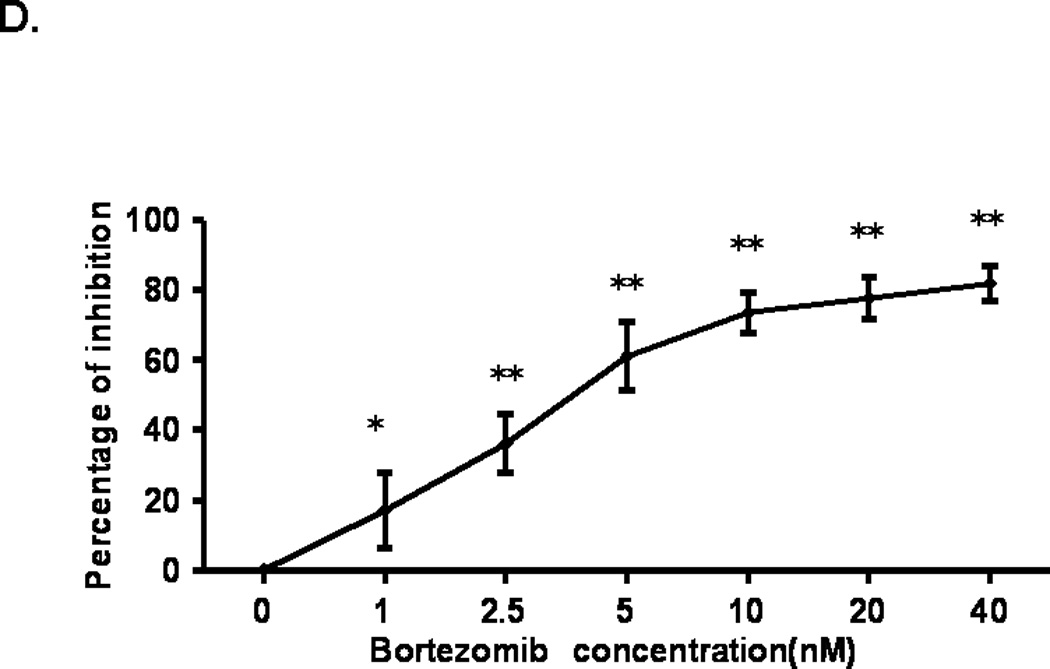
The effect of BTZ on the differentiation and viability of C2C12 cells. (A). C2C12 cells begin to form myotubes after adding the differentiation medium. The number, as well as the width and length of myotubes gradually increased over time. (B). C2C12 cells were cultured for 36 h in differentiation medium before BTZ was added and the cells were cultured for another 72 h in the presence of BTZ. (C). C2C12 cells were treated with BTZ at 10 nM for 24 h after being cultured in differentiation medium for several days. The protein levels of myogenin were assessed by immunoblotting. The bands were quantified by ImageJ and normalized to GAPDH. The graph represents the quantification from 3 independent blots. H: horse serum, D: day, B•10: bortezomib 10 nM. (D). MTT assay of C2C12 cells. C2C12 cells were planted into a 96-well plate and incubated overnight, followed by addition of various concentrations of BTZ and incubated for 24 h. MTT assay was performed as described in materials & methods. *P<0.05, **P<0.001.
BTZ reduces C2C12 cell viability
It has been shown previously that BTZ induces growth inhibition in tumor cells [20]. It is important to know whether BTZ’s growth inhibitory effect was unique to tumor cells or to muscle cells as well before BTZ could be used to treat patients with muscle wasting. As shown in Fig. 1D, BTZ caused a dose dependent growth inhibition of C2C12 cells. The inhibition rate of BTZ was 16.9% at 1 nM and went up to 81.8% at 40 nM.
BTZ induced G2/M phase cell cycle arrest
Growth inhibition usually results from cell cycle arrest. We examined the effects of BTZ on cell cycle progression in C2C12 cells by flow cytometry. As shown in Fig. 2A, treatment of C2C12 cells with BTZ at 10 nM for 24 h led to a decrease of the percentage of cells in G1 phase and an increase in S and G2/M phase (Fig.2B). Compared to the control, the percentage of cells in G1 phase decreased 41.65%, whereas the percentage of cells in S and G2 /M phases increased 11.00% and 27.01%, respectively. The data indicate that a significant number of cells were arrested at G2/M phase. We next examined the protein levels of cdc2 which controls the G2/M transition. Our data revealed a decrease of cdc2 expression in response to BTZ treatment (Fig. 2C), suggesting that BTZ induced cell cycle arrest at G2/M phase may be mediated by inhibition of cdc2 expression.
Fig. 2.
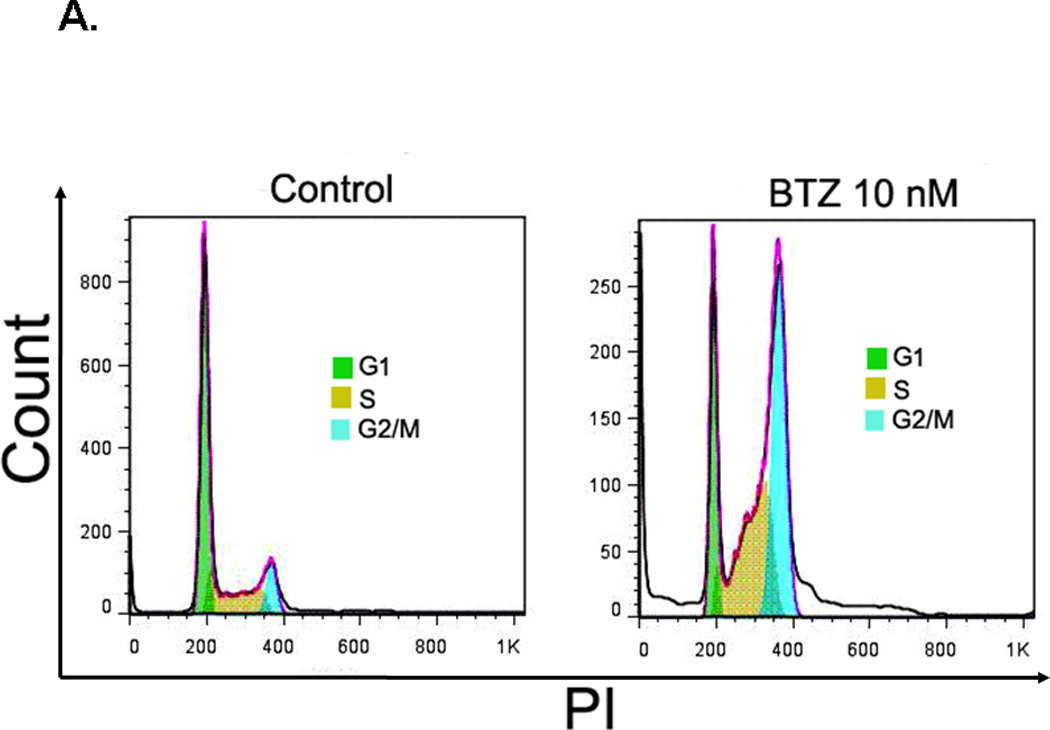
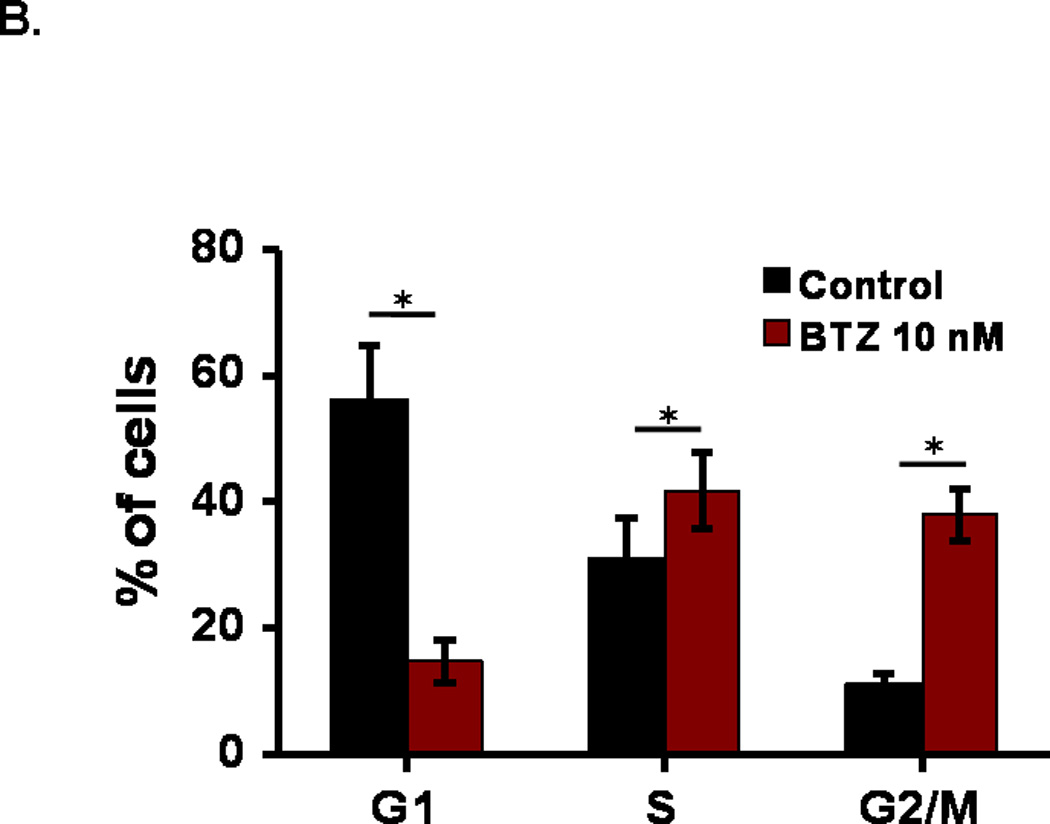
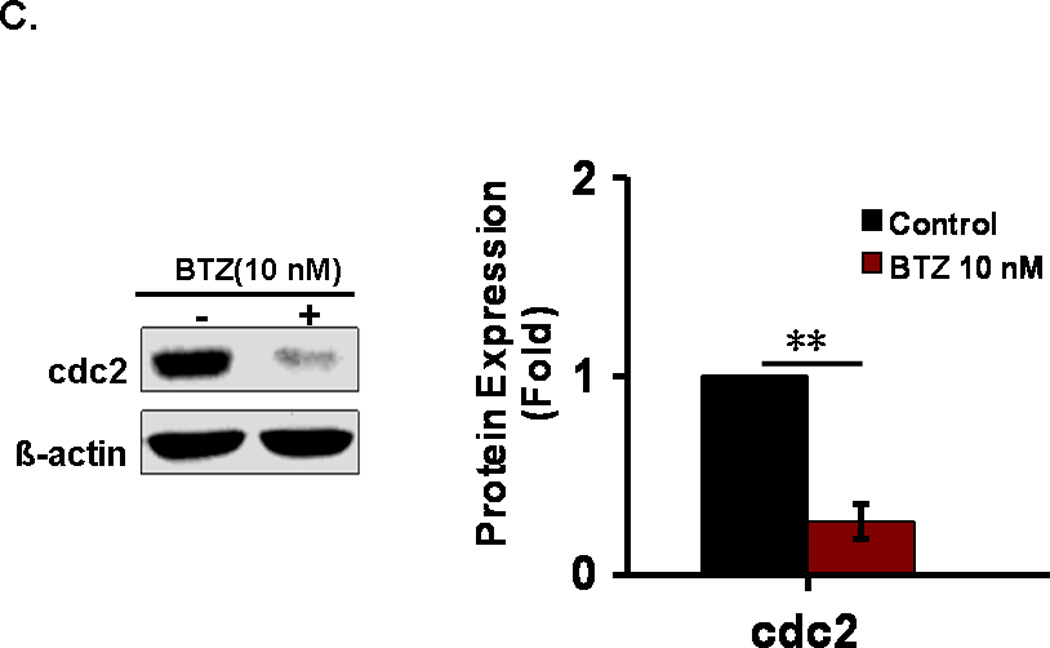
Cell cycle analysis. (A). C2C12 cells were stained with PI and then analyzed by flow cytometry as described in the materials and methods. C2C12 cells were exposed to 10 nM BTZ for 24 h. (B). Bar graph showing the percentage of cell cycle at G1, S, and G2/M phases. (C). The protein levels of cdc2 in C2C12 cells treated by BTZ (10 nM, 24h) were examined by immunoblotting. The bands were quantified by ImageJ and the expression levels of cdc2 were normalized to beta-actin. *P<0.05, **P<0.01.
BTZ induced apoptosis in C2C12 cells
Annexin V/ PI staining assay showed that BTZ induced apoptosis of C2C12 cells (Fig. 3A). The percentage of cells undergoing apoptosis in response to BTZ treatment was 9.8%, whereas the control was only1.8% (Fig. 3B). BTZ induced apoptosis was confirmed by caspase-3 cleavage (Fig. 3C,D). It has been shown previously that ERK signaling was involved in the anti-apoptotic action of 17β-Estradiol in C2C12 cells [21]. This has led us to assess the effect of MAPK inhibitors U0126 and PD98059 on BTZ induced apoptosis. Our data showed that both inhibitors induced further apoptosis when combined with BTZ (Fig. 3B). Consistent with these findings, immunoblotting results showed that expression of p-ERK was reduced by BTZ (Fig. 4).
Fig. 3.
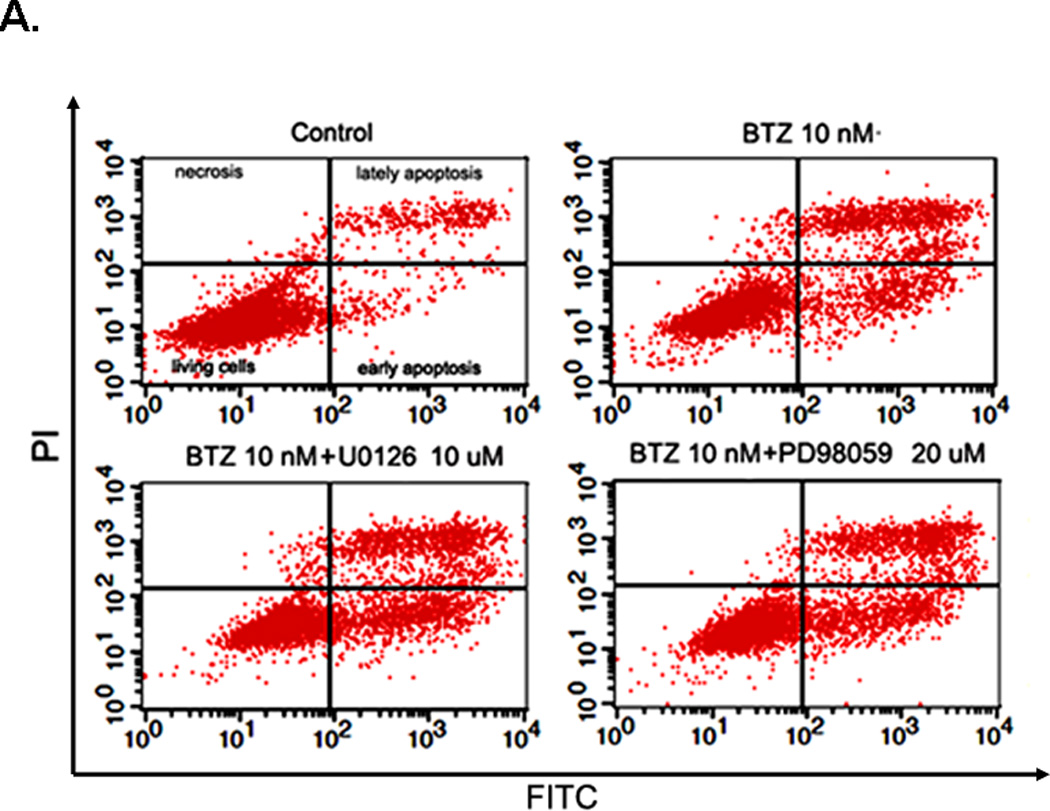
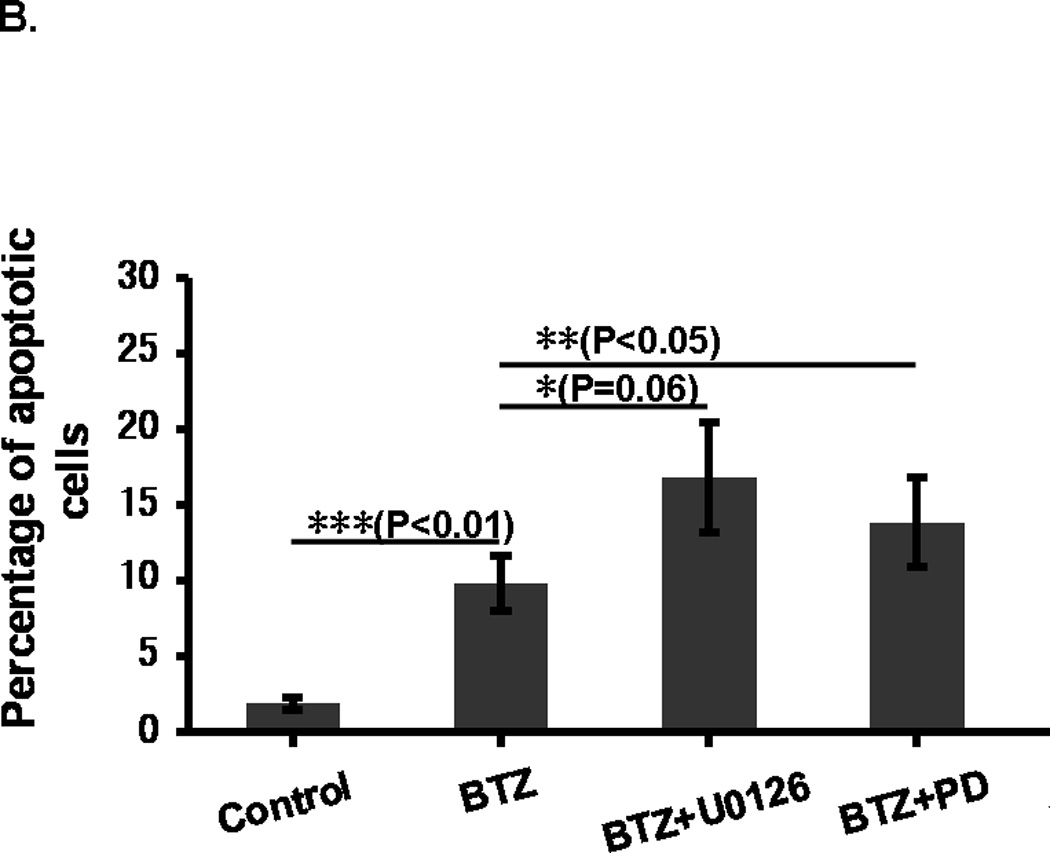
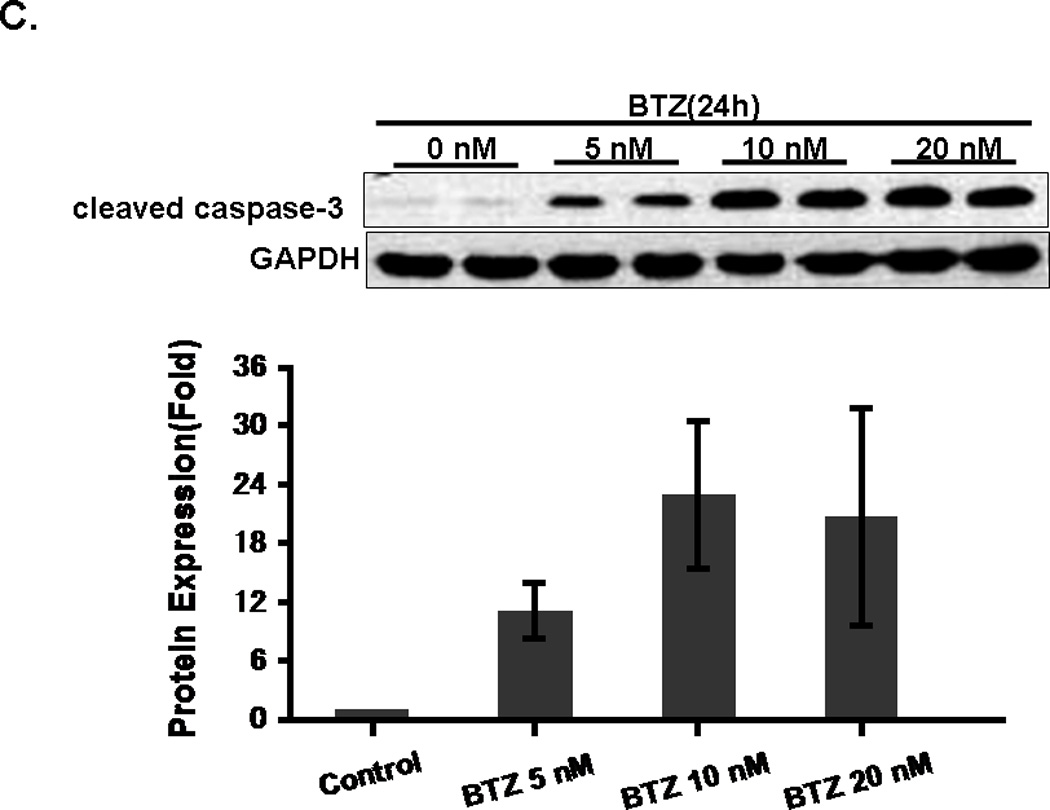
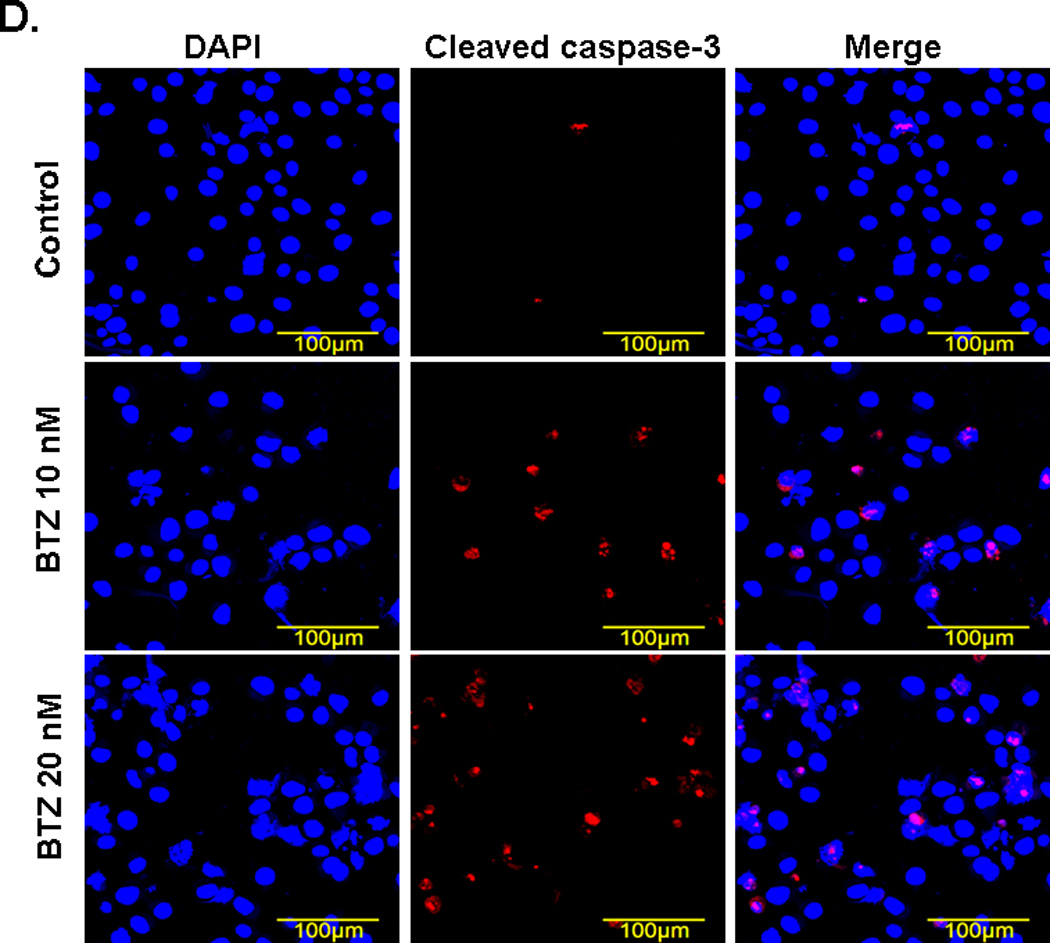
BTZ induces apoptosis in C2C12 cells. (A). Flow cytometry chart of C2C12 cells stained with Annexin V and propidium iodide (PI). C2C12 cells were either treated with BTZ alone or in combination with MAPK pathway inhibitors U1026 or PD98059 (PD) for 24 h. (B). Bar graph showing the percentage of cells undergoing apoptosis. (C). The level of cleaved caspase-3 was assessed by immunoblotting. The bands were quantified by ImageJ and normalized to GAPDH. The graph represents the quantification from 3 independent blots. (D). Immunofluorescence staining of cleaved caspase-3 in C2C12 cells after exposing to BTZ for 24 h.
Fig. 4.
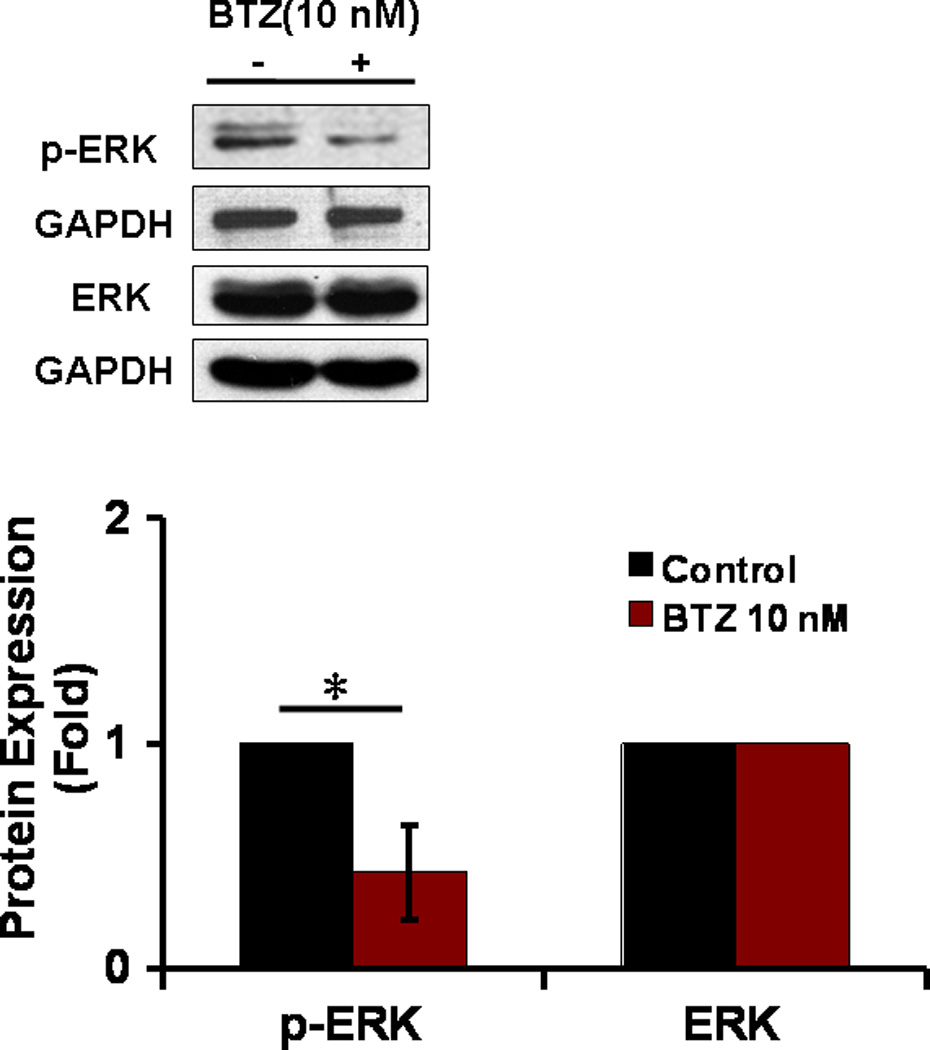
The expression of ERK in C2C12 cells. The expression of both phospho- and total ERK was assessed by immunoblotting. The bands were quantified by ImageJ and normalized to GAPDH. *P<0.01.
Discussion
Recently, proteasome inhibitors have been used to treat muscle wasting conditions in various animal models with some success. Using a hamster emphysema model, van Hees et al showed that BTZ treatment significantly increased diaphragm-specific force-generating capacity and completely restored myosin concentration 9-months after induction of emphysema [15]. In another study, BTZ prevented muscle loss induced by a 40% total body surface area full-thickness scald burn [16]. Beehler et al studied the effect of the BTZ in a rat denervation model. After 7 days of treatment, BTZ significantly reduced denervation-induced atrophy in the soleus but not in the EDL muscle [14]. However, Krawiec et al showed that BTZ only partially prevented the weight loss of gastrocnemius muscle associated with 3 days of immobilization [22]. Similarly, Supinski et al showed that BTZ treatment prevented endotoxin-induced muscle loss in the diaphragm but did not prevent endotoxin induced reductions in diaphragm-specific force.
The reduction of specific force was associated with elevated caspase-3 activity, which was not inhibited by BTZ [23]. The authors have shown previously that a specific caspase-3 inhibitor (DEVD-CHO) prevented endotoxin induced reduction of muscle force [24,25]. A recent study showed that caspase-3 activation is also involved in immobilization induced muscle atrophy [26].
Our data revealed that caspase-3 is activated in C2C12 cells in response to BTZ treatment. Caspase-3 activation led to apoptosis, as shown by our Annex V assay. Whether BTZ could induce apoptosis in myofibers remains unknown. It has been shown that proteasome inhibitors induce apoptosis in proliferating rather than quiescent cells [19,27]. Because skeletal muscle fibers are not proliferating cells, it is possible that BTZ may not have any major cytotoxic effect in vivo when used to treat muscle wasting conditions as reported by others. However, caspase-3 activation may lead to the cleavage of muscle fibers, which could trigger the cascade of muscle breakdown and result in reduced muscle force. Furthermore, BTZ may cause apoptosis in muscle progenitor/stem cells which will proliferate in response to injury.
In summary, although our data suggest that BTZ can induce apoptosis in C2C12 cells. These data alone do not preclude the potential use for treatment of muscle wasting. Nonetheless, caspase-3 activation may cause muscle weakness even though it may not induce apoptosis in muscle fibers. Caution should be taken when using BTZ in the clinic and to select the lowest dose possible to avoid potential side effect. More preclinical studies using different models of wasting are certainly warranted.
Highlights.
Bortezomib (BTZ) inhibits myogenin expression and myotube formation.
BTZ causes cell cycle arrest in C2C12 cells.
BTZ reduces C2C12 cell viability.
BTZ induced apoptosis in C2C12 cells.
The apoptosis induced by BTZ was associated with reduced expression of p-ERK.
Acknowledgments
This work was supported by the project for the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and by grants from National Institute of Health R01HL080682 and R01 HL070241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chauhan D, Bianchi G, Anderson KC. Targeting the UPS as therapy in multiple myeloma. BMC Biochem. 2008;9(Suppl 1):S1. doi: 10.1186/1471-2091-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 3.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 4.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 5.Elliott PJ, Ross JS. The proteasome: a new target for novel drug therapies. Am J Clin Pathol. 2001;116:637–646. doi: 10.1309/44HW-5YCJ-FLLP-3R56. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park) 2004;18:14–21. [PubMed] [Google Scholar]
- 7.San-Miguel JF, Richardson PG, Gunther A, Sezer O, Siegel D, Blade J, Leblanc R, Sutherland H, Sopala M, Mishra KK, Mu S, Bourquelot PM, Victoria Mateos M, Anderson KC. Phase ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013;31:3696–3703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 9.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 10.Agten A, Maes K, Thomas D, Cielen N, Van Hees HW, Dekhuijzen RP, Decramer M, Gayan-Ramirez G. Bortezomib partially protects the rat diaphragm from ventilator-induced diaphragm dysfunction. Crit Care Med. 2012;40:2449–2455. doi: 10.1097/CCM.0b013e3182553a88. [DOI] [PubMed] [Google Scholar]
- 11.Bach HHt, Laporte HM, Wong YM, Gamelli RL, Majetschak M. Proteasome inhibition prolongs survival during lethal hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;74:499–507. doi: 10.1097/TA.0b013e31827d5db2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayavarapu S, Coley W, Van der Meulen JH, Cakir E, Tappeta K, Kinder TB, Dillingham BC, Brown KJ, Hathout Y, Nagaraju K. Activation of the ubiquitin proteasome pathway in a mouse model of inflammatory myopathy: a potential therapeutic target. Arthritis Rheum. 2013;65:3248–3258. doi: 10.1002/art.38180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madeddu C, Mantovani G. An update on promising agents for the treatment of cancer cachexia. Curr Opin Support Palliat Care. 2009;3:258–262. doi: 10.1097/SPC.0b013e3283311c6f. [DOI] [PubMed] [Google Scholar]
- 14.Beehler BC, Sleph PG, Benmassaoud L, Grover GJ. Reduction of skeletal muscle atrophy by a proteasome inhibitor in a rat model of denervation. Exp Biol Med (Maywood) 2006;231:335–341. doi: 10.1177/153537020623100315. [DOI] [PubMed] [Google Scholar]
- 15.van Hees H, Ottenheijm C, Ennen L, Linkels M, Dekhuijzen R, Heunks L. Proteasome inhibition improves diaphragm function in an animal model for COPD. Am J Physiol Lung Cell Mol Physiol. 2011;301:L110–L116. doi: 10.1152/ajplung.00396.2010. [DOI] [PubMed] [Google Scholar]
- 16.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292:R328–R336. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 17.Voortman J, Giaccone G. Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report. BMC Cancer. 2006;6:129. doi: 10.1186/1471-2407-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacihanefioglu A, Tarkun P, Gonullu E. Acute severe cardiac failure in a myeloma patient due to proteasome inhibitor bortezomib. Int J Hematol. 2008;88:219–222. doi: 10.1007/s12185-008-0139-7. [DOI] [PubMed] [Google Scholar]
- 19.Nowis D, Maczewski M, Mackiewicz U, Kujawa M, Ratajska A, Wieckowski MR, Wilczynski GM, Malinowska M, Bil J, Salwa P, Bugajski M, Wojcik C, Sinski M, Abramczyk P, Winiarska M, Dabrowska-Iwanicka A, Duszynski J, Jakobisiak M, Golab J. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol. 2010;176:2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeki I, Terai S, Fujisawa K, Takami T, Yamamoto N, Matsumoto T, Hirose Y, Murata Y, Yamasaki T, Sakaida I. Bortezomib induces tumor-specific cell death and growth inhibition in hepatocellular carcinoma and improves liver fibrosis. J Gastroenterol. 2013;48:738–750. doi: 10.1007/s00535-012-0675-z. [DOI] [PubMed] [Google Scholar]
- 21.Ronda AC, Vasconsuelo A, Boland R. 17beta-Estradiol Protects Mitochondrial Functions through Extracellular-Signal-Regulated Kinase in C2C12 Muscle Cells. Cell Physiol Biochem. 2013;32:1011–1023. doi: 10.1159/000354502. [DOI] [PubMed] [Google Scholar]
- 22.Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab. 2005;289:E969–E980. doi: 10.1152/ajpendo.00126.2005. [DOI] [PubMed] [Google Scholar]
- 23.Supinski GS, Vanags J, Callahan LA. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. 2009;296:L994–L1001. doi: 10.1152/ajplung.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–R1597. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- 25.Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol. 2006;100:1770–1777. doi: 10.1152/japplphysiol.01288.2005. [DOI] [PubMed] [Google Scholar]
- 26.Talbert EE, Smuder AJ, Min K, Kwon OS, Powers SK. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J Appl Physiol. 1985;114(2013):1482–1489. doi: 10.1152/japplphysiol.00925.2012. [DOI] [PubMed] [Google Scholar]
- 27.Drexler HC, Risau W, Konerding MA. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. FASEB J. 2000;14:65–77. doi: 10.1096/fasebj.14.1.65. [DOI] [PubMed] [Google Scholar]


