Abstract
The 10 different genes associated with the deaf/blind disorder, Usher syndrome, encode a number of structurally and functionally distinct proteins, most expressed as multiple isoforms/protein variants. Functional characterization of these proteins suggests a role in stereocilia development in cochlear hair cells, likely owing to adhesive interactions in hair bundles. In mature hair cells, homodimers of the Usher cadherins, cadherin 23 and protocadherin 15, interact to form a structural fiber, the tip link, and the linkages that anchor the taller stereocilia's actin cytoskeleton core to the shorter adjacent stereocilia and the elusive mechanotransduction channels, explaining the deafness phenotype when these molecular interactions are perturbed. The conundrum is that photoreceptors lack a synonymous mechanotransduction apparatus, and so a common theory for Usher protein function in the two neurosensory cell types affected in Usher syndrome is lacking. Recent evidence linking photoreceptor cell dysfunction in the shaker 1 mouse model for Usher syndrome to light-induced protein translocation defects, combined with localization of an Usher protein interactome at the periciliary region of the photoreceptors suggests Usher proteins might regulate protein trafficking between the inner and outer segments of photoreceptors. A distinct Usher protein complex is trafficked to the ribbon synapses of hair cells, and synaptic defects have been reported in Usher mutants in both hair cells and photoreceptors. This review aims to clarify what is known about Usher protein function at the synaptic and apical poles of hair cells and photoreceptors and the prospects for identifying a unifying pathobiological mechanism to explain deaf/blindness in Usher syndrome.
Keywords: Usher syndrome, Cochlear hair cell, Photoreceptor, Mechanotransduction, Ribbon synapse
1. Introduction
Usher syndrome is a genetically heterogeneous disease affecting neurosensory cells in the cochlea, the retina, and for some clinical sub-types, the vestibular system. Ten genes have been associated to the 12 loci thus far identified in families with the syndrome (Adato et al., 2002; Fields et al., 2002; Weil et al., 1995, 2003; Ahmed et al., 2001; Bitner-Glindzicz et al., 2000; Bolz et al., 2001; Bork et al., 2001; Eudy et al., 1998; Verpy et al., 2000; Weston et al., 2004; Riazuddin et al., 2012). These genes encode proteins with a wide range of functions including an actin-binding molecular motor (myosin VIIA, USH1B), complex transmembrane cell adhesion molecules (cadherin 23, USH1D), protocadherin 15, USH1F), (Usherin long and short isoforms, USH2A), scaffold proteins (Harmonin, USH1C), (SANS, USH1G), (whirlin, USH2D), a G-protein coupled receptor (VLGR1, USH2C), a calcium binding protein (CIB2, USH1J) and a tetraspanin transmembrane protein (clarin-1, USH3A). All of these proteins are expressed in both hair cells and photoreceptors. In mouse models harboring mutations in the various genes associated with Usher syndrome (Table 1), there is a common developmental defect in stereocilia organization. In some cases the three rows are properly oriented within the cuticular plate, but the lengths are variable and the stereocilia have a splayed appearance. In others, the number, length, and orientation within the cuticular plate are affected. The universal observation of stereocilia abnormalities led earlier work to conclude that Usher proteins function in the development of the hair cell stereocilia bundles. This hypothesis was reinforced by the presence of the long isoform of Usherin and VLGR1 (very large G-protein coupled receptor type 1) at the ankle links during stereocilia development in the mouse. These structures are known to be transiently present during maturation of the bundles, and thought to be essential to proper bundle development. Further reinforcement was provided by definitive demonstration that protocadherin 15 and cadherin 23 form the tip links in stereocilia, and that harmonin links cadherin 23 to stereociliary F-actin at the upper tip link density (UTLD) (Grillet et al., 2009). While disruption of harmonin in the deaf circler mouse (dfcr) abolishes the UTLD, the tip links remain intact. Mechanotransduction is lost in this mouse, demonstrating an essential role for the harmonin/F-actin linkage in this process. More recently myosin VIIA (USH1B) and SANS (USH1G) were shown to localize to the UTLD in vestibular hair cells of the guinea pig, implicating these Usher proteins as structural components of the mechanotransduction system (Grati and Kachar, 2011). In addition to the protocadherin 15/cadherin 23/harmonin protein interactions, a number of other Usher protein interactions have been inferred through pull-down assays using heterologous cell lines and co-immunolocalization studies in both cochlear hair cells and photoreceptors, inferring the existence of one or more Usher protein interactomes, with the scaffold proteins, harmonin, whirlin and SANS playing key roles as protein linkers (reviewed in: El-Amraoui and Petit, 2005; Reiners et al., 2006; Kremer et al., 2006). In this regard, while harmonin and whirlin interact with Usher and non-Usher proteins mostly through their PDZ domains, SANS interactions take place through the ankyrin repeats present at its N-terminus. Mutations associated to these domains results in loss of specific interactions between the different Usher proteins (Siemens et al., 2002; El-Amraoui and Petit, 2005; Adato et al., 2005; Reiners et al., 2005a,b; Pan et al., 2009; van Wijk et al., 2009; Grillet et al., 2009; Yan et al., 2010; Kersten et al., 2010, 2012; Bahloul et al., 2010; Caberlotto et al., 2011; Wright et al., 2012). Combined, these reports have led to an emergent model for Usher protein function in vivo involving a role in the development of stereocilia hair bundles and as proteins comprising key structures of the mechanosentitive apparatus of stereociliary tip links. While this may be true, it is notable that photoreceptors do not have stereocilia or mechanosensitive apparatuses, yet photoreceptors express all of the Usher proteins (Reiners et al., 2006; Maerker et al., 2008) and progressively degenerate in Usher patients, establishing a critical function for these proteins in photoreceptor cell health. Furthermore, all of the Usher proteins (but not all variants) have been localized to the synapses of photoreceptors (Reiners et al., 2006) and most have also been demonstrated to be present at the synapses of cochlear hair cells (Kremer et al., 2006). In photoreceptors, besides at the synapse, Usher proteins localize to the region of the connecting cilia at the juncture of the inner and outer segments. Recently it was shown that harmonin associates with and inhibits Cav1.3 calcium channels at the presynaptic region of inner hair cell ribbon synapses (Gregory et al., 2011). It was recently demonstrated that both clarin1 and protocadherin 15 mutant mice show a delay in synaptic maturation (Zallocchi et al., 2012a). This same paper and several other reports (Reiners et al., 2003, 2005b; Lillo et al., 2006; Lagziel et al., 2009; Zallocchi et al., 2012b) demonstrated the existence of synaptic Usher protein complexes comprised of distinct Usher protein variants as well as the existence of specific synaptic vesicle trafficking machinery that directs the movement of complexes to either apically targeted or basally targeted regions of cochlear hair cells.
Table 1.
Usher genes and their associated disease.
| Usher gene | Protein | Associated diseases | Mouse models | Zebrafish models | References |
|---|---|---|---|---|---|
| MY07A | Myosin VIIA | USH1 BDFNA11 DFNB2, non-syndromic RP | shaker1; Myo7A null | mariner | Sang et al. (2013); Ammar-Khodja et al. (2009); Weil et al. (1995); Ernest et al. (2000); Ben Rebeh et al. (2010); Lopes et al. (2013) |
| USH1C | Harmonin | USH1C DFNB18 | dfcr; ush1c−/−; harmonin-PDZ2; Ush1c216AA | Ush1cfh293 | Johnson et al. (2003); Verpy et al. (2000); Lefevre et al. (2008); Grillet et al. (2009); Lentz et al. (2010); Tian et al. (2001); Seiler et al. (2004); |
| CDH23 | Cadherin 23 | USH1D DFNB12 | Waltzer | sputnik | Bolz et al. (2001); Schultz et al. (2011); Sollner et al. (2004) |
| PCDH15 | Protocadherin15 | USH1F DFNB23 | Ames Waltzer; noddy; pcdh15-CD1/CD2/CD3 | arbiter | Ahmed et al. (2003); Ahmed et al. (2001); Seiler et al. (2004); Webb et al. (2011); Geng et al. (2013) |
| USH1G | SANS | USH1G DFNA20/26 | Jackson shaker/Ush1gjs/js | Weil et al. (2003); Mustapha et al. (2006); Liu et al. (2007); | |
| CIB2 | Calcium integrin binding protein 2 | USH1F DFNB48 | Cib2tm1a(EUC0MM)Wtsi | Riazuddin et al., 2012; MGI | |
| USH2A | Usherin | USH2A non-syndromic RP (RP15) | Ush2a−/− | Ush2asa1881 | Ebermann et al. (2009); Kremer et al. (2006); Liu et al. (2007); Xu et al. (2011); ZIRC |
| GPR98 | Vlgr1/Gpr98 | USH2C | VLGR1/del7TM; VLGR1 ko | Weston et al. (2004); McMillan and White (2004); Yagi et al. (2005) | |
| DFNB31 | Whirlin | USH2D DFNB31 | Whirler; whirlinko | van Wijk et al. (2006); Mogensen et al. (2007); Mburu et al. (2006); Zou et al. (2011) | |
| CLRN1 | Clarin-1 | USH3A | Clrn1(−/−) | Adato et al. (2002); Zallocchi et al. (2009) |
Collectively, these studies suggest that Usher protein function in neurosensory cells may be more complex than previously thought. Indeed, numerous studies have demonstrated multiple protein variants for harmonin, cadherin 23, protocadherin 15, Usherin, whirlin, and VLGR1 (Reiners et al., 2003; Lagziel et al., 2005, 2009; Ahmed et al., 2006, 2008; Zallocchi et al., 2012a,b; van Wijk et al., 2006; Adato et al., 2005; Yagi et al., 2005; Wright et al., 2012). It is likely there are many more than currently identified; since they have been explored using a small number of available well qualified specific antibodies. Based on recent work, there is an emergent functional role for unique complexes of Usher proteins at the ribbon synapses as well. In this review, we summarize what is currently known about Usher protein function in stereocilia development, the hair cell mechanotransduction apparatus, in ribbon synaptogenesis and function, and at the periciliary region of the photoreceptor inner segments. The goal is to explain what is established and what is still speculative, with a special emphasis on addressing the consistencies and inconsistencies in our understanding of Usher protein functions in the two neurosensory cells affected by Usher syndrome, the hair cell and the photoreceptor.
2. Usher protein interactions and putative function in stereocilia development
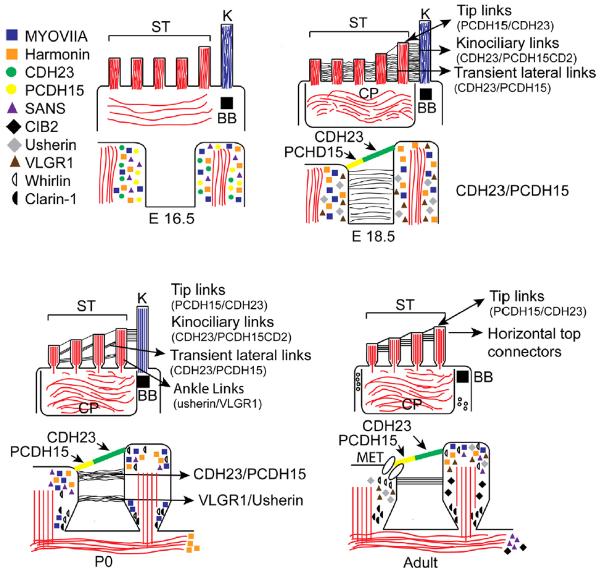
A common feature of all Usher animal models studied to date is some degree of stereociliary dysmorphology, presumably due to defective stereocilia development. A summary of the transient and permanent stereocilia links, and developmental patterns for localization of Usher proteins in the stereocilia is summarized in Fig. 1. In the mouse, stereocilia first emerge along with the kinocilium from developing hair cells at embryonic day 15 (E15) (Nishida et al., 1998; Holme et al., 2002). Between E15 and E18.5, the kinocilium migrates along with the stereocilia establishing the polarity of the stereocilia bundles. This process in perturbed in many of the Usher type I mouse models, where the kinocilium is mis-positioned, and the stereocilia bundles become fragmented, with abnormal numbers, lengths, and orientation (Lefèvre et al., 2008). As early as E16.5 many of the proteins encoded by genes associated with Usher type I are observed in the stereocilia tips with more uniform distribution along the lengths of the stereocilia by E17.5 (Lefèvre et al., 2008). This is the same timepoint when a dense network of transient lateral links are observed by scanning electron microscopy (Goodyear et al., 2005), and immunolocalization studies demonstrate that cadherin 23 and protocadherin 15 contribute to this dense cohesive network (Michel et al., 2005; Lefèvre et al., 2008; Goodyear et al., 2010), although which isoforms of these molecules are present in these electron-dense structures is not known. Between E16.5 and E18.5 stereocilia elongate, first nearest the kinocilium, and sequentially to the stereocilia most distal to the kinocilium, resulting in the “staircase” arrangement of stereocilia in the hair cell bundle (Nayak et al., 2007).
Fig. 1.
Early and late stages of cochlear hair bundle maturation showing expression and distribution of Usher proteins. The stereocilia (ST) that will form the mature hair bundle are held together by different links that vary during development. The first links can be detected at E18.5. Only the tip links and top connectors will persist in the adult hair cell bundle. By P0 the core of actin filaments insert rootlets into the cuticular plate (CP). In the adult cochlear hair cell bundle the kinocilia (K) regress and only its basal body (BB) remains. PCDH15, the lower component of the tip links, gates the mechanoelectrical transduction channels (MET). Usher protein localizations based on the following references: (Lagziel et al., 2005; Lefèvre et al., 2008; Boëda et al., 2002; Caberlotto et al., 2011; Grillet et al., 2009; Adato et al., 2005; Zallocchi et al., 2009, 2012a; McGee et al., 2006; Michalski et al., 2007; Reiners et al., 2005; van Wijk et al., 2006; Delprat et al., 2005; Kazmierczak et al., 2007).
By post-natal day 0 (P0) the ankle links are formed (Goodyear et al., 2005). These are transient structures located near the base of the stereocilia that disappear by P10. VLGR1 and the long isoforms of Usherin (isoforms B) have been shown to be structural components of these links (McGee et al., 2006; Michalski et al., 2007), and their expression patterns are temporally and spatially consistent with this notion. Recently, it has been shown that the Usher type 2 genetic modifier, PDZD7, co-localizes with Usherin and VLGR1 at the ankle link region, suggesting its possible involvement in stereocilia development (Ebermann et al., 2010; Grati et al., 2012). Mutations in the Usher type 2 genes result in a much milder stereocilia dysmorphic phenotype, and consistent with this, USH2A patients are not completely deaf, and can often function well with hearing aids. The long isoform of the protein encoded by the USH2D gene, whirlin, has been shown to co-localize with Usherin and VLGR1 at the ankle links and interact with them (van Wijk et al., 2006). A knockout mouse for whirlin shows whirlin dependency for localization of Usherin and VLGR1 at the ankle links, which could be interpreted as either interdependency for stable integration in the stereocilia or for directed transport to the apical domains of the hair cells (Yang et al., 2010). Earlier studies of whirler mice showed that the stereocilia were shorter and thicker, consistent with the knockout mouse, but curiously not consistent with the phenotype of VLGR1 knockout mice (Holme et al., 2002; Yagi et al., 2007). This discrepancy in phenotypes might be due to the recent finding that whirlin interacts with the actin bundling protein espin (Wang et al., 2012), and the functional role for this interaction might explain the blunted elongation and thickening of stereocilia in whirler and whirlin knockout mice. In adult mice, Usher proteins play a role in the molecular architecture of the tip links and the mechanotransduction apparatus which are discussed in detail below.
A large number of specific interactions between Usher type 1 and type 2 proteins have been inferred, primarily through a combination of co-localization by immunohistochemistry combined with GST pull down assays. In the latter, domains of Usher proteins are expressed as GST fusion peptides in a heterologous (non-neurosensory) cell culture system. This combination of approaches established a number of potential specific interactions that have been summarized in detail in earlier reviews (Kremer et al., 2006; Reiners et al., 2006). The interaction of harmonin with the actin cytoskeleton, where it functions in actin bundling, and the cytoplasmic tail of cadherin 23 has been confirmed in vivo, where harmonin is a critical component of the UTLD. Tip links form in the absence of harmonin interaction with cadherin 23, as demonstrated in the dfcr mouse model, but the animals are deaf, demonstrating that this complex is essential to mechanotransduction (Grillet et al., 2009). Likewise, the specific interaction between recombinant homodimers of cadherin 23 and protocadherin 15 as the structural components of the tip links has been firmly established (Kazmierczak et al., 2007; Lelli et al., 2010). More recently the functional role of the cytoplasmic domain 2 (CD2) of protocadherin 15 in cell polarity and stereocilia development was explored using a transgenic approach. Among the many variants of protocadherin 15 are those produced through alternative splicing of three distinct cytoplasmic domains, CD1, CD2, and CD3. Using a gene targeting approach, Webb et al. (2011) produced mutants that lacked either CD1, CD2, or CD3 domains. The CD1 and CD3 deletion mutants showed no developmental or functional defects in hair cell stereocilia. The CD2 deletion mutant showed defects in kinociliary links and hair bundle orientation, however all three mutants formed functionally normal tip links. In contrast, the av3J protocadherin 15 mouse mutant (a null allele) shows severe defects in stereocilia development. This study underscores the limitations of our current understanding of Usher protein function in stereocilia development. There are clearly additional protocadherin 15 isoforms that contribute to earlier mechanisms of stereocilia development. Recent biochemical analysis documents a number of protein variants for VLGR1, cadherin 23, and protocadherin 15 in the cochlea and the retina (Lagziel et al., 2009; Zallocchi et al., 2012a). Importantly, most of the variants in the two organs are the same molecular weights, which portends to similar functional roles for these proteins in the cochlea and the retina. A related study showed that specific protein variants of VLGR1 and protocadherin 15 are selectively and differentially trafficked to either the apical or basal aspect of cochlear hair cells (Zallocchi et al., 2012b). In this study, antibody preparations against two different regions of each protein were used for immunoblotting analysis using isolated P1 organs of Corti from the mouse, demonstrating variants recognized by both antibodies as well as variants recognized specifically by one or the other. While this work provides good information as to which protein variants are likely to be important to stereocilia development and/or maintenance, it underscores another significant limitation of our understanding of Usher protein function; the limited number of antibody preparations against these proteins are not likely to be comprehensive enough to identify all protein variants present in hair cells and photoreceptors. Thus we may miss functionally important Usher protein variants due to the limited number of antibodies available. An example of this is the presence of a VLGR1 variant recognized by an antibody against the EAR domain in the middle of the VLGR1 protein, in the stereocilia tips of mature (P30) hair cells (Zallocchi et al., 2012a, supplemental Figure 2). As mentioned, prior reports documented the presence of VLGR1 only in the ankle links of hair cell stereocilia and only from P2 to P10 (McGee et al., 2006; Michalski et al., 2007). The function of the variant found in the mature stereocilia remains unknown.
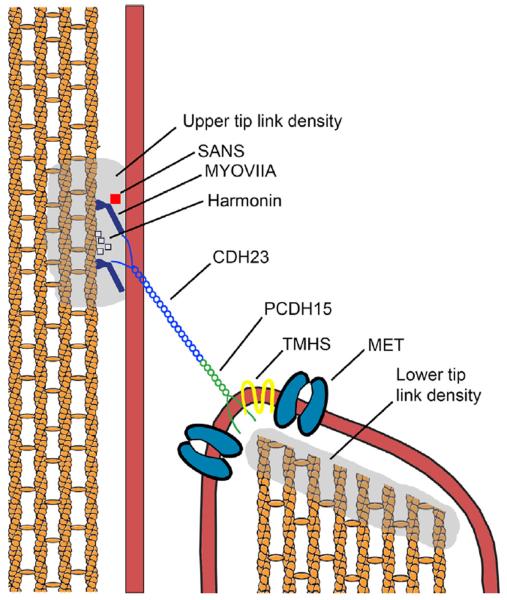
Fig. 2.
Usher proteins are building blocks of the hair cell mechanotransduction apparatus. Cadherin 23 homodimers and protocadherin 15 homodimers interact at their amino-terminal ends to form a rigid fiber that is anchored at the taller stereocilia via interaction of the carboxy-terminal ends of cadherin 23 with harmonin, which anchors the filament to the actin core. SANS and myosin VIIA are also implicated in the formation of this anchor at the upper tip link density. Protocadherin 15 carboxy termini are linked to the adjacent shorter stereocilia at the lower tip link density, where the as of yet unidentified mechanotransduction channels reside. Thus far, all that is known about this complex is that there is a functionally obligatory interaction with the tetraspanin TMHS (Xiong et al., 2012). The proteins that regulate the channel, as well as the channel itself, are an area of intense interest.
The differential Usher protein trafficking previously described (Lagziel et al., 2009; Zallocchi et al., 2012b) raises another important issue. Several examples, based on immunofluorescence analysis, have documented where the absence of one Usher protein from the stereocilia is associated with the absence of other Usher proteins (Lefèvre et al., 2008; Caberlotto et al., 2011; Yang et al., 2010). While this data is most often interpreted to mean that the protein interaction is required for stable integration of both proteins in stereocilia (or the periciliary region of the connecting cilia of photoreceptors), it might also indicate that the interaction is essential for proper directional transport of the complex to the stereocilia. Along these lines, recent data showed that the tetraspanin TMHS, which is an integral component of the mechanotransduction apparatus, must associate with protocadherin 15 to be properly targeted to the plasma membrane in cultured cells (Xiong et al., 2012). The data linking Usher protein function to the mechanotransduction apparatus is remarkably detailed and solid (described below). In contrast, data supporting the role of Usher proteins in cohesion of the stereocilia as the mechanism underlying the severe stereocilia phenotypes observed in most Usher type I mouse models is less rigorous. It is interesting that two different missense mutations in protocadherin 15 that affect the stability of tip link formation resulted in two very different phenotypes. The noddy mutation in the PCDH15 gene is in the region of interaction between the amino terminal domains of protocadherin 15 and cadherin 23 and disrupts the formation of tip links. This mouse has significant stereocilia dysmorphology (Geng et al., 2013). Another missense mutation in a calcium binding motif of the CDH23 gene of the salsa mouse model is the same mutation associated with DFNB12 (non syndromic hearing loss). Here the tip links form, but are gradually lost (Schwander et al., 2009). Both models show significant staining for their respective cadherins at a time point when interciliary links should be present, which have been shown to be comprised of cadherin 23 and protocadherin 15 in the chick (Goodyear et al., 2010). It would be of interest to know whether interciliary links are intact in either model (which was not reported) since it has been proposed that such links might regulate appropriate maturation of the hair bundle. Alternatively, hair bundle dysmorphology in the noddy mouse might be due to other protein interactions essential for proper bundle maturation. In either respect, the noddy mouse could provide a fruitful medium for exploring mechanisms underlying abnormal bundle development.
The example of the CD2 deletion mouse model for protocadherin 15, while supporting this cytoplasmic domain module in an important adhesive interaction interface between the kinocilium and the tallest stereocilia (Webb et al., 2011) demonstrates that the many isoforms of this protein, as well as the other Usher proteins, may have distinct functions, most of which are not yet known. Ultrastructural studies of hair cell development in the Ames Waltzer mouse model noted considerable disruption of the apical cytoskeleton as early as E16, a stage where the stereocilia are just beginning to emerge from the cuticular plate (Kikkawa et al., 2008). Thus it is possible that the severe stereocilia phenotypes reflect functional defects upstream of the cohesive interactions. There are a large number of endogenously expressed Usher protein variants that are selectively transported to the apical domain of cochlear hair cells that remain functionally uncharacterized (Zallocchi et al., 2012b).
3. Usher proteins, mechanotransduction, and the upper and lower tip link densities
Cochlear hair cells are unique in their ability to translate noise-initiated mechanical vibrations propagated across the basilar membrane into neurosensory input that is decoded in the cochlear nucleus into what we perceive as sound. This process is mediated through an apparatus comprised of stereociliary tip links that are anchored to the actin cytoskeleton of the taller stereocilia at one end, and to the mechanotransduction channel in the shorter adjacent stereocilia at the other. In the last seven years it has become clear that many of the proteins that comprise the mechanotransduction apparatus are associated with Usher syndrome (Sakaguchi et al., 2009). Biochemical analysis showed that the actual tip link itself is comprised of homodimers of cadherin 23, bound at its carboxy-terminal ends to the upper tip link density (UTLD) and protocadherin 15, bound at its carboxy-terminal ends to the lower tip link density (LTLD). The two homodimers interact at their amino-terminal ends forming the structural link between adjacent stereocilia and between the tallest stereocilia and the kinocilium (Kazmierczak et al., 2007; Indzhykulian et al., 2013). Myosin VIIA, SANS, and harmonin localize to the UTLD of mature cochlear hair cells, and mutations in the USH1C gene encoding harmonin abolish the UTLD and reduce the sensitivity of hair bundles to mechanical stimulation (Grillet et al., 2009; Grati and Kachar, 2011). In vitro studies show an interaction between myosin VIIA and myosin IC cytoplasmic tails and PHR1, suggesting a role for myosin VIIa in mechanotransduction slow adaptation (Etournay et al., 2010). The cytoplasmic domains of the cadherin 23 homodimer interact with harmonin-b and harmonin-b interacts with SANS which interacts with the MyTH4 domain and FERM domain of myosin VIIA. These specific interactions have been resolved by X-ray crystallography (Pan et al., 2009; Yan et al., 2010; Wu et al., 2011), and the ultra-high affinity of the harmonin-b multidomain interaction with cadherin 23 has been determined by surface plasmon resonance assay (Bahloul et al., 2010). Harmonin anchors the complex to the actin cytoskeleton (for a review, see Schwander et al., 2010). Collectively, these data provide a compelling model for the role of this Usher protein complex UTLD that anchors the cadherin 23 homodimer to the actin cytoskeleton.
The molecular composition of the lower tip link density is much less well characterized. There is an electron dense LTLD region that is thought to contain as of yet unidentified elastic filaments that allow fast adaptation to occur following the mechanical deflection of stereocilia in the direction of the tallest stereocilia (Eatock, 2000). The tip link itself is a rigid structure that does not have the elastic properties to confer the fast adaptation (Sotomayor et al., 2010). The cytoplasmic domain of protocadherin 15 is anchored to the LTLD, where it interacts with the tetraspanin TMHS. TMHS mutant mice are deaf, and fast adaptation is abolished (Longo-Guess et al., 2005; Xiong et al., 2012). It has been proposed that TMHS may facilitate both the transport and assembly of the, as of yet unidentified mechanotransduction channel which would be functionally coupled to the tip link apparatus via protocadherin 15 interaction (Xiong et al., 2012).
The well characterized molecular assemblage of the tip link, its connection to the actin cytoskeleton, and its clear role in mechanotransduction (summarized in Fig. 2) begs an important question: since mechanotransduction does not occur in photoreceptors, what is the cause of deafness for syndromic Usher missense mutations that specifically disrupt the mechanotransduction apparatus? Do these same mutations disrupt one function in cochlear hair cells, and another unrelated function in photoreceptors?
4. Usher proteins in synaptogenesis/maintenance
Even though early immunolocalization studies identified expression of Usher proteins at the synapse of both cochlear hair cells and photoreceptors, the function of the Usher proteins at these synapses has remained relatively unexplored, perhaps because of the aberrant morphology of the hair cell bundle, that is easily observed in the different Usher models by common microscopy techniques (Lefèvre et al., 2008; Geng et al., 2012; Riazuddin et al., 2012; Webb et al., 2011; Mogensen et al., 2007; Michalski et al., 2007; Ernest et al., 2000; Phillips et al., 2011; Seiler et al., 2004; Söllner et al., 2004). These early observations created a paradigm in which only the full length Usher variants were the ones associated with this syndrome and by transitive properties the focus of study at the hair cell stereocilia and at the connecting cilium of photoreceptor cells (Michalski et al., 2007; Maerker et al., 2008). However the emergence of a more complex picture with a diversity of Usher variants playing roles at both the apical and basal aspects of hair cells and photoreceptors is challenging this paradigm.
Hair cells and photoreceptors are sensory neurons that can transmit a broad range of information for long periods of time due to a specialized organelle termed the synaptic ribbon (reviewed in Zanazzi and Matthews, 2009). The function of the synaptic ribbon is to tether a large number of synaptic vesicles to the active zone where the neurotransmitter, mainly glutamate, can be released in response to calcium influx. Except perhaps for CIB2 (USH1J) distinct variants for all the known Usher associated proteins are present at the hair cell and photoreceptor synapses and at the neuronal terminals that innervate the base of cochlear and vestibular hair cells. This has been demonstrated by different groups (Lagziel et al., 2009; Kersten et al., 2010; Reiners et al., 2003, 2005a,b; Gregory et al., 2011; Zallocchi et al., 2009, 2012a,b; Williams et al., 2009; Phillips et al., 2011; Adato et al., 2002; Overlack et al., 2008; van Wijk et al., 2006; Yagi et al., 2007). The full length and small variants of cadherin 23, protocadherin 15 and VLGR1 can be detected in synaptosome preparations from mouse organ of Corti as well as neuroretina and by the use of antibodies recognizing specific domains within these molecules (Lagziel et al., 2009; Zallocchi et al., 2012a,b). The variant V3 of cadherin 23 (~30 kDa) is present at the synapses and neuronal terminals, where it co-localizes with SNAP25, a component of the SNARE complex (Lagziel et al., 2009; Zallocchi et al., 2012b). The small variants of protocadherin 15-CD1 and VLGR1 (~30 kDa) along with isoform 2 of clarin-1 are present at the hair cell and photoreceptor synapses and afferent neuronal fibers where they interact with each other and with cadherin 23-V3 forming a small synaptic complex. This complex associates with SNAP25 through VLGR1 suggesting their involvement in vesicle docking and fusion (Lagziel et al., 2009; Zallocchi et al., 2012a,b). The absence or loss of function of one of the components of the complex, i.e. clarin-1 or protocadherin 15 respectively, produces a delay in synaptic maturation and in type I afferent remodeling at the base of the outer hair cells (Zallocchi et al., 2012a), reinforcing the notion that some of these variants play a role in synaptogenesis. Because clarin-1 is a member of the hyperfamily of small tetraspanin proteins with a significantly degree of homology to stargazin, a protein that controls the expression and mobilization of the AMPA glutamate receptors to the synaptic cleft, a similar function for clarin-1 has been suggested at the hair cell and photoreceptor synapses (Adato et al., 2002).
In the case of harmonin (USH1C), isoform “a” is expressed at the base of hair cells and photoreceptors (Reiners et al., 2003; Gregory et al., 2011). Recent work by Gregory et al. (2011) demonstrates a presynaptic association between harmonin-a and Cav1.3 Ca2+ channels in mouse inner ear. This association (which increases with the maturation state of the hair cells) reduces the availability of the channels and therefore their functional levels at the cell surface, revealing a novel function for harmonin that goes beyond the scaffolding properties to a regulator of the electrical and calcium signals in hair cells. In zebrafish ush1c morphants (harmonin knockdowns) floating ribbon synapses are observed, which suggests a key role in retinal synaptogenesis and maintenance (Phillips et al., 2011). In mouse retina, whirlin (USH2D) shows an association with the Cav1.3 Ca2+ channels which suggests a potential role in calcium channel organization and membrane fusion (Kersten et al., 2010). Collectively, these studies not only demonstrate the importance of Usher protein synaptic function per se but also underscore the complexity of the syndrome where different variants are differentially trafficked to the apical or basal aspect of the cell to exert their specific functions through Usher and non-Usher protein interactions.
5. Usher protein function in photoreceptors
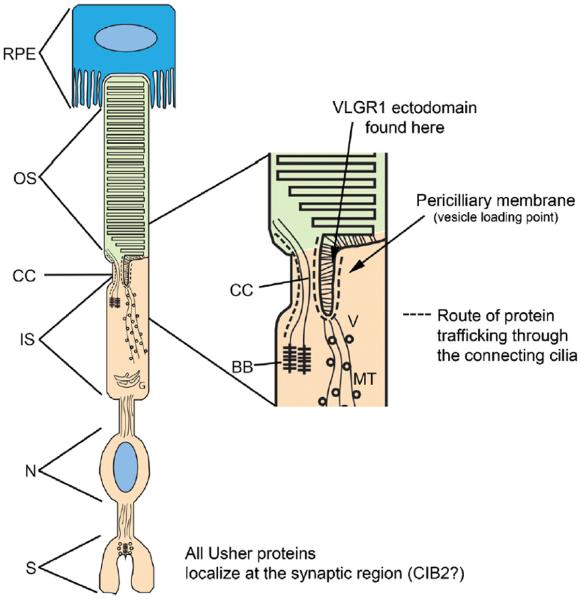
Usher protein function in retinal photoreceptors has been more difficult to explore, largely due to the mild or absent photoreceptor degeneration phenotype in the Usher mouse models (see Williams, 2008 review). Usher proteins localize to the periciliary region and basal bodies near the connecting cilia and to the synaptic region of the photoreceptors (Williams et al., 2009; Reiners et al., 2006; Maerker et al., 2008). Many of the same Usher protein variants for protocadherin 15, cadherin 23 and VLGR1 found in the cochlea are also found in the retina, providing evidence for the potential functional significance of these variants in both neurosensory cell types (Lagziel et al., 2009; Zallocchi et al., 2012a,b). The trafficking of these variants has not been explored in the photoreceptor. A summary of Usher protein localization in the photoreceptor is shown in Fig. 3.
Fig. 3.
Usher protein localization in rod photoreceptors. All Usher proteins identified thus far have been shown to localize at two regions in the photoreceptors by immunofluorescence microscopy; the region near the connecting cilium and the synaptic region. Two Usher proteins, myosin VIIA and usherinisoform a localize to the retinal pigment epithelium as well (Bhattacharya et al., 2001; Gibbs et al., 2003). Usherinisoform b, VLGR1, whirlin, and SANS have been shown to localize to the periciliary membrane region by immunogold labeling studies, which is a docking area for vesicular trafficking (Maerker et al., 2008). The EAR domain of VLGR1, which is in the middle of the molecule, has been shown to localize to the space between the periciliary basement membrane and the ciliary plasma membrane (McGee et al., 2006). Based on the presence of Usher proteins near the vesicle loading point at the periciliary membrane it has been proposed that they may play a role in vesicle transport between the inner segments and the outer segments of photoreceptors. BB, basal bodies; CC, connecting cilium; G, Golgi; IS, inner segment; MT, microtubules; N, nucleus; OS, outer segment; RPE, retinal pigment epithelium; S, synaptic region; V, vesicles.
Based on the protein interactions and localization at the periciliary membrane complex, an area near the connecting cilium implicated in membrane trafficking to the outer segments, it has been proposed that the Usher protein complexes might play a role in the trafficking of vesicular cargo between the inner and outer segments of photoreceptors (Reiners et al., 2006; Maerker et al., 2008). Consistent with this notion, it has been reported that opsin accumulates in the connecting cilium of shaker 1 mice (Liu et al., 1999). While this observation was interpreted as suggesting a direct role for the actin binding molecular motor, myosin VIIA, in opsin transport through the cilium, it may reflect a general role for Usher proteins in the transport of cargo between the inner and outer segments of photoreceptors. A recent study demonstrated that shaker 1 mice have an elevated light threshold, following dark adaptation, for activating the transport of α-transducin from the outer segments to the inner segments of rod photoreceptors (Peng et al., 2011). The light dependent translocation of α-transducin is thought to play an important role in buffering the photoresponse from high light sensitivity to low light sensitivity (Sokolov et al., 2002). Shaker-1 mice are susceptible to light induced rod photoreceptor degeneration under conditions that do not affect strain matched wild type mice (Peng et al., 2011), suggesting that the defective transport and photoreceptor degeneration might be functionally linked in the shaker-1 mice. Myosin VIIA functions in the retinal pigment epithelium in the localization and motility of melanosomes (Gibbs et al., 2004). More recently it was shown that myosin VIIA functions light-dependent localization of the isomerase RPE65 to the central region of the retinal pigment epithelium. Mice lacking functional myosin VIIA were shown to have reduced RPE65 activity (Lopes et al., 2011). Thus, it is clear that myosin VIIA is likely functionally important for both the RPE cells and the photoreceptor cells. Additional work will be required to determine whether photoreceptor degeneration in USH1B is caused by the RPE defects, the photoreceptor cell defects, or both.
It has been suggested that the mouse is not a good model for retinal degeneration associated with USH1D because the longest isoform of cadherin 23, which is an essential component of the tip links in cochlear hair cells, is not expressed in the mouse photoreceptors, while it is expressed in primate photoreceptors (Lagziel et al., 2009). The functional importance of this longest isoform of cadherin 23 in retinal photoreceptors is unknown, so this conclusion is based on untested assumptions. Among the Usher mouse models that do show a retinal degeneration phenotype, the knock-in mutation for the c.216G > A cryptic splice site mutation in exon 3 of the USH1C gene is an exception. This mouse shows abnormal electroretinograms by 1 month of age, and significant loss of photoreceptors between 6 and 12 months of age (Lentz et al., 2010). The dfcr and ush1c−/− mice, also harboring mutations in the USH1C gene do not have a strong retinal phenotype (Williams, 2008; Williams et al., 2009; Tian et al., 2010), but are deaf. These disparate observations suggest that the functionally important isoform(s) for harmonin in the retina and in the cochlea may be distinct.
A recent report suggests that calycal processes in photoreceptor cone cells are synonymous structures to hair cell stereocilia (Sahly et al., 2012). This same paper demonstrates, using immunofluorescence and scanning electron microscopy imaging, that the calycal processes present in frog and monkey cone photoreceptors are enriched in Usher type 1 proteins, while mouse cone photoreceptors, which do not have calycal processes, show a different distribution pattern of the Usher type I proteins, and complete absence of harmonin and cadherin 23. Based on these findings, the authors suggest that this is why Usher mouse models do not have a retinal degeneration phenotype. The published literature suggests problems with this assumption. First, several studies have demonstrated that all Usher proteins, and in most cases several protein variants, are indeed expressed in mouse photoreceptors. Most of these variants are present at the connecting cilia, periciliary region and photoreceptor synapses and in contrast to what was suggested by Sahly et al., 2012, Usher protein presence at the calycal processes is not restricted to type I only (Goodyear and Richardson, 1999; Reiners et al., 2003, 2005a,b, 2006; Lillo et al., 2006; Maerker et al., 2008; Lagziel et al., 2009; Williams et al., 2009; Zallocchi et al., 2010, 2012a,b). Importantly, recent studies demonstrate a robust light-dependent retinal phenotype for the shaker 1 mice (Peng et al., 2011). Finally, a harmonin knock-in mouse mutant showed a robust retinal degeneration phenotype (Lentz et al., 2010). Collectively, these studies clearly show that Usher proteins are expressed and functionally important in mouse photoreceptors.
6. Therapeutic strategies in Usher syndrome
The retina is an attractive target for therapeutic intervention due to its immune-privileged nature and its accessibility for mildly invasive sub-retinal delivery of therapeutic agents. There have been several attempts to validate this approach for the treatment of retinal disease associated with Usher syndrome. Lentiviral-based gene delivery of human myosin VIIA has been shown to rescue the melanosome migration and opsin-mislocalization phenotypes in a knockout mouse model for USH1B (Hashimoto et al., 2007). Sub-retinal delivery of a functional whirlin construct transcribed from a photoreceptor cell-specific rhodopsin kinase promoter demonstrated the restoration of a VLGR1/whirlin/UsherinisoformB complex at the connecting cilium of the mouse (Zou et al., 2011), suggesting that this approach might rescue the retinal degeneration phenotype in humans. Although gene therapy is potentially suitable for some types of Usher syndrome, it is not suitable for the very large Usher genes, Usherin and VLGR1 (Liu et al., 2007; McGee et al., 2006). The use of aminoglycosides (specifically NB30 and NB54) and a chemically unrelated molecule, PTC124, has been shown in vitro to induce translational read through of stop codons caused by missense mutations in both the USH1C (Goldmann et al., 2010, 2012) and USH1D (Rebibo-Sabbah et al., 2007) genes. These translational read-through-inducing drugs have the advantages of not being gene-specific, allowing the treatment of a diverse range of genetic diseases; the size of the mutated gene is not important as well as the number and expression of the mutated isoforms and the expression of the gene is under endogenous control.
More recently, a targeted zinc finger nuclease was successfully used to actually correct a point mutation in the USH1C gene in vitro, providing proof of concept for the emergent technology of gene correction (Overlack et al., 2012). Just months ago, the first example of a therapy that successfully rescues both hearing and balance defects for an Usher gene was demonstrated. Here antisense oligonucleotide formulations directed at a splice mutation in the USH1C gene were injected intraperitoneally and shown to induce a sustained (several months) rescue of both phenotypes (Lentz et al., 2013). Collectively, these works predict that therapeutic intervention to slow or arrest the retinal (and possibly the inner ear) defects associated with Usher syndrome are forthcoming.
7. Concluding remarks
The major focus of Usher protein research on stereocilia development and mechanotransduction has yielded a wealth of evidence regarding the molecular origin of developmentally dynamic hair bundle links as well as an exquisite emergent molecular design for the hair cell mechanotransduction apparatus. It is clear from these works that Usher protein function is essential for both the development and function of the stereocilia. The role of these specific Usher protein variants in synaptic development and function is only beginning to be explored, although recent reports portend these distinct Usher protein complexes do play roles in the development and function of ribbon synapses. Although the delayed onset and progressive nature of the retinal phenotype in Usher patients make this organ a suitable target for therapeutic intervention, very little is known about Usher protein function in these cells beyond immunolocalization studies and hypothetical models based largely on in vitro protein interactions. Progress has been hampered by the weak to absent retinal phenotype in Usher mouse models, however recent data demonstrating protein transport defects and sensitivity to moderate light induced retinal degeneration in the shaker 1 mouse model could change this if indeed these phenotypes are generally applicable to other or all Usher mouse models. Finally, the highly dysmorphic stereocilia in Usher type 1 mice suggest that molecular functions of Usher proteins upstream of the initiation of stereocilia development (earlier than E16.5 in the mouse) might be at play, for example regulated trafficking of proteins to the apical microdomain of cochlear hair cells. Such a mechanism might apply to the protein trafficking defects that have been documented in photoreceptors of the shaker 1 mouse (and now whirler, manuscript under revision), which could explain the retinal degeneration phenotype, and thus provide a shared mechanistic paradigm for Usher protein function and pathology in both hair cells and photoreceptors.
Acknowledgements
This work was supported by NIH grants R01DC004844 and 5 P20 RR018788. The authors thank John (Skip) Kennedy for help in the preparation of figures.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CD
cytoplasmic domain
- CDH23
cadherin 23
- CIB2
calcium integrin binging protein 2
- dfcr
deaf circler
- EAR
epilepsy associated repeat
- FERM
4.1 protein, ezrin, radixin, moesin
- GST
glutathione S-transferase
- LTLD
lower tip link density
- MyTH4
myosin tail homology 4
- PCDH15
protocadherin 15
- PDZ
post synaptic density protein (PSD95)
- (Dlg1)
Drosophila disk large tumor suppressor
- (zo-1)
Zonula occludens-1 protein
- PDZD7
PDZ domain containing 7
- RP
Retinitis pigmentosa
- RPE
retinal pigment epithelium
- RPE65
retinal pigment epithelium-specific 65 kDa protein
- SNAP25
synaptosomal-associated protein 25
- SNARE
soluble NSF attachment protein receptor
- TMHS
tetraspan membrane protein of hair cell stereocilia
- USH
Usher syndrome
- UTLD
upper tip link density
- VLGR1
very large G-coupled protein receptor type 1.
References
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, Belenkiy O, et al. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur J Hum Genet. 2002;10:339–50. doi: 10.1038/sj.ejhg.5200831. [DOI] [PubMed] [Google Scholar]
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005;14:347–56. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–34. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, et al. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet. 2003;12:3215–23. doi: 10.1093/hmg/ddg358. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Aye S, Ali RA, Venselaar H, Anwar S, et al. Gene structure and mutant alleles of PCDH15: nonsyndromic deafness DFNB23 and type 1 Usher syndrome. Hum Genet. 2008;124:215–23. doi: 10.1007/s00439-008-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar-Khodja F, Faugère V, Baux D, Giannesini C, Léonard S, Makrelouf M, et al. Molecular screening of deafness in Algeria: high genetic heterogeneity involving DFNB1 and the Usher loci, DFNB2/USH1B DFNB12/USH1D and DFNB23/USH1F. Eur J Med Genet. 2009;52:174–9. doi: 10.1016/j.ejmg.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Bahloul A, Michel V, Hardelin JP, Nouaille S, Hoos S, Houdusse A, et al. Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet. 2010;19:3557–65. doi: 10.1093/hmg/ddq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Rebeh I, Morinière M, Ayadi L, Benzina Z, Charfedine I, Feki J, et al. Reinforcement of a minor alternative splicing event in MYO7A due to a missense mutation results in a mild form of retinopathy and deafness. Mol Vis. 2010;16:1898–906. [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya G, Miller C, Kimberling WJ, Jablonski MM, Cosgrove D. Localization and expression of Usherin: a novel basement membrane protein defective in people with Usher's syndrome type IIa. Hearing Res. 2001;163:1–11. doi: 10.1016/s0378-5955(01)00344-6. [DOI] [PubMed] [Google Scholar]
- Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat Genet. 2000;26:6–7. doi: 10.1038/79178. [DOI] [PubMed] [Google Scholar]
- Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–99. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, Nothwang HG, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27:108–12. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet. 2001;68:26–37. doi: 10.1086/316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E, Michel V, Foucher I, Bahloul A, Goodyear RJ, Pepermans E, et al. Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc Natl Acad Sci. 2011;108:5825–30. doi: 10.1073/pnas.1017114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprat B, Michel V, Goodyear R, Yamasaki Y, Michalski N, El-Amraoui A, et al. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum Mol Genet. 2005;14:401–10. doi: 10.1093/hmg/ddi036. [DOI] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Ebermann I, Koenekoop RK, Lopez I, Bou-Khzam L, Pigeon R, Bolz HJ. An USH2A founder mutation is the major cause of Usher syndrome type 2 in Canadians of French origin and confirms common roots of Quebecois and Acadians. Eur J Hum Genet. 2009;17:80–4. doi: 10.1038/ejhg.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, et al. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest. 2010;120:1812–23. doi: 10.1172/JCI39715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Petit C. Usher I syndrome: unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118:4593–603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–96. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Etournay R, Lepelletier L, Boutet de Monvel J, Cayet N, Leibovici M, Weil D, et al. Cochlear outer hair cells undergo an apical circumference remodeling constrained by the hair bundle shape. Development. 2010;137:1373–83. doi: 10.1242/dev.045138. [DOI] [PubMed] [Google Scholar]
- Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type lla. Science. 1998;280:1753–7. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- Fields RR, Zhou G, Huang D, Davis JR, Moller C, Jacobson SG, et al. Usher syndrome type III: revised genomic structure of the USH3 gene and identification of novel mutations. Am J Hum Genet. 2002;71:607–17. 23. doi: 10.1086/342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng R, Melki S, Chen DH, Tian G, Furness DN, Oshima-Takago T, et al. The mechanosensory structure of the hair cell requires clarin-1, a protein encoded by Usher syndrome III causative gene. J Neurosci. 2012;32:9485–98. doi: 10.1523/JNEUROSCI.0311-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng R, Sotomayor M, Kinder KJ, Gopal SR, Gerka-Stuyt J, Chen DH, et al. Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells. J Neurosci. 2013;33:4395–404. doi: 10.1523/JNEUROSCI.4514-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, et al. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci. 2004;117:6473–83. doi: 10.1242/jcs.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci. 2003;100:6481–6. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Overlack N, Moller F, Belakhov V, van Wyk M, et al. Baasov T, et al. A comparative evaluation of NB30, NB54 and PTC124 in translational read-through efficacy for treatment of an USH1C nonsense mutation. EMBO Mol Med. 2012;4:1186–99. doi: 10.1002/emmm.201201438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Rebibo-Sabbah A, Overlack N, Nudelman I, Belakhov V, Baasov T, et al. Beneficial readthrough of a USH1C nonsense mutation by designed aminoglycoside NB30 in the retina. Invest Ophthalmol Vis Sci. 2010;51:6671–80. doi: 10.1167/iovs.10-5741. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Forge A, Legan PK, Richardson GP. Asymmetric distribution of cadherin 23 and protocadherin 15 in the kinocilial links of avian sensory hair cells. J Comp Neurol. 2010;518:4288–97. doi: 10.1002/cne.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. The ankle-link antigen: an epitope sensitive to calcium chelation associated with the hair-cell surface and the calycal processes of photoreceptors. J Neurosci. 1999;19:3761–72. doi: 10.1523/JNEUROSCI.19-10-03761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci. 2011;108:11476–81. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati M, Shin JB, Weston MD, Green J, Bhat MA, Gillespie PG, et al. Localization of PDZD7 to the stereocilia ankle-link associates this scaffolding protein with the Usher syndrome protein network. J Neurosci. 2012;32:14288–93. doi: 10.1523/JNEUROSCI.3071-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory FD, Bryan KE, Pangršič T, Calin-Jageman IE, Moser T, Lee A. Harmonin inhibits presynaptic Cav1.3 Ca2+ channels in mouse inner hair cells. Nat Neurosci. 2011;14:1109–11. doi: 10.1038/nn.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, et al. Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron. 2009;62:375–87. doi: 10.1016/j.neuron.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14:584–94. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme RH, Kiernan BW, Brown SDM, Steel KP. Elongation of hair cell stereocilia is defective in the mouse mutant whirler. J Com Neurol. 2002;450:95–102. doi: 10.1002/cne.10301. [DOI] [PubMed] [Google Scholar]
- Indzhykulian A, Stepanyan R, Nelina A, Spinelli K, Ahmed Z, Belyantseva IA, et al. Molecular remodeling of tip links underlies mechanosensory regeneration in auditory hair cells. PLoS Biol. 2013;11:e1001583. doi: 10.1371/journal.pbio.1001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Hawes NL, et al. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet. 2003;12:3075–86. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–97. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kersten FFJ, van Wijk E, Hetterschijt L, Bauβ K, Peters TA, Aslanyan MG, et al. The mitotic spindle protein SPAG5/Astrin connects to the Usher protein network postmitotically. Cilia. 2012;1:2. doi: 10.1186/2046-2530-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten FFJ, van Wijk E, van Reeuwijk J, van der Zwaag B, Märker T, Peters TA, et al. Association of whirlin with Cav1.3 (α1D) channels in photoreceptors, defining a novel member of the Usher protein network. Invest Ophthalmol Vis Sci. 2010;51:2338–46. doi: 10.1167/iovs.09-4650. [DOI] [PubMed] [Google Scholar]
- Kikkawa YS, Pawlowski KS, Wright CG, Alagramam KN. Development of outer hair cells in Ames waltzer mice: mutation in protocadherin 15 affects development of cuticular plate and associated structures. Anat Rec (Hoboken) 2008;291:224–32. doi: 10.1002/ar.20632. [DOI] [PubMed] [Google Scholar]
- Kremer H, van Wijk E, Märker T, Wolfrum U, Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet. 2006;15:R262–70. doi: 10.1093/hmg/ddl205. [DOI] [PubMed] [Google Scholar]
- Lagziel A, Ahmed ZM, Schultz JM, Morell RJ, Belyantseva IA, Friedman TB. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lagziel A, Overlack N, Bernstein SL, Morell RJ, Wolfrum U, Friedman TB. Expression of cadherin 23 isoforms is not conserved: implications for a mouse model of Usher syndrome type 1D. Mol Vis. 2009;15:1843–57. [PMC free article] [PubMed] [Google Scholar]
- Lefèvre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, et al. A core cochlear phenotype in USH1 mouse mutants implicated fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–37. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- Lelli A, Kazmierczak P, Kawashima Y, Müller U, Holt JR. Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15. J Neurosci. 2010;30:11259–69. doi: 10.1523/JNEUROSCI.1949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz JJ, Gordon WC, Farris HE, MacDonald GH, Cunningham DE, Robbins CA, et al. Deafness and retinal degeneration in a novel USH1C knock-in mouse model. Dev Neurobiol. 2010;70:253–67. doi: 10.1002/dneu.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz JJ, Jodelka FM, Hinrich AJ, McCaffrey KE, Farris HE, Spalitta MJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med. 2013 doi: 10.1038/nm.3106. http://dx.doi.org/10.1038/nm.3106 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, et al. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci USA. 2007;104:4413–8. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–74. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C, Kitamoto J, Williams D. Roles and interactions of Usher 1 proteins in the outer retina. Adv Exp Med Biol. 2006;572:341–8. doi: 10.1007/0-387-32442-9_48. [DOI] [PubMed] [Google Scholar]
- Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zhend QY, Johnson KR. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci USA. 2005;102:7894–9. doi: 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes V, Boye S, Louie C, Boye S, Dyka F, Chiodo V, et al. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013 doi: 10.1038/gt.2013.3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Gibbs D, Libby RT, Aleman TS, Welch DL, Lillo C, et al. The Usher 1B protein, MYO7A, is required for normal localization and function of the visual retinoid cycle enzyme, RPE65. Hum Mol Genet. 2011;20:2560–70. doi: 10.1093/hmg/ddr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerker T, van Wijk E, Overlack N, Kersten FFJ, McGee J, Goldmann T. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17:71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- McMillan D, White P. Loss of the transmembrane and cytoplasmic domains of the very large G-proteincoupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol Cell Neurosci. 2004;26:322–9. doi: 10.1016/j.mcn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet. 2006;34:421–8. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- McGee J, Goodyear RJ, McMillan DR, Stauffer EA, Holt JR, Locke KG, et al. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J Neurosci. 2006;26:6543–53. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski N, Michel V, Bahloul A, Lefèvre G, Barral J, Yagi H. molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27:6478–88. doi: 10.1523/JNEUROSCI.0342-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Goodyear RJ, Weil D, Marcotti W, Perfettini I, Wolfrum U, et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev Biol. 2005;280:281–94. doi: 10.1016/j.ydbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Mogensen MM, Rzadzinska A, Steel KP. The deaf mouse mutant whirler suggests a role for whirlin in actin filament dynamics and stereocilia development. Cell Motil Cytoskeleton. 2007;64:496–508. doi: 10.1002/cm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M, Chouery E, Torchard-Pagnez D, Nouaille S, Khrais A, Sayegh FN, et al. A novel locus for Usher syndrome type I USH1G, maps to chromosome 17q24–25. Hum Genet. 2002;110:348–50. doi: 10.1007/s00439-002-0690-x. [DOI] [PubMed] [Google Scholar]
- Nayak GD, Ratnayaka HSK, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51:597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Rivolta MN, Holley MC. Timed markers for the differentiation of the cuticular plate and stereocilia in hair cells from the mouse inner ear. J Comp Neurol. 1998;395:18–28. [PubMed] [Google Scholar]
- Overlack N, Goldmann T, Wolfrum U, Nagel-Wolfrum K. Gene repair of an Usher syndrome causing mutation by zinc-finger nuclease mediated homologous recombination. Invest Ophthalmol Vis Sci. 2012;53:4140–6. doi: 10.1167/iovs.12-9812. [DOI] [PubMed] [Google Scholar]
- Overlack N, Maerker T, Latz M, Nagel-Wolfrum K, Wolfrum U. SANS (USH1G) expression in developing and mature mammalian retina. Vision Res. 2008;48:400–12. doi: 10.1016/j.visres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Pan L, Yan J, Wu L, Zhang M. Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23. Proc Natl Acad Sci. 2009;106:5575–80. doi: 10.1073/pnas.0901819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YW, Zallocchi M, Wang WM, Delimont D, Cosgrove D. Moderate light-induced degeneration of rod photoreceptors with delayed transducin translocation in shaker1 mice. Invest Ophthalmol Vis Sci. 2011;52:6421–7. doi: 10.1167/iovs.10-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JB, Blanco-Sanchez B, Lentz JJ, Tallafuss A, Khanobdee K, Sampath S, et al. Harmonin (Ush1c) is required in zebrafish Müller glial cells for photoreceptor synaptic development and function. Dis Model Mech. 2011;4:786–800. doi: 10.1242/dmm.006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebibo-Sabbah A, Nudelman I, Ahmed ZM, Baasov T, Ben-Yosef T. In vitro and ex vivo suppression by aminoglycosides of PCDH15 nonsense mutations underlying type 1 Usher syndrome. Hum Genet. 2007;122:373–81. doi: 10.1007/s00439-007-0410-7. [DOI] [PubMed] [Google Scholar]
- Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Reiners J, Reidel B, El-Amraoui A, Boëda B, Huber I, Petit C, et al. Differential distribution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Invest Ophthalmol Vis Sci. 2003;44:5006–15. doi: 10.1167/iovs.03-0483. [DOI] [PubMed] [Google Scholar]
- Reiners J, van Wijk E, Märker T, Zimmermann U, Jürgens K, te Brinke H, et al. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum Mol Genet. 2005a;14:3933–43. doi: 10.1093/hmg/ddi417. [DOI] [PubMed] [Google Scholar]
- Reiners J, Märker T, Jürgens K, Reidel B, Wolfrum U. Photoreceptor expression of the Usher syndrome type 1 protein protocadherin 15 (USH1F) and its interaction with the scaffold protein harmonin. Mol Vis. 2005b;11:347–443. [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, et al. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet. 2012;44:1265–71. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I, et al. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol. 2012;199:381–99. doi: 10.1083/jcb.201202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Tokita J, Müller U, Kachar B. Tip links in hair cells: molecular composition and role in hearing loss. Curr Opin Otolaryngol Head Neck Surg. 2009;17:388–93. doi: 10.1097/MOO.0b013e3283303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Yan X, Wang H, Feng R, Fei X, Ma D, et al. Identification and functional study of a new missense mutation in the motor head domain of myosin VIIA in a family with autosomal dominant hearing impairment (DFNA11) PLoS ONE. 2013;8:e55178. doi: 10.1371/journal.pone.0055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JM, Bhatti R, Madeo AC, Turriff A, Muskett JA, Zalewski CK, et al. Allelic hierarchy of CDH23 mutations causing non-syndromic deafness DFNB12 or Usher syndrome USH1D in compound heterozygotes. J Med Genet. 2011;48:767–75. doi: 10.1136/jmedgenet-2011-100262. [DOI] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Müller U. The cell biology of hearing. J Cell Biol. 2010;190:9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, et al. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci. 2009;106:5252–7. doi: 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, Burnside B, et al. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–38. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Siemens J, Kazmierczak P, Reynolds A, Sticker M, Littlewood-Evans A, Müller U, et al. The Usher syndrome proteins cadherin 23 and harmonin form a complex by means of PDZ-domain interactions. Proc Natl Acad Sci USA. 2002;99(23):14946–51. doi: 10.1073/pnas.232579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, et al. Massive lightdriven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Söllner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Müller U, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–9. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structural determinants of cadherin-23 function in hearing and deafness. Neuron. 2010;66:85–100. doi: 10.1016/j.neuron.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Liu X, Han F, Yu H, Longo-Guess C, Yang B, et al. Ush1c gene expression levels in the ear and eye suggest different roles for Ush1c in neurosensory organs in a new Ush1c knockout mouse. Brain Res. 2010;1328:57–70. doi: 10.1016/j.brainres.2010.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk E, Kersten FF, Kartono A, Mans DA, Brandwijk K, Letteboer SJ, et al. Usher syndrome and Leber congenital amaurosis are molecularly linked via a novel isoform of the centrosomal ninein-like protein. Hum Mol Genet. 2009;18:51–64. doi: 10.1093/hmg/ddn312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk E, van der Zwaag B, Peters T, Zimmermann U, Te Brinke H, Kersten FF, et al. The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum Mol Genet. 2006;15:751–65. doi: 10.1093/hmg/ddi490. [DOI] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet. 2000;26:51–5. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- Wang L, Zou J, Shen Z, Song E, Yang J. Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II. Hum Mol Genet. 2012;21:692–710. doi: 10.1093/hmg/ddr503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SW, Grillet N, Andrade LR, Ziong W, Swarthout L, Della Santina CC, et al. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development. 2011;138:1607–17. doi: 10.1242/dev.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Lainé S, et al. Usher syndrome type 1 G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet. 2003;12:463–71. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, et al. Defective myosin VIIIA gene responsible for Usher syndrome type 1B. Nat Genet. 1995;374:60–1. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Weston MD, Luijendijk MWJ, Humphrey KD, Möller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74:357–66. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433–41. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Aleman TS, Lillo C, Lopes VS, Hughes LC, Stone EM, et al. Harmonin in the murine retina and the retinal phenotypes of Ush1c-mutant mice and human USH1C. Invest Ophthalmol Vis Sci. 2009;50:3881–9. doi: 10.1167/iovs.08-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RN, Hong DH, Perkins B. RpgrORF15 connects to the Usher protein network through direct interactions with multiple whirlin isoforms. Invest Ophthalmol Vis Sci. 2012;53:1519–29. doi: 10.1167/iovs.11-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Pan L, Wei Z, Zhang M. Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo. Science. 2011;331:757–60. doi: 10.1126/science.1198848. [DOI] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, et al. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–95. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dai H, Lu T, Zhang X, Dong B, Li Y. Seven novel mutations in the long isoform of the USH2A gene in Chinese families with nonsyndromic retinitis pigmentosa and Usher syndrome Type II. Mol Vis. 2011;17:1537–52. [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Takamura Y, Yoneda T, Konno D, Akagi Y, Yoshida K, et al. Vlgr1 knockout mice show audiogenic seizure susceptibility. J Neurochem. 2005;92:191–202. doi: 10.1111/j.1471-4159.2004.02875.x. [DOI] [PubMed] [Google Scholar]
- Yagi H, Tokano H, Maeda M, Takabayashi T, Nagano T, Kiyama H, et al. Vlgr1 is required for proper stereocilia maturation of cochlear hair cells. Genes Cells. 2007;12:235–50. doi: 10.1111/j.1365-2443.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Pan L, Chen X, Wu L, Zhang M. The structure of the harmonin/sans complex reveals an unexpected interaction mode of the two Usher syndrome proteins. Proc Natl Acad Sci. 2010;107:4040–5. doi: 10.1073/pnas.0911385107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, et al. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet. 2010;6:e1000955. doi: 10.1371/journal.pgen.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi J, Delimont D, Meehan DT, Cosgrove D. Regulated vesicular trafficking of specific PCDH15 and VLGR1 variants in auditory hair cells. J Neurosci. 2012a;32:13841–59. doi: 10.1523/JNEUROSCI.1242-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Meehan DT, Delimont D, Askew C, Garige S, Gratton MA, et al. Localization and expression of clarin-1, the Clrn1 gene product, in auditory hair cells and photoreceptors. Hear Res. 2009;255:109–20. doi: 10.1016/j.heares.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Meehan DT, Delimont D, Rutledge J, Gratton MA, Flannery J, et al. Role for a novel Usher protein complex in hair cell synaptic maturation. PLoS ONE. 2012b;7:e30573. doi: 10.1371/journal.pone.0030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Sisson J, Cosgrove D. Biochemical characterization of native Usher protein complexes from a vesicular subfraction of tracheal epithelial cells. Biochemistry. 2010;49:1236–47. doi: 10.1021/bi9020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Mol Neurobiol. 2009;39:130–48. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Luo L, Shen Z, Chiodo VA, Ambati BK, Hauswirth WW, et al. Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors. Invest Ophthalmol Vis Sci. 2011;52:2343–51. doi: 10.1167/iovs.10-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]