Abstract
Principle of oocyte cryoinjury is first overviewed and then research history of cryopreservation using bovine oocytes is summarized for the last two decades with a few special references to recent progresses. Various types of cryodevices have been developed to accelerate the cooling rate and applied to the oocytes from large domestic species enriched with cytoplasmic lipid droplets. Two recent approaches include the qualitative improvement of IVM oocytes prior to the vitrification and the short-term recovery culture of vitrified-warmed oocytes prior to the subsequent IVF. Supplementation of L-carnitine to IVM medium of bovine oocytes has been reported to reduce the amount of cytoplasmic lipid droplets and improve the cryotolerance of the oocytes, but it is still controversial whether the positive effect of L-carnitine is reproducible. Incidence of multiple aster formation, a possible cause for low developmental potential of vitrified-warmed bovine oocytes, was inhibited by a short-term culture of the postwarm oocytes in the presence of Rho-associated coiled-coil kinase (ROCK) inhibitor. Use of an antioxidant α-tocopherol, instead of the ROCK inhibitor, also supported the revivability of the postwarm bovine oocytes. Further improvements of the vitrification procedure, combined with pre- and postvitrification chemical treatment, would overcome the high sensitivity of bovine oocytes to cryopreservation.
1. Introduction
Many reproductive biotechnologies have been applied to efficient production of large domestic animals, such as pigs and cattle with the high economic importance. In cattle, those originally developed in the decades of 1950s to 1970s include artificial insemination and multiple ovulations/embryo transfer combined with or without cryopreservation of spermatozoa [1] and preimplantation-stage embryos [2, 3], respectively. Successful cryopreservation of spermatozoa and embryos made these technologies more practical and available for commercial use, because of their potential advantages to allow long-distance transportation and to omit estrous synchronization, thus reducing the number of recipient female population to be maintained. Thereafter, embryo production by in vitro maturation (IVM) and in vitro fertilization (IVF) using immature oocytes from abattoir-derived ovaries and frozen-thawed spermatozoa became more or less routine since the decade of 1980s [2, 3], and production of cloned embryos has been promising with the progress of somatic cell nuclear transplantation [4].
Cryopreservation of unfertilized oocytes can be combined with these advanced reproductive technologies, in addition to its potential advantage as oocyte banking for preserving female genetic resources. Revivability of cryopreserved oocytes from small rodents and humans is extremely high, adapting well to the maintenance of the huge numbers of gene-modified transgenic strains and the efficient use in therapies for human infertility [5, 6]. However, in bovine species, the low fertilization rates and developmental competence of cryopreserved oocytes still need to be improved. Some review articles regarding this topic are available in cattle [7, 8] and pigs [8, 9]. In this paper, principle of oocyte cryoinjury is first overviewed. Then, research history of cryopreservation using bovine oocytes is summarized for the period from 1992 to 2013 with a few special references to very recent progress.
2. Basic Cryobiology for Oocytes
2.1. Events around Fertilization
Once oocytes resume the first meiotic division, the nuclear envelop (germinal vesicle: GV) is disintegrated, allowing the nuclear material to mix into the cytoplasm. Some alterations also occur in organelles such as mitochondria, cytoskeleton, and cortical granules. Microfilaments of actin are involved in cell shape modifications and movements, and microtubules (cylindrical bundle, composed from heterodimer of α- and β-tubulin) form the spindle apparatus [10]. A spermatozoon has a pair of distinct centriolar structures as the proximal centriole located within the connecting piece under the sperm head and the distal centriole organized vertically to the proximal counterpart and aligned with the sperm tail [11]. During fertilization in most mammalian species including cattle, spermatozoal centrosome, composed from the two centrioles and the pericentriolar materials such as γ-tubulin, centrin, and pericentrin, is brought into an oocyte. The centrosome plays a critical role in assembly of the microtubule network (sperm aster) that brings both male and female pronuclei to the center of the newly formed zygote [12]. Thus, the centrosome is considered to be the microtubule-organizing center (MTOC), with duplication during the pronuclear stage and the subsequent separation to serve as mitotic centers anchoring the chromosomes during the first cleavage [13, 14]. Abnormalities of the spindle/MTOC function/sperm aster have been shown to directly correlate with the loss of developmental potential after IVF, because they are crucial for completion of the second meiosis, extrusion of the polar body, migration of the pronuclei, and formation of the first mitotic spindle [15].
2.2. High Cryosensitivity of Oocytes
Biological activity is completely stopped at very low subzero temperature, and the cell viability and functional state may be preserved for long terms [16]. However, some physical stresses can damage cells at the various subzero temperatures. Intracellular ice formation is one of the biggest causes to cell damage; hence, the freezing protocols use a combination of dehydration, freezing point depression, supercooling, and intracellular vitrification in an attempt to avoid cell damages [17]. Therefore, it is important to use cryoprotective additive (CPA), such as dimethyl sulfoxide (DMSO), ethylene glycol (EG), or glycerol alone or in combination, when cryopreserving cells in any methods. Due to both of hydrophobic and hydrophilic characteristics, as well as the relatively small molecular weight, these CPAs are permeable to the plasma membrane. On the other hand, use of CPA induces some adverse effects such as osmotic injury and toxicity of the CPAs.
Incidence of cryoinjuries depends on the size and shape of the cell, the permeability of the cell membranes, and the quality of the cells. However, these factors differ from species, developmental stage, and origin [18]. Although offspring has been born using frozen-thawed oocytes from various species, the ability to support embryo development following cryopreservation procedures is still low. This may be attributed to the susceptibility of oocytes to damage during cooling and/or freezing and subsequent thawing because of their complex structure. Unfertilized oocytes are much larger than the blastomeres of an early embryo and therefore have a small surface to volume ratio [13]. This led to dehydration and penetration of CPA being difficult to achieve, which attributes to the difficulty in cryopreservation. Furthermore, the plasma membranes of oocytes differ significantly from those of embryos, partially due to the lack of aquaporin expression which affects the movement of water and CPAs [19]. There is a rise of intracellular free calcium during fertilization, which makes the ionic strength and membrane potential of the plasma membrane [20]. Other adverse effects of cryopreservation procedures include the fracture damage in zona pellucida [21] and the destruction of intercellular coupling via gap junctions between cumulus cells and the oocyte [22, 23]. In general, the primary criteria to assess postthaw viability of oocytes are the presence or absence of membrane degeneration, cytoplasmic abnormalities and zona pellucida fractures [24]. Recent studies in humans have examined the meiotic spindle using a polarized microscope apparatus, which allows the visualization of the polymerization of the meiotic spindle after vitrification and warming. However, this technique is difficult in domestic animal species due to their high cytoplasmic lipid content, which hinders spindle examination. Therefore, such dark oocytes from domestic species must be typically examined through invasive methods, such as fluorescence microscopy and biochemical or molecular analyses [25].
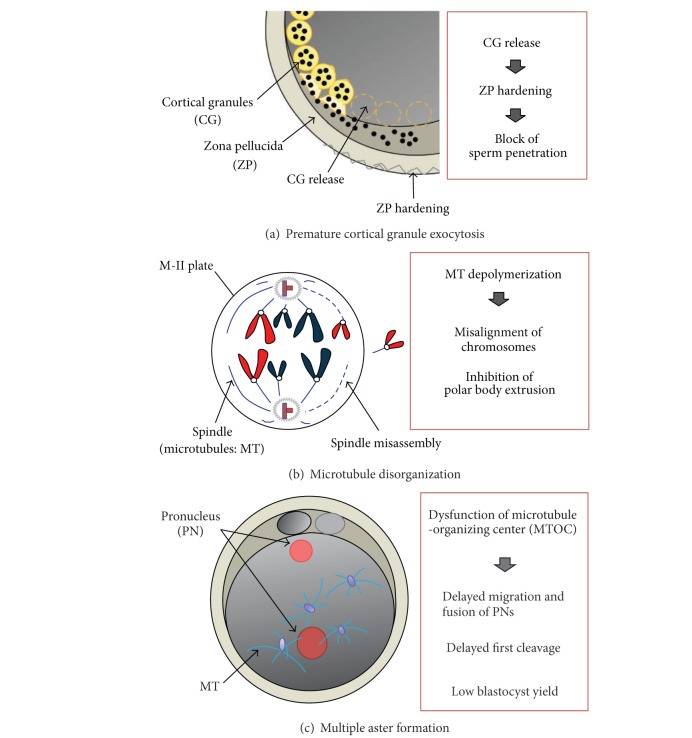
Low fertilization rates of cryopreserved oocytes were reported to be associated with chilling and freezing injuries, including zona hardening due to premature release of cortical granules [22, 26] and spindle disorganization and loss or clumping of microtubules [27, 28]. Briefly, exposure of mature oocytes to CPA and/or chilling procedure induced the transient rise of intracellular free calcium and prevented the sperm entry via block mechanisms at the level of plasma membrane or zona pellucida [29–32]. These processes also result in damage to the meiotic spindle, actin filaments, chromosomal dispersal, and microtubule depolymerization [33, 34]. In addition, Hara et al. [35] proposed a third hypothesis for cryodamage of bovine oocytes that multiple aster formation frequently observed in vitrified-warmed and fertilized oocytes may be related to loss of ooplasmic function responsible for normal microtubule assembly. These possible hypotheses responsible for cryodamages of the oocytes are shown in Figure 1.
Figure 1.
Hypotheses regarding cryoinjuries in mammalian oocytes. (a) Premature cortical granule exocytosis causes the hardening of zona pellucida, leading to block of sperm penetration. (b) Disorganization of microtubules means depolymerization of tubulin proteins, leading to misassembly of meiotic spindles, and subsequently resulting in misalignment of chromosomes and inhibition of the second polar body extrusion. (c) Multiple aster formation, resulting from ooplasmic dysfunction to support MTOC, is a possible cause of low blastocyst yield.
3. History
3.1. Learning from Embryo Cryoresearch
Knowledge can be concurrently accumulated from research history of embryo cryopreservation. Following the first successful freezing of mouse 8-cell stage embryos in 1972 [36], pregnancy from a cryopreserved cattle embryo was reported by Wilmut and Rowson [37]. These initial findings were then extended to embryos from several mammalian species including domestic animals [38–40] and human [41]. The protocol most commonly used for successful embryo cryopreservation at that time required slow cooling from upper −7°C to below −80°C in phosphate-buffered saline supplemented with DMSO or glycerol as a permeable CPA. During the slow cooling, embryonic blastomeres are dehydrated in response to the osmotic pressure that gradually increases with the formation of extracellular ice crystals after ice seeding. The frozen embryos were warmed very slowly to avoid the rapid influx of extracellular water into the dehydrated cells during warming. This earlier protocol was labor-intensive and time-consuming.
In 1977, a two-step freezing method was reported using sheep and cattle embryos [42]. The slow cooling of embryos is interrupted at around −30°C to −36°C, followed by rapid cooling to −196°C. The embryos in LN2 are believed to contain intracellular ice, although it is not detrimental at this point. But to survive, the frozen embryos must be warmed rapidly to avoid injury caused by recrystallization of the intracellular ice. This two-step freezing regimen allows the development of a temperature-controlled, programmable freezer and is still used widely for many mammalian species. In cattle, pregnancy rates following transfer of embryos frozen in this way range from 50 to 60% [43]. Additional progress resulted from the use of EG as a CPA for embryos from domestic species. Using sucrose as an osmotic buffer, direct transfer of postthaw embryos into recipients without expelling them from the straws was reported by Leibo [44].
Then, a great breakthrough for simple and efficient cryopreservation has been reported by a very high cooling rate of fully dehydrated mouse embryos in highly concentrated solutes. Rall and Fahy [53] developed a novel approach to cryopreserve mouse embryos in 1985. This protocol involves dehydration of the embryos by exposing them to highly concentrated CPAs prior to cooling them to low temperature, rather than during the cooling process itself. The dehydrated embryos are rapidly cooled by being directly plunged into LN2. Since the cryoprotective solution can be transformed into a stable glass without ice crystal formation during the rapid cooling process, this extremely rapid method of cryopreservation is referred to as “vitrification,” meaning “glass formation.” The application of vitrification as an alternative to conventional freezing can reduce the equipment required, but technician-dependent performance of vitrification process is the limited factor for its widespread use. So far, successful vitrification producing pregnancy and/or birth of live offspring has been reported with preimplantation embryos from various mammalian species including human. A wide variety of vitrification solutions and protocols have been employed even for the same type of embryo, that is, the same species and developmental stage.
3.2. Cryopreservation of Mature Oocytes
Cryopreservation of oocytes has short and less successful history when compared to the other reproductive cells as spermatozoa and embryos. The first successful IVF and birth of live offspring using frozen-thawed mouse oocytes was reported in 1976 by Parkening et al. [54], and followed by Whittingham [55] and Leibo et al. [56]. Other than the mouse, such a slow freezing procedure was acceptable for species whose oocytes are not sensitive to chilling, such as cat [57, 58] and human [59]. There are a few reports regarding successful pregnancies from frozen-thawed bovine oocytes [60, 61]. However, oocytes from the large domestic species are rich in cytoplasmic lipid droplets and very sensitive to chilling, resulting in the poor revivability following the slow cooling [62]. After the publication of innovative results by Rall and Fahy [53], vitrification has been attempted to apply to oocytes. Pregnancies or birth of live offspring have been published in mouse [63], human [64], and cattle [46], with an increased requirement for improving developmental competence of the vitrified-warmed oocytes.
In 1996, Martino et al. [47] reported that 15% of matured bovine oocytes developed into blastocysts following vitrification, under in vitro culture (IVC) conditions in which >40% of the non-treated fresh oocytes were able to develop to that stage. That protocol, a pioneer work opening the new window for oocyte cryobiology, is characterized by the extremely rapid cooling rate of oocytes suspended in <1 μL of a vitrification solution consisting of 30% EG plus 1.0 M sucrose placed onto electron microscope grids, a procedure derived from methods to cryopreserve Drosophila embryos [65]. The microgrids provide a cooling rate estimated to be <150,000°C/min, in contrast to 2,500°C/min with the conventionally used plastic straws. Vajta et al. [48] reported an alternative way of ultra-rapid cooling for vitrification of bovine oocytes. When the oocytes were aspirated with 20% EG and 20% DMSO solution into open-pulled straws (OPS) and cooled by directly plunging into LN2, 13% of the post-warm oocytes could develop into blastocysts after IVF and IVC. The OPS method has been improved to use open-pulled glass capillaries [66, 67] or commercially available gel-loading tips [68] using different CPA combinations. Other types of cryodevices so far reported for ultra-rapid cooling are the “Cryoloop” [21] and “Cryotop” [69]. Complete containerless methods have also been reported from two independent laboratories [49, 70]. Blastocyst yields from frozen-thawed or vitrified-warmed bovine metaphase-II oocytes, reported during the last two decades [45–52], are summarized in Table 1. There was no significant improvement on the blastocyst yield from cryopreserved bovine mature oocytes (commonly exceeding 10%), even after increased cleavage rates as >60% by using different cryodevices and vitrification protocols were obtained.
Table 1.
A list on Day-2 cleavage and Day-8 blastocyst yield from bovine mature oocytes cryopreserved and fertilized in vitro.
| Years | Method/device for cryopreservation |
Oocyte cryosurvival | Literature | |
|---|---|---|---|---|
| Cleavage rate | Blastocyst yield | |||
| 1992 | Freezing/French straw | 42% | 3% | Otoi et al. [45] |
| 1992 | Vitrification/French straw | 22% | 9% | Hamano et al. [46] |
| 1996 | Vitrification/EM-grid∗1 | 40% | 15% | Martino et al. [47] |
| 1998 | Vitrification/OPS∗2 | 50% | 13% | Vajta et al. [48] |
| 2000 | Vitrification/Microdrop | 62% | 11% | Dinnyes et al. [49] |
| 2004 | Vitrification/Cryotop | 70% | 7% | Chian et al. [50] |
| 2005 | Vitrification/GL-tip∗3 | 49% | 17% | Tominaga et al. [51] |
| 2010 | Vitrification/Cryotop | 76% | 12% | Zhou et al. [52] |
∗1EM-grid: electron microscope grid; ∗2OPS: open-pulled straw; ∗3GL-tip: gel-loading tip.
3.3. Cryopreservation of Immature Oocytes
Cryopreservation of immature oocytes at the GV stage is also the subject for challenging endeavour. Vajta et al. [48] reported that 25% of bovine oocytes vitrified-warmed using OPS system could develop into the blastocyst stage on Day 8. While it is still unclear that the high revivability of post-warm GV oocytes in the OPS system is reproducible, birth of calves following transfer of embryos derived from cryopreserved immature oocytes [71] encouraged such challenges. Abe et al. [72] reported that 8% of bovine oocytes developed into blastocysts when they were exposed to EG + Ficoll + sucrose-based solution in a stepwise manner and vitrified-warmed on nylon-mesh holder as a cryodevice, with successful data on a live calf after transfer. Bovine oocytes at the GV stage have homogenous (=less variable in size) lipid droplets that show little change following cooling, but intercellular coupling via gap junctions between cumulus cells and the GV-stage oocytes may be sensitive to osmotic stress. In addition, maintaining functional integrity of the cumulus cells after vitrification and warming is an important factor to harvest cytoplasmically-matured oocytes after subsequent IVM process.
4. Recent Improvement of Oocyte Cryosurvival
Our literature search failed to find recent papers published during 2011 to 2013 which described the significantly improved cryosurvival (blastocyst yield) of bovine oocytes by modifying the CPA composition, the cryodevice, or the CPA addition/dilution process in the vitrification procedures. Hence, a few chemical treatments of bovine oocytes during the IVM prior to vitrification and during the recovery culture after vitrification are highlighted in this section. Using mouse and porcine embryos, cellulose triacetate hollow fiber with a pore size of 7.5 nm has been proposed as a new device that can vitrify large amount of embryos without stepwise handlings of the embryos, reported recently by Matsunari et al. [73]. But this hallow fiber vitrification procedure has not yet been applied to the bovine oocytes.
4.1. Treatment During IVM
Large amount of cytoplasmic lipid droplets serves as energy resource but increases sensitivity of bovine oocytes to chilling injury during cryopreservation. Most of the lipid droplets locate at the periphery of plasma membrane or close proximal to organelles such as mitochondria and endoplasmic reticulum [74], both of which are the major target of cryodamage in oocyte organelles [75, 76]. Cytoplasmic lipid droplets can be partially removed from bovine oocytes by high magnitude centrifugation, and incidence of polyspermic penetration in the centrifuged and vitrified-warmed oocytes was significantly inhibited (blastocyst yield; 11% versus 7% in noncentrifuged control) [61]. It is also well known that vitrification of bovine oocytes induces mitochondrial dysfunction and loss of adenosine triphosphate (ATP) [77, 78]. L-Carnitine (Figure 2(a)), an active form of carnitine, can enhance lipid metabolism in animal cells and play an important role in the transportation of fatty acids from the cytoplasm to the mitochondria for β-oxidation [79]. Hence, L-carnitine can enhance ATP production in animal cells [80] and stimulate mitochondrial metabolism during maturation as firstly reported in mouse oocytes [81]. In porcine oocytes enriched with cytoplasmic lipid droplets, supplementation of L-carnitine into IVM medium reduced the amount of the lipid droplets and changed their distribution from the cortex to the medulla of oocyte cytoplasm [82]. Furthermore, supplementation of L-carnitine into IVC medium reduced the lipid content in bovine embryos and increased cryotolerance and developmental competence [83].
Figure 2.
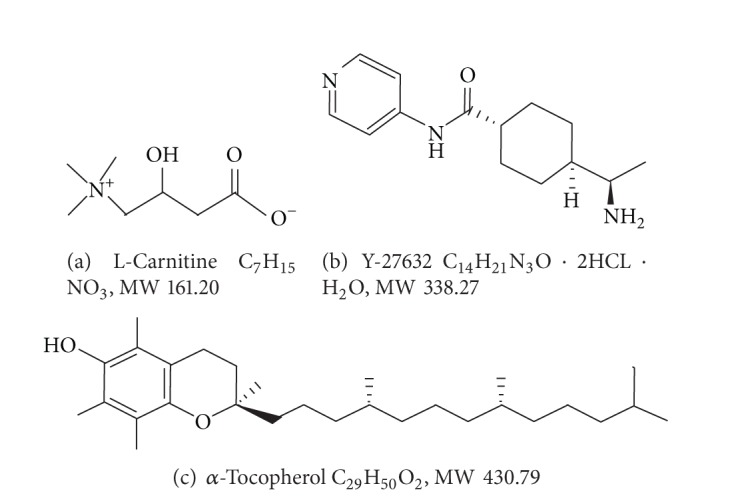
Structures of chemicals used for improvement of cryosurvival of bovine oocytes and resulted in significantly higher blastocyst yield in vitro [84, 85]. (a) L-carnitine, (b) ROCK inhibitor Y-27632, (c) α-tocopherol.
Two recent papers have described the effect of L-carnitine supplementation into IVM medium for bovine oocytes on their cryotolerance [84, 86], as summarized in Table 2. Both groups have employed Cryotop vitrification system for cryopreservation of bovine mature oocytes, but composition of the vitrification solution was different between the groups. Chankitisakul et al. [84] showed that bovine oocytes matured in the presence of 0.6 mg/mL; L-carnitine had the higher developmental potential to blastocyst stage 8 days after vitrification and IVF when compared to those matured in the absence of the L-carnitine (34% versus 20%, fresh control; 44%). No significant changes were found in nuclear maturation rate, ATP content, timing of first cleavage, and blastocyst quality, while dislocation of lipid droplets from the peripheral area to inner cytoplasm was observed in the L-carnitine treated oocytes. On the other hand, negative result of L-carnitine treatment during IVM on cryotorelance of bovine oocytes has been reported by Phongnimitr et al. [86]. The attempt of this research group in Thailand seemed to be conducted almost simultaneously with the above Chankitisakul's group (based on the date of initial paper submission). Supplementation of 0.6 mg/mL L-carnitine to the IVM medium significantly improved the nuclear maturation rate (78% versus 68%) and the Day-7 blastocyst yield from non-vitrified control oocytes (31% versus 24%), but did not contribute to improve the cryotolerance of the oocytes (Day-7 blastocyst yield; 13% versus 11%). Further research would be needed to clarify the effect of L-carnitine on bovine oocytes.
Table 2.
Supplementation effect of L-carnitine to IVM medium on the cryotolerance of bovine oocytes [84, 86].
| L-Carnitine | Vitrification | Nuclear maturation | Survival | Cleavage | Blastocyst yield |
|---|---|---|---|---|---|
| Chankitisakul et al. [84] | |||||
| − | − | 67% | 92%a | 84%a | 44%a |
| + | − | 65% | 93%a | 84%a | 45%a |
| − | + | 81%b | 57%b | 20%b | |
| + | + | 83%b | 63%b | 34%a | |
|
| |||||
| Phongnimitr et al. [86] | |||||
| − | − | 68%d | 100% | 78%c | 24%d |
| + | − | 78%c | 100% | 76%c | 31%c |
| − | + | 86% | 67%d | 11%e | |
| + | + | 88% | 69%d | 13%e | |
Superscripts a versus b, c versus d versus e in each column: P < 0.05.
Concentration of L-carnitine in the IVM medium: 0.6 mg/mL.
Glutathione (L-γ-glutamyl-L-cysteinyl-glycine; GSH), a major nonprotein sulfhydryl compound, plays an important role in protecting cells against the destructive effects of reactive oxygen species (ROS) and regulating syntheses of DNA and proteins [87]. GSH level increases during oocyte maturation in the ovary and reaches a peak at the metaphase-II stage [88]. However, the GSH levels of IVM oocytes are lower when compared with those of ovulated oocytes, as reported in some species [89–92]. GSH synthesis in oocytes during IVM may be disturbed by a low availability of cysteine [87, 93]. Low molecular weight thiol compounds, such as β-mercaptoethanol and cysteamine, can promote cysteine (cystine) uptake through formation of a mixed disulfide compound [94, 95]. In addition, such thiol compounds supplemented into IVM medium can increase intracellular GSH level and the developmental potential of the bovine oocytes [96]. It has been reported that GSH in bovine IVM-IVF oocytes can stimulate sperm aster formation [97]. Therefore, we have produced bovine IVM oocytes with 2.5-fold higher GSH content and then vitrified-warmed using Cryotop [98]. However, the high content of GSH in mature oocytes did not result in suppression of the high incidence of multiple aster formation (vitrified; 61% versus 53%, fresh control; 16% versus 17%) and improvement of developmental potential into Day-8 blastocysts (17% versus 16%, fresh control; both 41% regardless of thiol treatment for GSH).
4.2. Postvitrification Treatment
Generally, increased apoptosis in embryonic cells by oocyte vitrification procedure results in a decrease of developmental competence [99, 100]. Rho-associated coiled-coil kinase (ROCK), which is a kinase belonging to the AGC (PKA, PKG, and PKC) family of serine-threonine kinases, was realized as a downstream target of the small GTP-binding protein Rho [101], which could regulate growth, adhesion, migration, metabolism, and apoptosis of cells through controlling the actin-cytoskeletal assembly and contraction of cells [102]. Inhibition of the ROCK activity was involved in decrease of apoptosis in embryonic stem cell-derived neural cells [103]. Inhibition of the ROCK activity was also effective to improve the plating efficiency of dissociated human pluripotent stem cells after cryopreservation [104–108] and the revivability of in vitro produced bovine blastocysts after vitrification and warming [109].
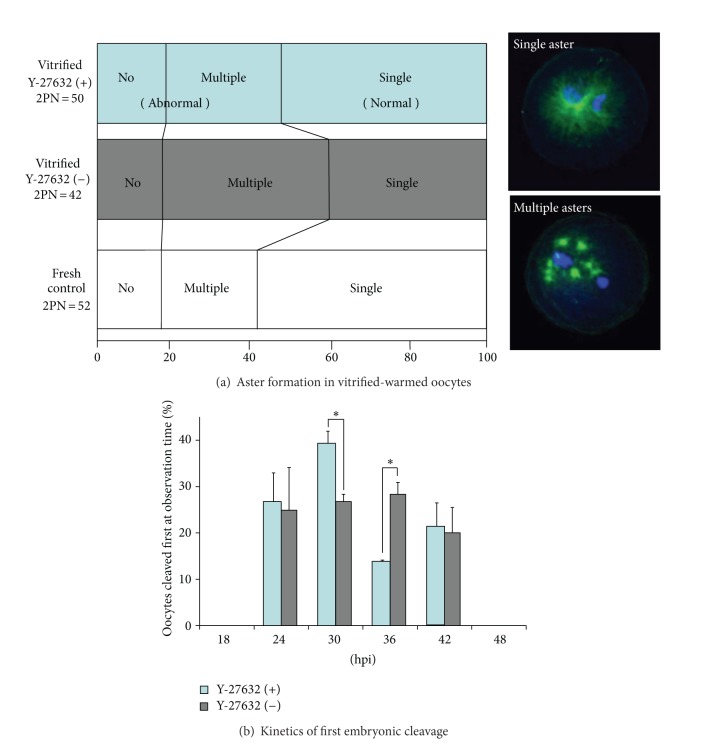
Therefore, we used the ROCK inhibitor (Y-27632; Figure 2(b)) to improve the developmental competence of vitrified-warmed oocytes during 2 hours of recovery culture after Cryotop vitrification [85]. The vitrification solution consisted of 15% EG, 15% DMSO, and 0.5 M sucrose, and oocytes retrieved from 1-day-stored ovaries (10–12°C) were subjected to the IVM. As summarized in Table 3, treatment of the postwarm mature oocytes with 10 μM Y-27632 resulted in significantly higher oocyte survival rate prior to the IVF, Day-2 cleavage rate, and Day-8 blastocyst yield (21% versus 14%, fresh control; 34%). The resultant blastocysts in Y-27632-treated group had better quality in terms of total cell number and apoptotic cell ratio. Time-dependent change in mitochondrial activity of the vitrified-warmed oocytes was not influenced by ROCK inhibition during the period of recovery culture. However, the ability of ooplasm to support single-aster formation was improved by the ROCK inhibition (Figure 3(a)). Timing of first cleavage in the bovine oocytes vitrified-warmed and treated with Y-27632 was accelerated (Figure 3(b)), which may be favourable because bovine oocytes cleaving earlier are more likely to become blastocysts [110, 111]. Thus, inhibition of ROCK activity in vitrified-warmed bovine oocytes during short-term recovery culture could lead to higher developmental competence, probably due to decreased apoptosis and normalized function of the MTOC.
Table 3.
Rescue of vitrified-warmed bovine oocytes with ROCK inhibitor (Y-27632) [85].
| Vitrification | Y-27632 | Survival | Cleavage | Blastocysts | ||
|---|---|---|---|---|---|---|
| Yield | Cell number | Apoptotic cell ratio | ||||
| − | − | 100%a | 71%a | 34%a | 135.7a | 1.8%a |
| + | − | 90%b | 56%b | 14%c | 97.5b | 4.0%b |
| + | + | 98%a | 72%a | 21%b | 124.6a | 2.2%a |
Superscript a versus b versus c in each column: P < 0.05.
Concentration of Y-27632 in recovery culture medium: 10 µM.
Figure 3.
Effect of ROCK inhibition during postwarm recovery culture on revivability of bovine mature oocytes [85]. (a) Proportion of vitrified-warmed bovine oocytes exhibiting the formation of no, single, or multiple sperm aster(s). The abnormal incidence of multiple aster formation was inhibited by the recovery culture with Y-27632. Immunostaining against α-tubulin (green) and nuclear staining with DAPI (blue) were performed at 10-hour post-insemination (hpi). (b) Accelerated timing of first cleavage in bovine oocytes vitrified-warmed and rescued with Y-27632. Asterisks indicate significant difference at P < 0.05.
Using the same strategy, effect of two antioxidants, 10 μM α-tocopherol (Figure 2(c)) or 250 μM ascorbic acid, on rescuing vitrified-warmed bovine oocytes has been investigated in our laboratory. Oxidative stress by ROS must be one of the causes which may induce lipid peroxidation and/or organelle damage in bovine oocytes [112]. Interestingly, the supplementation of α-tocopherol, not ascorbic acid, to the recovery culture medium resulted in a significantly higher blastocyst yield from the postwarm oocytes as 37% versus 26% in the postwarm control oocytes (fresh control; 53%) (unpublished data of I. Yashiro and S. Hochi). The improved baseline of blastocyst yield in the nonvitrified control group was due to the availability of the fresh (=within 6 h after slaughter) bovine ovaries for recent experiments.
5. Conclusion
Ultrarapid vitrification procedure, originally reported using electron microscope grid as cryodevice [47], has become a standard approach for cryopreservation of bovine oocytes with some modifications. Due to numerous efforts, as the development of novel cryodevice such as OPS [48] or Cryotop [69] and the preloading with low concentration permeable CPA [49, 70, 113], blastocyst yields at >10% have been commonly reported by several laboratories during the last decade. Two recent attempts to improve cryosurvival of bovine oocytes have been focused on; the qualitative improvement of IVM oocytes prior to the vitrification and the short-term recovery culture of vitrified-warmed oocytes prior to the subsequent IVF. Supplementation of L-carnitine to the IVM medium of bovine oocytes has been reported to redistribute cytoplasmic lipid droplets and improve the cryotolerance of the oocytes after Cryotop vitrification as the blastocyst yield of 34% (comparable to fresh control) [84]. However, it is still unclear whether the positive effect of L-carnitine is reproducible. Incidence of multiple aster formation, a possible cause for low developmental potential of vitrified-warmed bovine oocytes [35], can be inhibited by a short-term culture of the postwarm oocytes in the presence of ROCK inhibitor, with a blastocyst yield of 21% after the Cryotop vitrification (>10% less than fresh control) [85]. Use of an antioxidant α-tocopherol during the recovery culture also rescued the postwarm bovine oocytes as the maximum blastocyst yield at 37% (>10% less than fresh control). Thus, chemical treatment of bovine oocytes before or after the vitrification protocol made it possible to increase their revivability to 20–40% when evaluated with blastocyst yield. Further improvements of the vitrification procedure, combined with pre- and postvitrification chemical treatment, would overcome the high sensitivity of bovine oocytes to cryopreservation and provide valuable information for biomedical experts working in human infertility clinic.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Singleton EF. Field collection and preservation of bovine semen for artificial insemination. Australian Veterinary Journal. 1970;46(4):160–163. doi: 10.1111/j.1751-0813.1970.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright RW, Jr., Anderson GB, Cupps PT, Drost M. Successful culture in vitro of bovine embryos to the blastocyst stage. Biology of Reproduction. 1976;14(2):157–162. doi: 10.1095/biolreprod14.2.157. [DOI] [PubMed] [Google Scholar]
- 3.Wright RW., Jr. Successful culture in vitro of swine embryos to the blastocyst stage. Journal of Animal Science. 1977;44(5):854–858. doi: 10.2527/jas1977.445854x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell KHS, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380(6569):64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri R, Porcu E, Marsella T, et al. Oocyte cryopreservation. Human Reproduction. 1998;13(supplement 4):98–108. doi: 10.1093/humrep/13.suppl_4.98. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri R, Porcu E, Marsella T, et al. Technical aspects of oocyte cryopreservation. Molecular and Cellular Endocrinology. 2000;169(1-2):39–42. doi: 10.1016/s0303-7207(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou GB, Li N. Bovine oocytes cryoinjury and how to improve their development following cryopreservation. Animal Biotechnology. 2013;24(2):94–106. doi: 10.1080/10495398.2012.755466. [DOI] [PubMed] [Google Scholar]
- 8.Mullen SF, Fahy GM. A chronologic review of mature oocyte vitrification research in cattle, pigs, and sheep. Theriogenology. 2012;78(8):1709–1719. doi: 10.1016/j.theriogenology.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Somfai T, Kikuchi K, Nagai T. Factors affecting cryopreservation of porcine oocytes. Journal of Reproduction and Development. 2012;58(1):17–24. doi: 10.1262/jrd.11-140n. [DOI] [PubMed] [Google Scholar]
- 10.Fuge H. Ultrastructure and function of the spindle apparatus. Microtubules and chromosomes during nuclear division. Protoplasma. 1974;82(4):289–320. doi: 10.1007/BF01275726. [DOI] [PubMed] [Google Scholar]
- 11.Sathananthan AH, Ratnam SS, Ng SC, Tarín JJ, Gianaroli L, Trounson A. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Human Reproduction. 1996;11(2):345–356. doi: 10.1093/humrep/11.2.345. [DOI] [PubMed] [Google Scholar]
- 12.Navara CS, First NL, Schatten G. Phenotypic variations among paternal centrosomes expressed within the zygote as disparate microtubule lengths and sperm aster organization: correlations between centrosome activity and developmental success. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(11):5384–5388. doi: 10.1073/pnas.93.11.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing—a review article. Molecular and Cellular Endocrinology. 2003;202(1-2):101–107. doi: 10.1016/s0303-7207(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 14.Schatten H, Sun Q-Y. The role of centrosomes in mammalian fertilization and its significance for ICSI. Molecular Human Reproduction. 2009;15(9):531–538. doi: 10.1093/molehr/gap049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schatten G, Simerly C, Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(12):4152–4156. doi: 10.1073/pnas.82.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 17.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141(1):1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 18.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65(1):236–244. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Jin B, Kawai Y, Hara T, et al. Pathway for the movement of water and cryoprotectants in bovine oocytes and embryos. Biology of Reproduction. 2011;85(4):834–847. doi: 10.1095/biolreprod.110.088641. [DOI] [PubMed] [Google Scholar]
- 20.Gook DA, Osborn SM, Johnston WIH. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Human Reproduction. 1993;8(7):1101–1109. doi: 10.1093/oxfordjournals.humrep.a138201. [DOI] [PubMed] [Google Scholar]
- 21.Lane M, Schoolcraft WB, Gardner DK, Phil D. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertility and Sterility. 1999;72(6):1073–1078. doi: 10.1016/s0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 22.Fuku E, Xia L, Downey BR. Ultrastructural changes in bovine oocytes cryopreserved by vitrification. Cryobiology. 1995;32(2):139–156. doi: 10.1006/cryo.1995.1013. [DOI] [PubMed] [Google Scholar]
- 23.Hochi S, Kozawa M, Fujimoto T, Hondo E, Yamada J, Oguri N. In vitro maturation and transmission electron microscopic observation of horse oocytes after vitrification. Cryobiology. 1996;33(3):300–310. doi: 10.1006/cryo.1996.0030. [DOI] [PubMed] [Google Scholar]
- 24.Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their developmental competence. Molecular Reproductioin and Development. 45(4):503–512. doi: 10.1002/(SICI)1098-2795(199612)45:4<503::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Ledda S, Bogliolo L, Succu S, et al. Oocyte cryopreservation: oocyte assessment and strategies for improving survival. Reproduction, Fertility and Development. 2007;19(1):13–23. doi: 10.1071/rd06126. [DOI] [PubMed] [Google Scholar]
- 26.Carroll J, Depypere H, Matthews CD. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. Journal of Reproduction and Fertility. 1990;90(2):547–553. doi: 10.1530/jrf.0.0900547. [DOI] [PubMed] [Google Scholar]
- 27.Magistrini M, Szollosi D. Effects of cold and of isopropyl-N-phenylcarbamate on the second meiotic spindle of mouse oocytes. European Journal of Cell Biology. 1980;22(2):699–707. [PubMed] [Google Scholar]
- 28.Aman RR, Parks JE. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biology of Reproduction. 1994;50(1):103–110. doi: 10.1095/biolreprod50.1.103. [DOI] [PubMed] [Google Scholar]
- 29.Morató R, Izquierdo D, Albarracín JL, et al. Effects of pre-treating in vitro-matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Molecular Reproduction and Development. 2008;75(1):191–201. doi: 10.1002/mrd.20725. [DOI] [PubMed] [Google Scholar]
- 30.Rojas C, Palomo MJ, Albarracín JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology. 2004;49(3):211–220. doi: 10.1016/j.cryobiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Succu S, Leoni GG, Bebbere D, et al. Vitrification devices affect structural and molecular status of in vitro matured ovine oocytes. Molecular Reproduction and Development. 2007;74(10):1337–1344. doi: 10.1002/mrd.20693. [DOI] [PubMed] [Google Scholar]
- 32.Pereira RM, Marques CC. Animal oocyte and embryo cryopreservation. Cell and Tissue Banking. 2008;9(4):267–277. doi: 10.1007/s10561-008-9075-2. [DOI] [PubMed] [Google Scholar]
- 33.Massip A. Cryopreservation of bovine oocytes: current status and recent developments. Reproduction Nutrition Development. 2003;43(4):325–330. doi: 10.1051/rnd:2003024. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa B, Ueno S, Nakayama N, et al. Developmental ability of porcine in vitro matured oocytes at the meiosis II stage after vitrification. Journal of Reproduction and Development. 2010;56(3):356–361. doi: 10.1262/jrd.10-005h. [DOI] [PubMed] [Google Scholar]
- 35.Hara H, Hwang IS, Kagawa N, Kuwayama M, Hirabayashi M, Hochi S. High incidence of multiple aster formation in vitrified-warmed bovine oocytes after in vitro fertilization. Theriogenology. 2012;77(5):908–915. doi: 10.1016/j.theriogenology.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Whittingham DG, Leibo SP, Mazur P. Survival of mouse embryos frozen to −196° and −269°C. Science. 1972;178(4059):411–414. [PubMed] [Google Scholar]
- 37.Wilmut I, Rowson LEA. Experiments on the low temperature preservation of cow embryos. The Veterinary Record. 1973;92(26):686–690. doi: 10.1136/vr.92.26.686. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto Y, Oguri N, Tsutsumi Y, Hachinohe Y. Experiments in the freezing and storage of equine embryos. Journal of Reproduction and Fertility. 1982;32:399–403. [PubMed] [Google Scholar]
- 39.Hayashi S, Kobayashi K, Mizuno J, Saitoh K, Hirano S. Birth of piglets from frozen embryos. Veterinary Record. 1989;125(2):43–44. doi: 10.1136/vr.125.2.43. [DOI] [PubMed] [Google Scholar]
- 40.Willadsen P, Williams PG. Isolation and partial characterization of an antigen from the cattle tick, Boophilus microplus . Immunochemistry. 1976;13(7):591–597. doi: 10.1016/0019-2791(76)90171-3. [DOI] [PubMed] [Google Scholar]
- 41.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305(5936):707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 42.Willadsen SM. Factors affecting the survival of sheep embryos during-freezing and thawing. Ciba Foundation Symposium. 1977;(52):175–201. doi: 10.1002/9780470720332.ch9. [DOI] [PubMed] [Google Scholar]
- 43.Niemann H, Sacher B, Elsaesser F. Pregnancy rates relative to recipient plasma progesterone levels on the day of nonsurgical transfer of frozen/thawed bovine embryos. Theriogenology. 1985;23(4):631–639. doi: 10.1016/0093-691x(85)90197-9. [DOI] [PubMed] [Google Scholar]
- 44.Leibo SP. A one-step method for direct nonsurgical transfer of frozen-thawed bovine embryos. Theriogenology. 1984;21(5):767–790. doi: 10.1016/0093-691x(84)90022-0. [DOI] [PubMed] [Google Scholar]
- 45.Otoi T, Tachikawa S, Kondo S, Suzuki T. Developmental capacity of bovine oocytes cryopreserved after maturation in vitro and of frozen-thawed bovine embryos derived from frozen mature oocytes. Theriogenology. 1992;38(4):711–719. doi: 10.1016/0093-691x(92)90033-n. [DOI] [PubMed] [Google Scholar]
- 46.Hamano S, Koikeda A, Kuwayama M, Nagai T. Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology. 1992;38(6):1085–1090. doi: 10.1016/0093-691x(92)90122-8. [DOI] [PubMed] [Google Scholar]
- 47.Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biology of Reproduction. 1996;54(5):1059–1069. doi: 10.1095/biolreprod54.5.1059. [DOI] [PubMed] [Google Scholar]
- 48.Vajta G, Holm P, Kuwayama M, et al. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Molecular Reproduction and Development. 1998;51(1):53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 49.Dinnyes A, Dai Y, Jiang S, Yang X. High developmental rates of vitrified bovine oocytes following parthenogenetic activation, in vitro fertilization, and somatic cell nuclear transfer. Biology of Reproduction. 2000;63(2):513–518. doi: 10.1095/biolreprod63.2.513. [DOI] [PubMed] [Google Scholar]
- 50.Chian RC, Kuwayama M, Tan L, Tan J, Kato O, Nagai T. High survival rate of bovine oocytes matured in vitro following vitrification. Journal of Reproduction and Development. 2004;50(6):685–696. doi: 10.1262/jrd.50.685. [DOI] [PubMed] [Google Scholar]
- 51.Tominaga K, Hamada Y, Hochi S. Gel-loading tip vitrification of in vitro-matured bovine oocytes and subsequent embryo production by IVF and nuclear transfer. Journal of Mammalian Ova Research. 2005;22(3):178–184. [Google Scholar]
- 52.Zhou XL, Al Naib A, Sun DW, Lonergan P. Bovine oocyte vitrification using the Cryotop method: effect of cumulus cells and vitrification protocol on survival and subsequent development. Cryobiology. 2010;61(1):66–72. doi: 10.1016/j.cryobiol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313(6003):573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 54.Parkening TA, Tsunoda Y, Chang MC. Effects of various low temperatures, cryoprotective agents and cooling rates on the survival, fertilizability and development of frozen-thawed mouse eggs. Journal of Experimental Zoology. 1976;197(3):369–374. doi: 10.1002/jez.1401970310. [DOI] [PubMed] [Google Scholar]
- 55.Whittingham DG. Fertilization in vitro and development to term of unfertilized mouse oocytes previously stored at −196°C. Journal of Reproduction and Fertility. 1977;49(1):89–94. doi: 10.1530/jrf.0.0490089. [DOI] [PubMed] [Google Scholar]
- 56.Leibo SP, McGrath JJ, Cravalho EG. Microscopic observation of intracellular ice formation in unfertilized mouse ova as a function of cooling rate. Cryobiology. 1978;15(3):257–271. doi: 10.1016/0011-2240(78)90036-6. [DOI] [PubMed] [Google Scholar]
- 57.Wolfe BA, Wild DE. Development to blastocysts of domestic cat oocytes matured and fertilized in vitro after prolonged cold storage. Journal of Reproduction and Fertility. 1996;106(1):135–141. doi: 10.1530/jrf.0.1060135. [DOI] [PubMed] [Google Scholar]
- 58.Cocchia N, Ciani F, Russo M, et al. Immature cat oocyte vitrification in open pulled straws (OPSs) using a cryoprotectant mixture. Cryobiology. 2010;60(2):229–234. doi: 10.1016/j.cryobiol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Chen C. Pregnancy after human oocyte cryopreservation. The Lancet. 1986;1(8486):884–886. doi: 10.1016/s0140-6736(86)90989-x. [DOI] [PubMed] [Google Scholar]
- 60.Fuku E, Kojima T, Shioya Y, Marcus GJ, Downey BR. In vitro fertilization and development of frozen-thawed bovine oocytes. Cryobiology. 1992;29(4):485–492. doi: 10.1016/0011-2240(92)90051-3. [DOI] [PubMed] [Google Scholar]
- 61.Otoi T, Yamamoto K, Koyama N, et al. Cryopreservation of mature bovine oocytes following centrifugation treatment. Cryobiology. 1997;34(1):36–41. doi: 10.1006/cryo.1996.1988. [DOI] [PubMed] [Google Scholar]
- 62.Ledda S, Leoni G, Bogliolo L, Naitana S. Oocyte cryopreservation and ovarian tissue banking. Theriogenology. 2001;55(6):1359–1371. doi: 10.1016/s0093-691x(01)00487-3. [DOI] [PubMed] [Google Scholar]
- 63.Nakagata N. High survival rate of unfertilized mouse oocytes after vitrification. Journal of Reproduction and Fertility. 1989;87(2):479–483. doi: 10.1530/jrf.0.0870479. [DOI] [PubMed] [Google Scholar]
- 64.Porcu E, Ciotti PM, Fabbri R, Magrini O, Seracchioli R, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertility and Sterility. 1997;68(4):724–726. doi: 10.1016/s0015-0282(97)00268-9. [DOI] [PubMed] [Google Scholar]
- 65.Steponkus PL, Myers SP, Lynch DV, et al. Cryopreservation of Drosophila melanogaster embryos. Nature. 1990;345(6271):170–172. doi: 10.1038/345170a0. [DOI] [PubMed] [Google Scholar]
- 66.Kong IK, Lee SI, Cho SG, Cho SK, Park CS. Comparison of open pulled straw (OPS) vs glass micropipette (GMP) vitrification in mouse blastocysts. Theriogenology. 2000;53(9):1817–1826. doi: 10.1016/s0093-691x(00)00317-4. [DOI] [PubMed] [Google Scholar]
- 67.Hochi S, Hirabayashi M, Hirao M, et al. Effects of cryopreservation of pronuclear-stage rabbit zygotes on the morphological survival, blastocyst formation, and full-term development after DNA microinjection. Molecular Reproduction and Development. 2001;60(2):227–232. doi: 10.1002/mrd.1082. [DOI] [PubMed] [Google Scholar]
- 68.Tominaga K, Hamada Y. Gel-loading tip as container for vitrification of in vitro-produced bovine embryos. Journal of Reproduction and Development. 2001;47(5):267–273. [Google Scholar]
- 69.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reproductive BioMedicine Online. 2005;11(3):300–308. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 70.Papis K, Shimizu M, Izaike Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Theriogenology. 2000;54(5):651–658. doi: 10.1016/S0093-691X(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 71.Vieira AD, Mezzalira A, Barbieri DP, Lehmkuhl RC, Rubin MIB, Vajta G. Calves born after open pulled straw vitrification of immature bovine oocytes. Cryobiology. 2002;45(1):91–94. doi: 10.1016/s0011-2240(02)00109-8. [DOI] [PubMed] [Google Scholar]
- 72.Abe Y, Hara K, Matsumoto H, et al. Feasibility of a nylon-mesh holder for vitrification of bovine germinal vesicle oocytes in subsequent production of viable blastocysts. Biology of Reproduction. 2005;72(6):1416–1420. doi: 10.1095/biolreprod.104.037051. [DOI] [PubMed] [Google Scholar]
- 73.Matsunari H, Maehara M, Nakano K, et al. Hollow fiber vitrification: a novel method for vitrifying multiple embryos in a single device. Journal of Reproduction and Development. 2012;58(5):599–608. doi: 10.1262/jrd.2011-051. [DOI] [PubMed] [Google Scholar]
- 74.Kruip TAM, Cran DG, van Beneden TH, Dieleman SJ. Structural changes in bovine oocytes during final maturation in vivo. Gamete Research. 1983;8(1):29–47. [Google Scholar]
- 75.Jones A, van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Human Reproduction. 2004;19(8):1861–1866. doi: 10.1093/humrep/deh313. [DOI] [PubMed] [Google Scholar]
- 76.Lowther KM, Weitzman VN, Maier D, Mehlmann LM. Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biology of Reproduction. 2009;81(1):147–154. doi: 10.1095/biolreprod.108.072538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao XM, Du WH, Wang D, et al. Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Molecular Reproduction and Development. 2011;78(12):942–950. doi: 10.1002/mrd.21389. [DOI] [PubMed] [Google Scholar]
- 78.Zhao XM, Du WH, Wang D, et al. Effect of cyclosporine pretreatment on mitochondrial function in vitrified bovine mature oocytes. Fertility and Sterility. 2011;95(8):2786–2788. doi: 10.1016/j.fertnstert.2011.04.089. [DOI] [PubMed] [Google Scholar]
- 79.Kerner J, Minkler PE, Lesnefsky EJ, Hoppel CL. Fatty acid chain-elongation in perfused rat heart: synthesis of stearoylcarnitine from perfused palmitate. The FEBS Letters. 2007;581(23):4491–4494. doi: 10.1016/j.febslet.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vanella A, Russo A, Acquaviva R, et al. L-Propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biology and Toxicology. 2000;16(2):99–104. doi: 10.1023/a:1007638025856. [DOI] [PubMed] [Google Scholar]
- 81.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biology of Reproduction. 2010;83(6):909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 82.Somfai T, Kaneda M, Akagi S, et al. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reproduction, Fertility and Development. 2011;23(7):912–920. doi: 10.1071/RD10339. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi T, Inaba Y, Somfai T, et al. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reproduction, Fertility and Development. 2013;25(4):589–599. doi: 10.1071/RD11262. [DOI] [PubMed] [Google Scholar]
- 84.Chankitisakul V, Somfai T, Inaba Y, Techakumphu M, Nagai T. Supplementation of maturation medium with L-carnitine improves cryo-tolerance of bovine in vitro matured oocytes. Theriogenology. 2013;79(4):590–598. doi: 10.1016/j.theriogenology.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 85.Hwang IS, Hara H, Chung HJ, Hirabayashi M, Hochi S. Rescue of vitrified-warmed bovine oocytes with rho-associated coiled-coil kinase inhibitor. Biology of Reproduction. 2013;89(2):p. 26. doi: 10.1095/biolreprod.113.109769. [DOI] [PubMed] [Google Scholar]
- 86.Phongnimitr T, Liang Y, Srirattana K, et al. Effect of L-carnitine on maturation, cryo-tolerance and embryo developmental competence of bovine oocytes. Animal Science Journal. 2013;84(11):719–725. doi: 10.1111/asj.12067. [DOI] [PubMed] [Google Scholar]
- 87.Meister A. Selective modification of glutathione metabolism. Science. 1983;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 88.Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Developmental Biology. 1988;125(1):181–186. doi: 10.1016/0012-1606(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 89.Brad AM, Bormann CL, Swain JE, et al. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Molecular Reproduction and Development. 2003;64(4):492–498. doi: 10.1002/mrd.10254. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-González E, López-Bejar M, Mertens MJ, Paramio MT. Effects on in vitro embryo development and intracellular glutathione content of the presence of thiol compounds during maturation of prepubertal goat oocytes. Molecular Reproduction and Development. 2003;65(4):446–453. doi: 10.1002/mrd.10316. [DOI] [PubMed] [Google Scholar]
- 91.Kim MK, Hossein MS, Oh HJ, et al. Glutathione content of in vivo and in vitro matured canine oocytes collected from different reproductive stages. Journal of Veterinary Medical Science. 2007;69(6):627–632. doi: 10.1292/jvms.69.627. [DOI] [PubMed] [Google Scholar]
- 92.Ge L, Sui H-S, Lan G-C, Liu N, Wang J-Z, Tan J-H. Coculture with cumulus cells improves maturation of mouse oocytes denuded of the cumulus oophorus: observations of nuclear and cytoplasmic events. Fertility and Sterility. 2008;90(6):2376–2388. doi: 10.1016/j.fertnstert.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 93.Furnus CC, de Matos DG. The availability of cysteine in culture medium appears to be the limiting factor for glutathione synthesis in mammalian oocytes. Theriogenology. 1999;51(1):p. 373. [Google Scholar]
- 94.Ishii T, Hishinuma I, Bannai S, Sugita Y. Mechanism of growth promotion of mouse lymphoma L1210 cells in vitro by feeder layer or 2-mercaptoethanol. Journal of Cellular Physiology. 1981;107(2):283–293. doi: 10.1002/jcp.1041070215. [DOI] [PubMed] [Google Scholar]
- 95.Ohmori H, Yamamoto I. A mechanism of the augmentation of antibody response in vitro by 2-mercaptoethanol: facilitation of cystine uptake in murine lymphocytes. International Journal of Immunopharmacology. 1982;4(5):475–479. doi: 10.1016/0192-0561(82)90023-6. [DOI] [PubMed] [Google Scholar]
- 96.de Matos DG, Furnus CC, Moses DF, Martinez AG, Matkovic M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Molecular Reproduction and Development. 1996;45(4):451–457. doi: 10.1002/(SICI)1098-2795(199612)45:4<451::AID-MRD7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 97.Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biology of Reproduction. 1997;56(6):1503–1512. doi: 10.1095/biolreprod56.6.1503. [DOI] [PubMed] [Google Scholar]
- 98.Hara H, Yamane I, Noto I. Microtubule assembly and in vitro development of bovine oocytes with increased intracellular glutathione level prior to vitrification and in vitro fertilization. Zygote. 2013 doi: 10.1017/S0967199413000105. [DOI] [PubMed] [Google Scholar]
- 99.Morató R, Izquierdo D, Paramio MT, Mogas T. Survival and apoptosis rates after vitrification in cryotop devices of in vitro-produced calf and cow blastocysts at different developmental stages. Reproduction, Fertility and Development. 2010;22(7):1141–1147. doi: 10.1071/RD10013. [DOI] [PubMed] [Google Scholar]
- 100.Li L, Zhang X, Zhao L, Xia X, Wang W. Comparison of DNA apoptosis in mouse and human blastocysts after vitrification and slow freezing. Molecular Reproduction and Development. 2012;79(3):229–236. doi: 10.1002/mrd.22018. [DOI] [PubMed] [Google Scholar]
- 101.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. The EMBO Journal. 1996;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 102.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nature Reviews Molecular Cell Biology. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 103.Koyanagi M, Takahashi J, Arakawa Y, et al. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. Journal of Neuroscience Research. 2008;86(2):270–280. doi: 10.1002/jnr.21502. [DOI] [PubMed] [Google Scholar]
- 104.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 105.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Molecular Reproduction and Development. 2009;76(8):722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gauthaman K, Fong CY, Bongso A. Effect of ROCK inhibitor Y-27632 on normal and variant human embryonic stem cells (hESCs) in vitro: its benefits in hESC expansion. Stem Cell Reviews and Reports. 2010;6(1):86–95. doi: 10.1007/s12015-009-9107-8. [DOI] [PubMed] [Google Scholar]
- 107.Gauthaman K, Fong CY, Subramanian A, Biswas A, Bongso A. ROCK inhibitor Y-27632 increases thaw-survival rates and preserves stemness and differentiation potential of human Wharton’s jelly stem cells after cryopreservation. Stem Cell Reviews and Reports. 2010;6(4):665–676. doi: 10.1007/s12015-010-9184-8. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Human Reproduction. 2009;24(3):580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 109.Hochi S, Abdalla H, Hara H, et al. Stimulatory effect of Rho-associated coiled-coil kinase (ROCK) inhibitor on revivability of in vitro-produced bovine blastocysts after vitrification. Theriogenology. 2010;73(8):1139–1145. doi: 10.1016/j.theriogenology.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 110.Lonergan P, Khatir H, Piumi F, Rieger D, Humblot P, Boland MP. Effect of time interval from insemination to first cleavage on the developmental characteristics, sex ratio and pregnancy rate after transfer of bovine embryos. Journal of Reproduction and Fertility. 1999;117(1):159–167. doi: 10.1530/jrf.0.1170159. [DOI] [PubMed] [Google Scholar]
- 111.Ward F, Rizos D, Corridan D, Quinn K, Boland M, Lonergan P. Paternal influence on the time of first embryonic cleavage post insemination and the implications for subsequent bovine embryo development in vitro and fertility in vivo. Molecular Reproduction and Development. 2001;60(1):47–55. doi: 10.1002/mrd.1060. [DOI] [PubMed] [Google Scholar]
- 112.Gutnisky C, Morado S, Dalvit GC, Thompson JG, Cetica PD. Glycolytic pathway activity: effect on IVM and oxidative metabolism of bovine oocytes. Reprodduction, Fertility, and Development. 2013;25(7):1026–1035. doi: 10.1071/RD12193. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Al Naib A, Sun D-W, Lonergan P. Membrane permeability characteristics of bovine oocytes and development of a step-wise cryoprotectant adding and diluting protocol. Cryobiology. 2010;61(1):58–65. doi: 10.1016/j.cryobiol.2010.05.001. [DOI] [PubMed] [Google Scholar]