Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) is associated with difficulty inhibiting impulsive, hyperactive, and off-task behavior. However, no studies have examined whether a distinct pattern of brain activity precedes inhibitory errors in typically developing (TD) children and children with ADHD. In healthy adults, increased activity in the default mode network, a set of brain regions more active during resting or internally focused states, predicts commission errors, suggesting that momentary lapses of attention are related to inhibitory failures.
Method
Event-related functional magnetic resonance imaging and a go/no-go paradigm were used to explore brain activity preceding errors in 13 children with ADHD and 17 TD controls.
Results
Comparing pre-error with pre-correct trials, TD children showed activation in the precuneus/posterior cingulate cortex and parahippocampal and middle frontal gyri. In contrast, children with ADHD demonstrated activation in the cerebellum, dorsolateral prefrontal cortex (DLPFC), and basal ganglia. Between-group comparison for the pre-error versus pre-correct contrast showed that children with ADHD showed greater activity in the cerebellum, DLPFC, and ventrolateral PFC compared with TD controls. Results of region-of-interest analysis confirmed that the precuneus/posterior cingulate cortex are more active in TD children compared with children with ADHD.
Conclusions
These preliminary data suggest that brain activation patterns immediately preceding errors differ between children with ADHD and TD children. In TD children, momentary lapses of attention precede errors, whereas pre-error activity in children with ADHD may be mediated by different circuits, such as those involved in response selection and control.
Keywords: ADHD, children, functional magnetic resonance imaging, commission error, go/no-go task
Difficulties in response inhibition and attentional deficits have been widely reported in children with attention-deficit/hyperactivity disorder (ADHD). These difficulties are indicated by the high commission error rates and intrasubject variability (ISV) of reaction time (RT) on a range of inhibitory control tasks.1–4 The go/no-go task is a response inhibition task in which go trials are presented in consecutive groups, with no-go trials rarely interspersed throughout the task. Behavioral performance in the go/no-go task has been shown to be related to the number of preceding go trials. Specifically, typically developing (TD) children are more likely to commit an error when larger numbers of go trials precede a no-go trial,5 suggesting that deficits in sustained attention may underline impaired inhibitory control in TD children during a go/no-no task. In contrast, children with ADHD do not show a significant effect of the number preceding go trials, with commission errors occurring even after a single go trial.5 Moreover, the use of a variable interstimulus interval (ISI) during go/no-go task performance has been shown to decrease ISV in children with ADHD but not in TD controls,6 suggesting that with constant ISI children with ADHD may rely on more automatic processes, such as time estimation, thus increasing the likelihood of being unprepared when a no-go trial is presented. Although limited, these behavioral data indicate that response inhibition errors in ADHD may relate to a more complex pattern of cognitive deficits in response timing, selection, and control. As such, response inhibition errors in ADHD may relate to frontostriatal abnormalities involved in response selection and control, resulting in failure to inhibit unwanted responses.3,5,7–9
Despite evidence for response inhibition deficits in children with ADHD, we are unaware of any studies that have examined whether a distinct pattern of brain activity precedes errors (pre-error). Examining the neural correlates of pre-error trials has implications for understanding brain systems that mediate cognitive and motor preparedness in ADHD.
Neural activation occurring before errors has been examined in healthy adults.10,11 Using a stop signal task, a standard response inhibition task, Li et al.11 reported increased activity in the posterior cingulate cortex (PCC) and the medial prefrontal cortex (mPFC) during trials immediately before commission errors compared with trials before correct inhibition (pre-correct). Based on these findings, the investigastors suggested that errors of commission are preceded by increased activity within the default mode network (DMN), a set of cortical regions, including the precuneus, PCC, and mPFC. Similarly, Eichele et al.10 used a speeded flanker task and independent components analysis to show that relatively greater activity within the precuneus, together with less activity within task-activated regions, increased the likelihood of subsequent error commission. In a study examining the neural basis of attentional lapse in healthy adults, Weissman et al.12 reported that slower RTs in a global-local selective attention task (on all trial types) were associated with increased activity in the PCC, precuneus, and middle temporal gyrus. Similarly, Mason et al.13 found that the DMN was relatively more active when individuals performed a rehearsed, attentionally undemanding working memory task compared with a novel working memory task. Moreover, the degree of activity in the precuneus/PCC and the mPFC was directly correlated with greater subject report of stimulus-independent thought and reflective of mind-wandering. Collectively, these data from healthy adults suggest increased DMN activity before attentional lapse and task-related errors, including commission errors. Consistent with this, Sonuga-Barke and Castellanos14 proposed a “default-mode interference hypothesis,” positing that increased DMN activity intrudes into task-specific processing, resulting in attentional lapse and compromised task performance. Increased DMN activity during task performance thus may contribute to deficits in attention and subsequent impairments in behavioral performance during a response inhibition task.14–16
Consistent with the adult data, if attentional lapses impair performance in TD children, as suggested by an increased commission error rate when larger numbers of go trials precede a no-go trial, increased DMN activity should be expected in trials preceding an error. However, behavioral data in ADHD do not show an effect of the number preceding go trials on commission errors,5 suggesting that commission errors may be related to deficits in response selection and control, rather than increased DMN activity.
In this study, previously acquired event-related functional magnetic resonance imaging (fMRI) data during go/no-go task performance were reanalyzed to investigate whether a particular neural signature precedes commission errors in children with ADHD and TD children. Based on the findings of adult studies,10,11 greater PCC and mPFC activity was expected during pre-error go trials compared with pre-correct go trials in TD children,14 but not in children with ADHD. Preliminary findings in ADHD suggest that response inhibition deficits in ADHD are related to a more complex pattern of cognitive deficits in response selection and control. Because there are no previous fMRI studies investigating the neural correlates of pre-error trials in ADHD, the present study had no specific a priori hypothesis regarding brain regions showing increased activity for the ADHD group.
METHOD
Participants
Thirteen children with ADHD (nine boys; mean age = 10.6 years, standard deviation [SD] = 1.4, range = 8–13 years; mean IQ = 109.2, SD = 5.2, range = 88–137; Conner's Parent Rating Scale–Revised [CPRS-R], mean = 74.3, SD = 10.9, range = 54–90) and 17 TD children (eight boys; mean age = 10.5 years, SD 1.2, range = 8–13 years; mean IQ = 108.8, SD = 15.4, range = 81–135; CPRS-R, mean = 45.1, SD = 4.3, range = 40–55) were matched for age, gender, IQ, and commission error rate in the go/no-go task. Only children with a commission error rate of 25% or higher were included in the analysis. These criteria were implemented to ensure sufficient statistical power for fMRI analyses and to limit performance variability across the two groups. Subjects were selected from a larger group of 26 children with ADHD and 42 TD children who successfully completed the go/no-go task during fMRI scanning in previous research studies.17–19 All children had a Full-Scale IQ higher than 80 as measured by the WISC (third or fourth edition).20,21
Three children with ADHD had comorbid oppositional-defiant disorder and one of these children also met criteria for simple phobia. Two children with ADHD were being treated with stimulant medication; their parents were asked to withhold the medication on the day before and the day of testing. Withholding of medication was confirmed for both children by parent report on the day of testing. Children included in the TD group had no evidence of psychopathology on any of the study measurements and were not taking any psychoactive medication (Supplement 1, available online).
The study was conducted at the Kennedy Krieger Institute in Baltimore. Written consent was provided by a parent or guardian for all subjects, and assent was obtained from the participating child. All study procedures were approved by the Johns Hopkins University School of Medicine institutional review board.
Psychiatric Measures
The Diagnostic Interview for Children and Adolescents, Fourth Edition (parent interview), and ADHD-specific behavior rating scales, the CPRS-R and Conners' Parent Teacher Rating Scale–Revised (CTRS-R, long form) were administered to both groups of children.22,23 Children were included in the ADHD group if the Diagnostic Interview for Children and Adolescents, Fourth Edition confirmed a DSM-IV-TR diagnosis and if at least one parent and one teacher rating scale was positive. A child neurologist (S.H.M.) confirmed that all children with ADHD met DSM-IV-TR criteria. The CPRS-R/CTRS-R and DSM-IV criteria were also used to evaluate ADHD subtype. Ten children met criteria for ADHD combined type and three met criteria for inattentive type. The Diagnostic Interview for Children and Adolescents, Fourth Edition was also used to assess the presence of comorbid psychiatric disorders.
Go/No-Go Paradigm
During the fMRI scan, children performed a simple go/no-go task. Green and red spaceships were presented one at a time on the screen; subjects were asked to push a button with the right index finger as quickly as possible each time a green spaceship appeared (“go” trials) and to refrain from pushing the button when a red spaceship appeared (“no-go” trials). Stimuli were presented for 300 ms, followed by a fixation cross that was displayed for 1,500 ms. Subjects had time to respond until the next stimulus appeared. Go trials were presented in consecutive groups of three to seven green ships, whereas no-go trials never appeared more than twice in a row. Thus, no-go stimuli were jittered, with a varying number of preceding go stimuli. The task was divided into two 5-minute runs, each with 95 go and 32 no-go trials. Each run began and ended with a 10-second rest period; four 10-second rest periods also occurred at irregular intervals during each run (see4,18,19 and Supplement 1 and Figure S1, available online).
Consistent with the study goal, the primary contrast examined brain regions that were more active during pre-error trials compared with pre-correct trials. For each subject, the mean RT and ISV were calculated for pre-error go trials and pre-correct go trials. ISV was calculated as the SD RT divided by the mean RT.24 Because of the small number of go trials preceding an omission and go trials preceding an anticipatory response, ISV and RT for these two trials types were not included in the behavioral analysis.
fMRI Procedures
Images were acquired on a 1.5-T ACS-NT Powertrack 6000 MRI scanner (Philips Medical Systems, Best, Netherlands) using a body coil transmission and quadrature end-capped head-coil reception. Image processing was carried out using SPM2 (Wellcome Department of Imaging Neuroscience, Wellcome Trust Centre for Neuroimaging, London, UK). No subjects demonstrated greater than 3.5 mm of motion in any direction (smaller than one voxel; Supplement 1).
Data Analysis
Demographic and behavioral data were examined using two-tailed independent-sample t tests. Separate repeated measurements analyses of variance (RM-ANOVA) was performed on RTs and ISVs with group (ADHD versus TD) as the independent variable and trial type (pre-error, pre-correct, go) as the within-subject variable. Fisher post hoc test was performed to examine main effects and interactions. Analyses were conducted using StatView 5.0.1 (SAS Institute, Inc., Cary, NC). All data were reported as mean ± standard error of the mean, and statistical significance was set at p < .05 two-tailed alternatives.
Functional data were analyzed using SPM5 to construct and examine the fit of the voxel-wise time course data to a general linear model. Instead of using the canonical hemodynamic response functional, an optimized hemodynamic impulse response latency was estimated for each subject to more accurately account for ISV in hemodynamic response latency. The general linear model coefficients were estimated for multiple hemodynamic response functional latencies (time to peak 2.5 seconds after stimulus to 9.5 seconds after stimulus in increments of 250 ms). The optimal hemodynamic response functional latency was estimated as the latency at which the activation amplitude was highest for the “correct go” trial in the left primary motor cortex (BA4). Optimal latencies were not significantly different in the TD children (mean = 5.34 seconds, SD = 0.40) and children with ADHD (mean = 5.04 seconds, SD = 0.46; unpaired t test, p > .5).
Using the optimized latency for each subject, event-related response amplitudes were estimated using the general linear model. Nine task-related regressors and six motion-related regressors were modeled. The task-related regressors included the two main trials of interest (pre-correct go trials and pre-error go trials), correct no-go trials, incorrect no-go trials (commission errors), omission errors, anticipatory responses (occurring <200 ms after stimulus presentation), correct go trials preceding another correct go trial, go trials preceding an omission, and go trials preceding an anticipatory response.
Contrast maps were created for each subject depicting brain activity during pre-error trials compared with pre-correct trials. Individual subject contrasts were entered into a second-level analysis to examine activation for each group separately (one-sample t tests) and group differences in activation (two-sample t test). Whole-brain random effects analyses were performed using a spatial extent cluster size threshold to achieve a corrected statistical threshold of p = .05 based on the number of voxels in the brain and the spatial smoothness of the data. In addition to the whole-brain contrasts, a region-of-interest (ROI) analysis was performed to pursue the hypothesis of group differences in activation within the PCC and mPFC during pre-error versus pre-correct trials. A 15-mm ROI sphere within the PCC area and the mPFC was extracted from the WFU Pickatlas of the SPM Toolbox25 (corresponding to RSN2 clusters 1 and 3 in Table 1, p1365).
TABLE 1.
Performance Measurements in the Go/No-No Task
| ADHD (n = 13) | TD (n = 17) | |
|---|---|---|
| % Commission errorsa | 40.7 (3.0) | 36.0 (2.2) |
| % Omission errorsa | 6.3 (1.5)c | 2.0 (0.7) |
| Go RT (ms) | 397.4 (10.3) | 377.7 (13.7) |
| Pre-error RT (ms)b | 330.8 (7.6) | 326.5 (10.1) |
| Pre-correct RT (ms) | 406.4 (17.0) | 369.6 (17.6) |
| Go ISV | 0.42 (0.04)c | 0.33 (0.01) |
| Pre-error ISVb | 0.28 (0.02)c | 0.23 (0.02) |
| Pre-correct ISV | 0.37 (0.04)c | 0.31 (0.04) |
Note: Results are reported as mean (standard error of the mean).
Groups were matched for percentage of commission error (p < .20).
Reaction time (RT) for pre-error was significantly faster than RT for pre-correct and go trials, and intrasubject variability (ISV) for pre-error was significantly lower than ISV for pre-correct and go trials (p < .001) for both groups.
Children with attention-deficit/hyperactivity disorder (ADHD) showed a larger percentage of omission errors (p < .02) and higher ISV for all trial types (p < .03) than typically developing (TD) children.
Location of voxels significantly associated with contrasts of interest were determined by summarizing local maxima separated by at least 8 mm and converting maxima from Montreal Neurological Institute to Talaraich coordinate space using formulas provided by Matthew Brett (Medical Research Council, Cognition and Brain Sciences Unit; www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
RESULTS
Behavioral Data
Table 1 present behavioral performance on the go/no-go task. Groups were matched for commission error rate (p > .2). The ADHD group showed a significantly larger percentage of omission errors than the TD group (t28 = 2.74, p < .02).
Analysis of RT using RM-ANOVA showed no effect of group (ADHD versus TD; p > .2), a main effect of trial type (F2,56 = 37.42, p < .001), but no group by trial type interaction (p > .1). Fisher post hoc analysis indicated that pre-error RT was significantly faster than pre-correct RT and go RT (p < .001).
Analysis of ISV using RM-ANOVA showed a main effect of group (F2,56 = 9.42, p < .03) as indicated by higher ISV in the ADHD group compared with controls and a main effect of trial type (F2,56 = 9.42, p < .001), indicating that pre-error ISV was significantly lower than pre-correct ISV and go ISV (p < .001). No group by trial type interactions (p > .7) were present.
fMRI Data
Within-Group Analysis of Blood Oxygenation Level Dependency Signal during Pre-Error Trials versus Pre-Correct Inhibition Trials
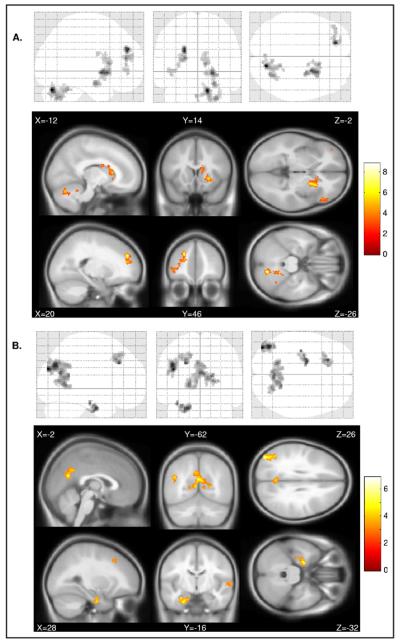
In the TD group, the one-sample t test showed greater activation during pre-error trials compared with pre-correct trials in four regions: the left superior occipital and angular gyrus (BA19/39), the right parahippocampal gyrus (BA28), the right middle frontal gyrus (BA8), and the precuneus/PCC (BA23/30; corrected p < .05, corresponding to a minimum cluster size of 148 at p < .005 uncorrected; Figure 1A, Table 2; and Figure S2, available online).
FIGURE 1.
Within-group glass brain representation in 13 children with attention-deficit/hyperactivity disorder (A) and 17 typically developing children (B) of blood oxygenation level dependency (BOLD) contrasts during trials preceding commission errors (pre-error) compared with trials preceding correct inhibition (pre-correct). Note: Neurologic convention is used (i.e., right = right hemisphere).
TABLE 2.
Regions of Activation Associated With Pre-Error versus Pre-Correct Inhibition Trials
| Extent (Voxels) | † (Peak Voxel) | Region | BA | x | y | z | Hem |
|---|---|---|---|---|---|---|---|
| TD group | |||||||
| 384 | 6.88 | superior occipital G | 19 | 42 | −82 | 28 | R |
| angular G | 39 | 44 | −70 | 34 | R | ||
| middle temporal G | 39 | 44 | −62 | 26 | R | ||
| 184 | 5.75 | parahippocampal G | 28 | 20 | −6 | −28 | R |
| hippocampus | 36 | −16 | −22 | R | |||
| hippocampus | 28 | −10 | −24 | R | |||
| 213 | 5.39 | middle frontal G | 8 | 22 | 30 | 36 | R |
| middle frontal G | 8 | 22 | 24 | 42 | R | ||
| middle frontal G | 8 | 14 | 30 | 48 | R | ||
| 922 | 5.10 | precuneus | 23 | −2 | −62 | 22 | L |
| posterior cingulate | 30 | 18 | −56 | 6 | R | ||
| ADHD group | |||||||
| 420 | 8.86 | cerebellum (declive) | −14 | −72 | −26 | L | |
| cerebellum (pyramis) | −28 | −66 | −38 | L | |||
| cerebellum (culmen) | −16 | −48 | −26 | L | |||
| 255 | 7.77 | superior frontal G | 9 | 20 | 46 | 28 | R |
| medial frontal G | 9 | 20 | 44 | 18 | R | ||
| 304 | 6.82 | putamen | −22 | 6 | 28 | L | |
| putamen | −24 | 14 | 18 | L | |||
| amygdala | −26 | −4 | 8 | L | |||
| 146 | 5.59 | caudate body | −12 | 18 | 10 | L | |
| caudate body | −12 | 12 | 18 | L | |||
| caudate body | −12 | 0 | 24 | L | |||
| ADHD > TD | |||||||
| 343 | 4.96 | superior frontal G | 10 | 22 | 48 | 28 | R |
| medial frontal G | 9 | 20 | 44 | 20 | R | ||
| superior frontal G | N/A | 24 | 50 | 2 | R | ||
| 243 | 4.76 | cerebellum (declive) | −18 | −64 | −30 | L | |
| cerebellum (dentate) | −18 | −54 | −28 | L | |||
| cerebellum | −22 | −58 | −38 | L | |||
| 254 | 4.31 | inferior frontal G | 45 | −50 | 30 | −2 | L |
| inferior frontal G | 47 | −46 | 36 | −14 | L | ||
| inferior frontal G | 47 | −54 | 36 | −10 | L |
Note: Coordinates are in Montreal Neurological Institute (MNI) space. ADHD = attention-deficit/hyperactivity disorder; BA = Brodmann area; G = gyrus; Hem = hemisphere; L = left; N/A = not applicable; R = right; TD = typically developing.
In the ADHD group, the one-sample t test of the pre-error versus pre-correct contrast revealed four regions of significant activation: the left cerebellum, the right superior/medial frontal gyrus (BA9), the left putamen, and the left cau-date (corrected p < .05, corresponding to a minimum cluster size of 128 at p < .005 uncorrected; Figure 1B, Table 2).
Between-Group Analysis of Blood Oxygenation Level Dependency Signal during Pre-Error Trials versus Pre-Correct Inhibition Trials
Two-sample t tests examining for group differences in the pre-error minus pre-correct contrast at the whole-brain level revealed three suprathreshold clusters of increased neural activity in the ADHD group (corrected p < .05, corresponding to a minimum cluster size of 202 at p < .005 uncorrected; Figure 2A, B, Table 2): the right superior/medial frontal gyrus (BA10/9, dorsolateral PFC [DLPFC]), the left cerebellum, and the left inferior frontal gyrus (BA45/47; ventrolateral PFC [VLPFC]). No significant suprathreshold cluster was found in the TD versus ADHD group contrast.
FIGURE 2.
(A) Glass brain and sectional representation of regions of increased activity in the attention-deficit/hyperactivity disorder (ADHD) group compared with the typically developing (TD) group in trials preceding commission errors (pre-error) compared with trials preceding correct inhibition (pre-correct). Children with ADHD showed higher activation in the right dorsolateral prefrontal cortex (DLPFC), left ventrolateral prefrontal cortex (VLPFC), and left cerebellum (CB) compared with TD children. Note: Neurological convention is used (i.e., right = right hemisphere). (B) Percentage of signal change in the right DLPFC, left VLPFC, and left CB for pre-error trials and pre-correct inhibition trials in the ADHD (blue) and TD (green) groups.
Results from this ROI analysis using a small volume correction indicated that TD subjects demonstrated greater activity in the PCC compared with the ADHD group at a corrected threshold of p = .05 (corresponding to a cluster size of 461 voxels at p < .05 uncorrected, t = 3.47, x =−12, y = −54, z = 16; x = −14, y = −54, z = 26; x = 10, y = −58, z = 18; Montreal Neurological Institute coordinates, BA30/31/23; Figure 3). No significant differences were found in the mPFC (Supplement 1).
FIGURE 3.

Percentage of signal change in the precuneus/posterior cingulate cortex for pre-error trials and pre-correct inhibition trials in attention-deficit/hyperactivity disorder (ADHD, blue) and typically developing (TD, green) groups.
DISCUSSION
This study examined error-predictive brain patterns in children with ADHD and TD children. Similar to data in healthy adults, TD children shoed activated regions of the DMN, including the precuneus/PCC and the parahippocampal gyrus, during pre-error trials compared with pre-correct trials. In contrast, children with ADHD showed a different pattern of pre-error activation, with increased activity in the cerebellum, the DLPFC, and the basal ganglia, specifically the caudate and putamen. Between-groups comparisons confirmed this pattern of findings. Children with ADHD demonstrated increased cerebellum, DLPFC, and VLPFC activation compared with TD controls during pre-error trials compared with pre-correct trials. Follow-up ROI analyses confirmed that activity in the precuneus/PCC was higher in TD children than in childred with ADHD during pre-error trials. Together, increased activity in cerebellar and PFC regions coupled with the lack of increased DMN activity in ADHD suggests that behavioral processes other than attentional lapses, such as deficits in response selection and control, may contribute to inhibitory errors in this group.
The left cerebellum, right DLPFC, and left VLPFC showed increased blood oxygenation level dependency (BOLD) signal in the ADHD compared with the TD groups during pre-error (versus pre-correct) trials. The left lateral cerebellum and right DLPFC are considered part of the neural network that, together with the basal ganglia, is implicated in time perception and estimation.26 In the present study, the regular presentation of stimuli every 1.5 seconds likely resulted in both groups relying on automatic motor responses, as manifested by the shorter RT for pre-error trials compared with pre-correct trials. However, relying on this constant temporal distance during go trials may increase the likelihood of motor unpreparedness for no-go trials, predisposing to a commission error. Although time processing during a go/no-go task may be relatively effortless in the TD group, this process may require a more active engagement of the cerebellum and DLPFC in the ADHD group. Deficits in temporal processing, such as impairments in time estimation and decreased tolerance to delays, and differences in cerebellar and prefrontal activity have been widely reported in children and adults with ADHD.27–30 Moreover, variability in timing of stimulus presentation during the go/no-go task has been shown to decrease ISV in children with ADHD but not in TD controls.6 It is, therefore, possible that during conditions of constant ISI, deficits in timing processing affected performance, whereas this played less of a role when ISI varied from trial to trial and a more automatic timing strategy could not be used.
Alternatively, it is possible that the increased DLPFC activation reflected the need for greater cognitive control and thus represented a compensatory mechanism engaged before error trials. We recently analyzed RT and BOLD signal changes related to error and post-error trials in same population reported in the present study.17 Although we did not find differences between the ADHD and TD groups during errors, the post-error trials analysis showed increased ISV and increased temporal cortex activation in children with ADHD compared with controls. Moreover, in ADHD there was a correlation between increased temporal cortex activity and lower variability. These findings are interpreted as indicative of a compensatory mechanism engaged, although with limited efficacy, to overcome for attentional deficits. In support of this hypothesis, it should be noted that, although omission errors were relatively low, the ADHD group showed a higher omission error rate (6.4%) compared with the TD group (2%), indicative of attentional deficits in children with ADHD. It is therefore possible that the increased cerebellum and DLPFC activity reported in the present study represented a compensatory strategy. However, although in the post-error trials analysis there was a clear correlation between ISV and temporal cortex activity, no significant correlation was found for ISV during pre-error trials and BOLD activity.
The cerebellum and DLPFC are also important for the control and selection of actions and higher-order behaviors. Several groups of investigators have found decreased activation of regions critical to response control during successful response inhibition, including the (pre-)supplementary motor areas and anterior cingulate in ADHD.19,31–33 It is possible that the main source of commission errors in children with ADHD was related to inefficient use of motor response selection and control mechanisms necessary for withholding an unwanted response.4,34,35 Previous studies examining RT distribution in children with and without ADHD have shown greater ISV in different behavioral tasks,2,3 and increased ISV in the ADHD group was also found in the present study for all trial types. It is likely that increased ISV in children with ADHD is related to deficits in different cognitive domains, including motor selection and sustained attention. Normal RTs combined with occasional long response times36,37 and greater variability when occasionally long RTs are excluded from the analysis3,38 have been reported in ADHD, indicating that lapses of attention and impaired response preparation contribute to greater performance variability in ADHD. The present data suggest that impaired timing processing may increase motor unpreparedness and thus contribute to increase RT variability.
Children with ADHD also showed increased VLPFC activity compared with TD children. Previous studies have suggested that increased VLPFC activity and higher commission errors are associated with the persistence of ADHD symptoms in adolescents with childhood ADHD when compared with remitted and TD adolescents39,40 during successful inhibition using the go/no-go task. In the present study, the ADHD and TD groups were selected to have a 25% commission error rate to provide sufficient power for the analyses. Although a correlation was not found between VLPFC activity and ADHD-specific behavior rating scales (data not shown), it is possible that the ADHD group selected for the present analysis represents a cohort of children with more severe symptoms and/or developmental outcome. This may limit the generalizability of the present findings. It is therefore important in future studies to use a task that manipulates difficulty level to a subject's ability, thus eliciting similar error rates in all participants.
Although the whole-brain analysis indicated no pattern of increased activity in the TD compared with the ADHD groups, the ROI analysis showed increased precuneus/PCC activity before inhibitory errors in the TD compared with the ADHD group. Abnormal DMN activity, including in the precuneus/PCC, has been reported in several fMRI studies in children and adults with ADHD. Comparing performance during a paced go/no-go task with low and high incentives, Liddle et al.41 reported increased DMN activity for the low incentive condition in children with ADHD across trial types (go, correct, and incorrect inhibitory responses). Moreover, DMN activity was normalized by stimulant medication. Similarly, increased DMN activity was reported during a Stroop task in off-medication adolescents with ADHD and was normalized by stimulants.42 In addition, decreased PCC activity was reported in adults with ADHD during task switching.43 In contrast, increased precuneus/PCC activity was found during unsuccessful response inhibition during the stop-signal task in TD adolescents compared with medication-naive adolescents with ADHD.32 Overall these results support the hypothesis that functional abnormalities in the precuneus/PCC are present in ADHD and need to be further investigated.
This study has several limitations. First, the relatively small size of the groups may have limited the ability to detect pre-error activation in the whole-brain analysis; thus, the present results need to be considered preliminary. Second, intersubject heterogeneity may be a factor in the present findings, because the mechanisms underlying commission error may vary across ADHD subtypes. For example, attentional deficits may be more prominent in the inattentive subtype, whereas response inhibition deficits or the degree of pre-error motor preparedness and timing deficits may play a greater role in the hyperactive/impulsive subtype.44 Analysis of the latter may have been underpowered because 10 children had combined type ADHD in the present sample. Third, the sample was restricted to children with commission error rates of 25% or greater, limiting the generalizability of the present findings. Fourth, we hypothesized that increased precuneus/PCC activity was indicative of lapses of attention in the TD group but lacked a behavioral measurement of attention to confirm this hypothesis. The go/no-go task was designed to study response inhibition; thus, rapid stimulus presentation was necessary. However, BOLD fMRI time courses of rapid event-related designs are extremely difficult to visually interpret because of the integration of the rapid events, thus limiting the ability to evaluate temporal information and to exclude the impact of spikes cause by movement on the results. However, the exclusion criteria applied and the analysis of the motion regressors suggest that motion artifacts were unlikely to affect the present results. Future studies using different paradigms and including measurements of stimulus-independent thought and eye tracking may help to link attentional lapse to brain activity. Fifth, the present findings are limited to prepubertal children with and without ADHD. Given developmental changes in brain structure and function and in the ADHD pheno-type, the mechanisms mediating error prediction may differ across age groups. Future developmental studies are therefore warranted.
In conclusion, this is the first study examining pre-error neural activity in children with ADHD and TD children. Distinct neural processes were found to predict errors in these two groups of children. Increased precuneus/PCC and parahippocampal activity was found during pre-error trials in TD children, suggesting that attentional shifts toward an internal focus may predict commission errors. In contrast, children with ADHD showed increased activity in the left cerebellum, right DLPFC, and basal ganglia, which may reflect more widespread deficits in response control, attention, and timing before errors. These results are preliminary and require replication using larger samples and different behavioral tasks.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health grants R01NS048527 (S.H.M.), R01MH085328 (S.H.M.), and K02NS044850 (S.H.M.), the Developmental Disabilities Research Center (HD-24061), the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, the NIH-NCRR CTSA Program UL1-RR025005, and the NIH-NCRR P41-RR15241.
Footnotes
Disclosure: Drs. Spinelli, Joel, Vasa, Pekar, and Mostofsky, and Ms. Nelson report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.de Zeeuw P, Aarnoudse-Moens C, Bijlhout J, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008;47:808–816. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- 2.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intraindividual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wodka EL, Mahone EM, Blankner JG, et al. Evidence that response inhibition is a primary deficit in ADHD. J Clin Exp Neuropsychol. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- 5.Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 6.Ryan M, Martin R, Denckla MB, Mostofsky SH, Mahone EM. Interstimulus jitter facilitates response control in children with ADHD. J Int Neuropsychol Soc. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 9.Roth RM, Saykin AJ. Executive dysfunction in attention-deficit/ hyperactivity disorder: cognitive and neuroimaging findings. Psychiatr Clin North Am. 2004;27:83–96. ix. doi: 10.1016/S0193-953X(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 10.Eichele T, Debener S, Calhoun VD, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 13.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Spinelli S, Vasa RA, Suresh J, Nelson TE, Pekar JJ, Mostofsky SH. Variability in post-error behavioral adjustment is associated with functional abnormalities in the temporal cortex in children with ADHD. J Child Psychol Psychiatry. doi: 10.1111/j.1469-7610.2010.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suskauer SJ, Simmonds DJ, Fotedar S, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. Harcourt Assessment; San Antonio, TX: 1991. [Google Scholar]
- 21.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 4rd ed. Harcourt Assessment; San Antionio, TX: 2003. [Google Scholar]
- 22.Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. J Abnorm Child Psychol. 1997;25:487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- 23.Reich W. Diagnostic Interview for Children and Adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 25.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Smith A, Taylor E, Lidzba K, Rubia K. A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. Neuroimage. 2003;20:344–350. doi: 10.1016/s1053-8119(03)00337-9. [DOI] [PubMed] [Google Scholar]
- 27.Pollak Y, Kroyzer N, Yakir A, Friedler M. Testing possible mechanisms of deficient supra-second time estimation in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2009;23:679–686. doi: 10.1037/a0016281. [DOI] [PubMed] [Google Scholar]
- 28.Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Phil Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vloet TD, Gilsbach S, Neufang S, Fink GR, Herpertz-Dahlmann B, Konrad K. Neural mechanisms of interference control and time discrimination in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:356–367. [PubMed] [Google Scholar]
- 30.Mulder MJ, Baeyens D, Davidson MC, et al. Familial vulnerability to ADHD affects activity in the cerebellum in addition to the prefrontal systems. J Am Acad Child Adolesc Psychiatry. 2008;47:68–75. doi: 10.1097/chi.0b013e31815a56dc. [DOI] [PubMed] [Google Scholar]
- 31.Booth JR, Burman DD, Meyer JR, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 32.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 33.Tamm L, Menon V, Ringel J, Reiss AL. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 34.Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Percept Mot Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- 35.Rubia K, Russell T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 36.Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 38.Hervey AS, Epstein JN, Curry JF, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 39.Schulz KP, Fan J, Tang CY, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am J Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 40.Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry. 2005;44:47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- 41.Liddle EB, Hollis C, Batty MJ, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. doi: 10.1111/j.1469-7610.2010.02333.x. published online ahead of print November 12, 2010. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson BS, Potenza MN, Wang Z, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dibbets P, Evers EA, Hurks PP, Bakker K, Jolles J. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 2010;24:413–423. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- 44.Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.