Abstract
Overproduction of reactive oxygen species (ROS) in vivo can result in damage associated with many aging-associated diseases. Defenses against ROS that have evolved include antioxidant enzymes, such as superoxide dismutases, peroxidases, and catalases, which can scavenge ROS. In addition, endogenous and dietary antioxidants play an important role in moderating damage associated with ROS. In this study, we use four common dietary antioxidants to demonstrate that, in the presence of copper (cupric sulfate and cupric gluconate) and physiologically relevant levels of hydrogen peroxide, these antioxidants can also act as pro-oxidants by producing hydroxyl radicals. Using electron spin resonance (ESR) spin trapping techniques, we demonstrate that the level of hydroxyl radical formation is a function of the pH of the medium and the relative amounts of antioxidant and copper. Based on the level of hydroxyl radical formation, the relative pro-oxidant potential of these antioxidants is: cysteine > ascorbate >EGCG > GSH. It has been reported that copper sequestered by protein ligands, as happens in vivo, loses its redox activity (diminishing/abolishing the formation of free radicals). However, in the presence of hydrogen peroxide, cysteine and GSH efficiently react with cupric sulfate sequestered with bovine serum albumin to generate hydroxyl radicals. Overall, the results demonstrate that, in the presence of copper endogenous and dietary antioxidants can also exhibit pro-oxidative activity.
Keywords: Antioxidant, electron spin resonance, Fenton-like reaction, hydroxyl radical, copper
INTRODUCTION
Reactive oxygen species (ROS) are by-products of cellular oxidative metabolism, much of which occurs in the mitochondria of cells. Biologically relevant ROS include hydrogen peroxide (H2O2), superoxide, hydroxyl radicals, and singlet oxygen. In addition to cellular metabolism, there are several other biological reactions that can generate ROS in vivo. Transition metals such as copper and iron are essential dietary minerals that play important roles in enzyme activity and oxygen transport. However, they can also participate in one-electron oxidation-reduction reactions, leading to formation of ROS. They are therefore usually sequestered by protein ligands in vivo to limit their redox activity.
Reactive oxygen species play a dual role, being both beneficial and harmful.1–3 They play beneficial physiological roles in cellular signaling systems and induction of mitogenic responses.2,3 However, overproduction of ROS can induce oxidative stress which is associated with many age-related degenerative diseases.2,4–6 These degenerative diseases have major public health significance and include cardiovascular disease,7 inflammation,8 Alzheimer’s disease,9 Parkinson’s disease,10 diabetes,11 and cancer.12 The mechanisms underlying the involvement of ROS and oxidative stress in disease development may include oxidative modification of proteins,4 oxidation of lipids,13–15 DNA strand breaks and modification to nucleic acids,16 modulation of gene expression through activation of redox-sensitive transcription factors,17,18 and modulation of inflammatory responses through signal transduction.8 Enzymatic defenses have evolved to protect against these harmful biological oxidants. Superoxide dismutases, peroxidases, and catalases are some of the prominent and extensively studied antioxidant enzymes. Antioxidants also play an important role in preventing/limiting the damage caused by ROS.
The hydroxyl radical possesses the highest one-electron reduction potential of all the physiologically relevant ROS, and is extremely reactive with almost every type of biomolecule.19,20 The presence and pathological role of hydroxyl radicals in vivo has been demonstrated. Targets for hydroxyl radicals include proteins and nucleic acids.16,21 Because of their reactivity and ability to damage biological targets, hydroxyl radicals can serve as a representative ROS for investigating dietary antioxidants for their potential to react directly with and to quench free radicals, as well as to protect important biomolecules from radical-mediated damage. Growing evidence suggests that dietary antioxidants may play an important role in limiting oxidative damage and reducing the risk of numerous chronic diseases related to advancing age.22,23 There is as yet no known enzymatic reaction that can detoxify the hydroxyl radical in vivo. The only known defense against hydroxyl radicals is from antioxidants.
Many antioxidants are reducing agents; they participate in redox reactions by donating electrons or hydrogen atoms. There are several biologically relevant antioxidants that act to restore oxidative balance in the cellular environment. For example, vitamin C (ascorbate, AscH−), can neutralize free radicals by donating a hydrogen atom, forming the ascorbyl radical, which readily reacts with NADH or NADPH-dependent reductases to regenerate ascorbate.24 Similar to ascorbate, reduced glutathione (GSH) can reduce free radicals by hydrogen atom donation.25 Similarly, because of the ability of thiols to undergo redox reactions, cysteine exhibits antioxidant properties by hydrogen atom donation. However, by virtue of their reducing ability, these beneficial antioxidant compounds can also activate transition metal ions (e.g. Fe3+ to Fe2+ or Cu2+ to Cu+), making them behave as pro-oxidants. This may proceed in a cyclical manner (redox cycling), leading to continuous stream of ROS that can cause damage to DNA and other biomolecules.
Recent research has focused mostly on discovering antioxidant compounds for use in foods, cosmetics, and other products. There is less literature on the possible crossover effect from antioxidant to pro-oxidant activity that may be attributed to many biologically relevant antioxidants. A review of ingredients in marketed dietary supplements reveals a growing trend where antioxidants and redox active metals (e.g. chromium, cobalt, copper and iron) are present in the same formulation. These metals may participate in the formation of free radicals by a Fenton-like reaction mechanism (Mx+ + H2O2 → Mx++ + OH· + OH−, where M is a transition metal).20,26 The presence of antioxidant reducing agents together with redox active metals may lead to pro-oxidant activity. Most evidence for the pro-oxidant effect of antioxidants in the presence of redox active metals comes from in vitro studies. However, there is some evidence that this effect may occur in vivo. It has been shown that hydroxyl radicals can be detected in the bile of rats following intragastric administration of copper sulfate and ascorbic acid.27,28 Slivka and Kang have provided evidence that hydroxyl radicals are generated in the gastrointestinal tract following oral administration of ferrous sulfate and ascorbic acid to rats.29 Naito et al.30 have shown that injection of ferrous sulfate and ascorbic acid into the gastric wall of rats results in gastric ulcers. While it is generally accepted that antioxidants can act as pro-oxidants under certain conditions, there is no clear delineation of what these conditions are, how they differ among antioxidants, and how the adverse pro-oxidative effect can be avoided.
In the current study, we have focused on copper as a redox active metal which is present in many dietary supplements. Copper is an essential trace element having a recommended dietary allowance for adults of 900 μg/day.31 In vivo, most copper is bound securely to ceruloplasmin, which renders it inactive in Fenton-like reactions. However, about 5 to 15% is loosely bound to plasma albumin and other small molecules.32 This copper has been termed free copper and there is concern that free copper, in the presence of biological reducing agents, may increase the formation of free radicals. It has been previously shown that inorganic copper (e.g., copper present in drinking water and dietary supplements) is processed differently than organically complexed copper (i.e., copper present in food).32 Inorganic copper when ingested in large part bypasses the liver, ending up in the blood stream and contributing to the free copper pool.32 Dietary supplements contain primarily inorganic copper and, therefore, may increase free copper levels in the body, increasing the risk of free radical formation. The role of copper as a catalyst for free radical generation is well established. In fact, copper (Cu2+) has been found to be a much more redox active metal than iron (Fe3+) in many in vitro systems.33 Free radical generation involving copper is thought to be associated with development of some types of cancer and the acceleration of aging and age associated degenerative diseases.32,34–41
L-Ascorbic acid (Vit. C), L-cysteine, L-glutathione (GSH, reduced), and (−)-epigallocatechin gallate (EGCG) are biological antioxidants commonly present in dietary supplements. Many such dietary supplements also contain transition metals like iron and copper. This combination raises the question of the potential generation of free radicals in a Fenton-type reaction. In this study, we examined free radicals generated via a copper-based Fenton-type reaction. We determined if, and under what conditions, the selected antioxidants quench and/or promote radical formation. Conditions simulating the physiological pH of the stomach (pH =1.2) and of cells and tissues (pH = 7.4) were investigated. We also investigated whether albumin-bound copper can be redox activated by the presence of these antioxidants.
MATERIALS AND METHODS
General materials and instrumentation
Bovine serum albumin (BSA), L-ascorbic acid (Vit. C), L-cysteine, L-glutathione (GSH, reduced), (−)-epigallocatechin gallate (EGCG), copper sulfate, copper gluconate, hydrogen peroxide (30 wt. %), and 5,5-dimethyl-1-pyrroline- N-oxide (DMPO) were purchased from Sigma (St. Louis, MO). Distilled deionized water (18.2 MΩ.cm) from a Milli-Q water purification system was used in all experiments. ESR spectra were recorded with a Bruker EMX ESR spectrometer (Bruker Biospin, Billerica, MA).
Chemical reaction preparation
Two sources of copper commonly found in dietary supplements (the sulfate and gluconate salts of copper) were used. Four water soluble antioxidants of biological importance were used (Vit. C, GSH, cysteine, and EGCG). These were selected based on their presence in dietary supplements and/or prevalence in biological systems. Samples were tested at different pHs (1.2 and 7.4) to simulate gastric and intracellular conditions, respectively. The spin trap, DMPO, was purified by charcoal decolorization and vacuum distillation. The purified DMPO did not contain any ESR detectable impurities. Phosphate buffered saline (PBS, pH 7.4, 50 mM phosphate, 137 mM NaCl) and an HCl/KCl (50 mM KCl) solution (pH 1.2) were used to simulate intercellular and stomach conditions, respectively. All the buffers used were treated with Chelex 100 (Bio-Rad Inc., Hercules, CA) to remove transition metal ion contaminants.
Fenton-like reaction
Reactions were conducted in PBS, pH 7.4 or HCl/KCl, pH 1.2 and contained 10 μL of 1 mM Cu2+ (as copper sulfate or copper gluconate), 10 μL of either 10 or 100 mM antioxidant, and 20 μL of 250 mM DMPO. Ten μL of 10 mM H2O2 was added to initiate the reaction. The final volume was made up to 100 μL with the appropriate pH 7.4 buffer or pH 1.2 solution. The final concentrations of the reactants were 0.1 mM Cu2+, 1 or 10 mM antioxidant, 50 mM DMPO, and 1 mM H2O2. 50 μL aliquots of the samples were put in glass capillary tubes with internal diameter of 1 mm, placed in the ESR cavity, and the spectra recorded 1, 5, 10 and 15 min after addition of H2O2. ESR instrument settings were: 20 mW microwave power, 1 G field modulation, 9.76 GHz microwave frequency, 6.32 × 104 receiver gain, 100 kHz modulation frequency, 40.96 ms time constant, and 100 G sweep width. All measurements were taken in duplicate at room temperature (27 °C). The time dependence of the ESR signal intensity was obtained by measuring the peak to peak height of the second line of ESR spectrum of the hydroxyl radical adduct with DMPO.
Copper-albumin complex
To determine whether copper-protein complexes may participate in redox reactions, and to determine if these reactions generate harmful free radicals, copper was complexed with albumin.42 This complex was prepared by dissolving CuSO4 in a pH 7.4 phosphate buffered solution of BSA to prevent the precipitation of Cu(OH)2.42 The spectrum of the paramagnetic Cu2+-albumin complex was recorded at room temperature after 30 min. The complex was then mixed with different concentrations of the antioxidants (final concentration 0.25 mM copper to 0.375 mM BSA in each reaction mixture) to determine if the Cu2+ in the complex could be reduced to the diamagnetic Cu+. ESR instrument settings were 20 mW microwave power, 1 × 104 receiver gain, 100 kHz modulation frequency, 7.94 G modulation amplitude, 3029 G center field, and 1000 G scan range with 81.98 ms scan time constant. To further confirm the reduction of the complex, and whether this reduction made the complex redox active, 50 mM DMPO and 1 mM H2O2 were added to the mixture containing the Cu/BSA complex and antioxidant and the reaction was monitored for radical generation in real time using ESR. DMPO was used as the spin trap. The ESR instrument settings were 20 mW microwave power, 1 G field modulation, and 100 G scan range. All experiments were repeated at least twice and where appropriate reported as mean ± standard deviation.
RESULTS AND DISCUSSION
In this study, two different sources of copper (copper sulfate and copper gluconate) were used. The ESR results obtained using copper sulfate and copper gluconate were not significantly different (the results using copper gluconate are not shown). Consequently, copper sulfate was used to obtain the results presented here. To participate in a typical Fenton-like reaction, the cupric cation (Cu2+) of copper sulfate must first be reduced to Cu+, which can then react with H2O2 to produce the observed hydroxyl radicals.43 The resulting hydroxyl radicals react with the spin trap DMPO to form the stable DMPO-OH adduct, identified by a characteristic four line spectrum with 1:2:2:1 hyperfine splitting.44 Ascorbate can reduce cupric cation to the cuprous (Cu+) cation which may then react with hydrogen peroxide to generate hydroxyl radicals (Scheme 1).
Scheme 1.
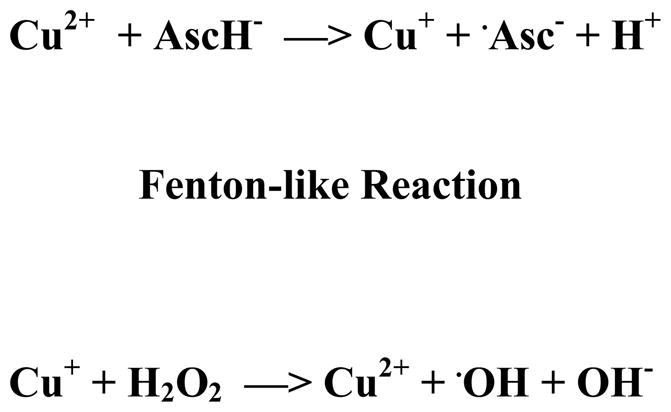
In the presence of cupric ion, ascorbate can act as a pro-oxidant.
Anti- and pro-oxidant activity of reducing agents in the presence of higher concentration of Cu2+
Figure 1 shows the results obtained when a 10 to 1 molar ratio of antioxidant (1 mM) to CuSO4 (0.1 mM) was used. Samples containing DMPO (50 mM) and, H2O2 (1 mM), without CuSO4, showed no radical production (data not shown). A weak ESR spectrum, characteristic of the spin adduct between DMPO and hydroxyl radical, was observed when DMPO was mixed with solutions containing CuSO4 and any of the antioxidants (data not shown). This observation is consistent with previous reports that Cu2+ can catalyze the oxidation of Vit. C, GSH, cysteine, and EGCG with concomitant formation of hydrogen peroxide and, subsequently, the hydroxyl radical.45–47
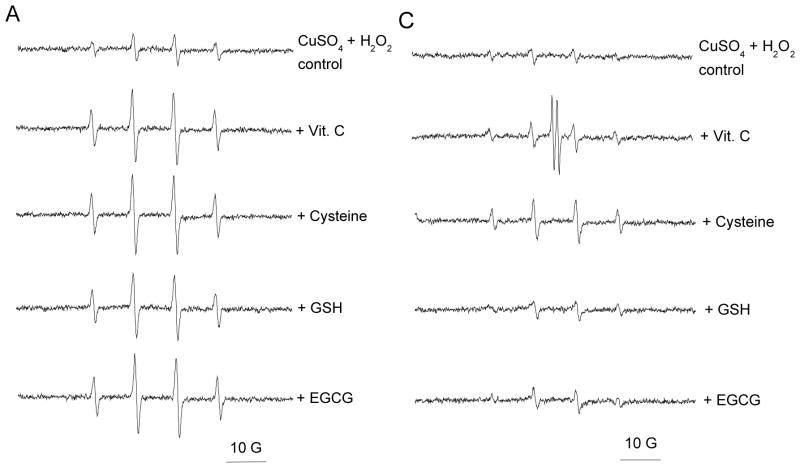
Figure 1. Activity of antioxidants at concentrations comparable to copper (10:1).
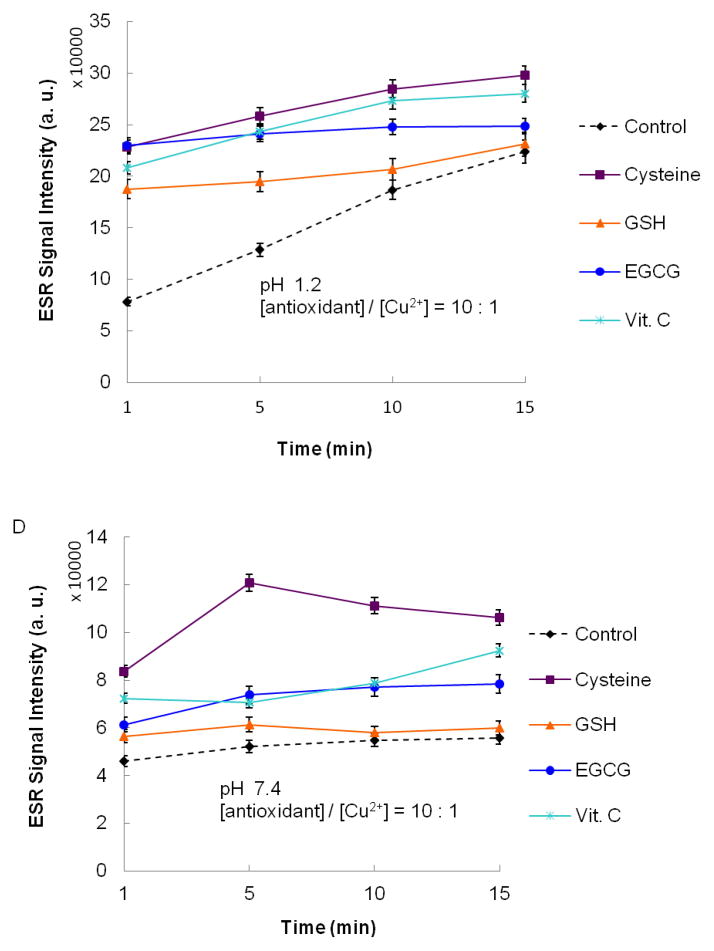
Final concentrations were 50 mM DMPO, 0.1 mM CuSO4, 1 mM antioxidant, and 1 mM H2O2. Reactions were conducted in buffered solutions with (A) pH 1.2 and (C) pH 7.4. ESR spectra were recorded after 1 min sample mixing. (B) and (D) show the effect of time on the progress of the reaction at pH 1.2 and 7.4, respectively. ESR instrument settings were: 20 mW microwave power, 1 G field modulation, and 100 G scan range. Measurements were taken at room temperature (27 °C). The time dependence of the ESR signal intensity was obtained by measuring the peak to peak height of the second line of ESR spectrum of the hydroxyl radical adduct with DMPO.
Figures 1A and 1B show the ESR spectra and time dependence for the ESR signal intensity obtained for solutions with pH 1.2. The ESR spectra shown in Figure 1A contain four-lines, having relative intensities of 1:2:2:1 and hyperfine splitting parameters aN = aH = 14.9 G and g = 2.005. This ESR spectrum is characteristic for the spin adduct between DMPO and the hydroxyl radical (DMPO-OH). The spin adduct, DMPO-OH, was observed 1 min after addition of H2O2 (1 mM) to the control sample, containing CuSO4 (0.1 mM) and DMPO (50 mM). Formation of DMPO-OH is attributable to reduction of Cu2+ by H2O2. The resulting Cu+ can then react with another H2O2 molecule to produce a hydroxyl radical and, subsequently, DMPO-OH.48,49 When one of the antioxidants is additionally present, an increase in the ESR signal intensity is observed (Figures 1A and 1B). This increase can be attributed to the antioxidants acting as pro-oxidants by reducing Cu2+ to the redox active Cu+. All four of the antioxidants elicited increases in the formation of DMPO-OH measured 1 min after addition of H2O2 (Figure 1A). The time dependence for the intensity of the ESR signal is shown in Figure 1B. When antioxidants were present, the major formation of DMPO-OH occurred within 1 min after addition of H2O2 with gradual increases in levels of DMPO-OH generally observed thereafter (Figure 1B). At 15 min after addition of H2O2, cysteine was observed to elicit the largest increase in the formation of DMPO-OH, followed by Vit. C, and EGCG. At 15 min following addition of H2O2, the signal intensity observed for samples containing GSH was similar to that observed for the control (Figure 1B).
Pro-oxidant activity was also observed for solutions containing antioxidants at pH 7.4 (Figures 1C and 1D). The signal intensities for all samples tested at pH 7.4 were significantly lower than samples tested at pH 1.2. This may be due to the poor solubility of Cu2+ at pH 7.4. The ESR spectra depicted in Figure 1C are consistent with enhanced hydroxyl radical formation in the presence of the antioxidants. An additional pair of lines appears in the ESR spectrum when samples contained Vit. C. These lines can be assigned to the ascorbyl radical (aH = 1.8 G, g = 2.005) resulting from the one electron reduction of Vit. C (Scheme 1). The ascorbyl radical is not observed in the ESR spectra obtained at pH 1.2 (Figure 1A). It has previously been reported that the ESR signal intensity for the ascorbyl radical is pH dependent with an optimum pH range of 7.2 – 7.4.50 The low ESR signal intensity for ascorbyl radical at low pH has been attributed to disproportionation of the ascorbyl radical in acidic solutions to form Vit. C and dehydroascorbic acid.51,52 Neither of these products can be detected by ESR. The time dependence for the intensity of the ESR signal at pH 7.4 is shown in Figure 1D. Similar to the trend observed at pH 1.2, hydroxyl radical production is evident 1 min after addition of H2O2, with only gradual increases thereafter. The relative pro-oxidant activities, observed 15 min after addition of H2O2, are the same as those observed at pH 1.2: cysteine > Vit. C > EGCG. The addition of GSH produced no significant effect on the ESR signal intensity, even after 15 min.
The results shown in Figure 1 demonstrate that crossover from antioxidant to pro-oxidant activity can occur for several antioxidants widely found in foods and in dietary supplements. Much is understood about the chemistry underlying the pro-oxidant activity of these antioxidants. The pro-oxidant activity of thiol compounds, such as cysteine and glutathione, results from the stable complexes formed between thiol groups and redox active transition metal ions, such as Cu2+ and Fe3+, and the reductive potential of the thiol group. Mechanistic studies of Cu2+-dependent oxidation of cysteine demonstrate that the formation of a Cu2+-cysteine complex is followed by reduction of Cu2+ to Cu+. Subsequent formation of H2O2 is observed and is thought to involve reduction of O2 by the thionyl radical formed during reduction of Cu2+.46 Both efficient reduction of Cu2+ to Cu+ and subsequent production of H2O2 contribute to the pro-oxidant activity of cysteine by supplying the reactants needed for a Fenton-like reaction. We found that cysteine, both in samples having pH of 1.2 and 7.4, had higher pro-oxidant activity than glutathione. This observation is consistent with reports that Cu2+-catalyzed oxidation of cysteine occurs more readily than Cu2+-catalyzed oxidation of glutathione.46,53 Copper-dependent oxidative damage to DNA is also enhanced more by cysteine than glutathione (Spear and Aust, 1995).54 In addition, in media supplemented with Cu+2, cytotoxicity elicited by cysteine (added as N-acetylcysteine) is greater than that elicited by GSH (Held and Biaglow, 1994).55 The relatively low pro-oxidant activity of GSH has been attributed to stabilization of Cu+ when complexed to GSH. This stable complex limits the reaction of Cu+ with H2O2 and reduces the formation of hydroxyl radical (Hanna and Mason, 1992).56 It is perhaps this property that allows GSH to function as the primary intracellular transporter of Cu+ without concomitant Cu+-catalyzed oxidative damage to cellular components (Vulpe and Packman, 1995).57
We also observed pro-oxidant activity for Vit. C in samples having pH 1.2 and 7.4. The pro-oxidant activity of Vit. C in the presence of redox active metal ions is well documented. As with thiol antioxidants, complex formation between the metal ion and Vit. C is an early event in the sequence of steps leading to Vit. C’s pro-oxidant activity. We observed pro-oxidant activity both at pH 7.4 and pH 1.2, which is well below the pKa for Vit. C (4.2). This result indicates that both undissociated ascorbic acid and the monobasic ascorbate ion can associate with Cu+2 to initiate free radical production. Reduction of Cu+2 to Cu+ by Vit. C is accompanied by production of the ascorbyl radical. In contrast to the analogous radical (thionyl radical) formed when cysteine reduces Cu+2, the ascorbyl radical is relatively stable and does not readily react to form ROS.20 When formed in vivo, the ascorbyl radical can react with NADH or NADPH-dependent reductases to regenerate ascorbate.24
The results in Figure 1 also show that EGCG, which is abundant in green tea and is available in dietary supplements, can exhibit pro-oxidant activity. This observation is consistent with previous reports describing the in vitro pro-oxidant activity of EGCG under a variety of experimental conditions.47,58–61 EGCG readily forms a complex with Cu2+.62 Subsequent reduction of complexed Cu2+ to Cu+, results in the formation of semiquinone radical due to oxidation of EGCG.63 This semiquinone radical, though relatively stable, has been shown under aerobic conditions to reduce O2 to ultimately form H2O2.
Hayakawa et al.59 have reported that EGCG has the lowest pro-oxidant activity of several catechins found in green tea. They have attributed this lower activity to the radical scavenging and metal chelating properties and of the additional gallate moiety in EGCG.
Anti- and pro-oxidant activity of reducing agents in the presence of lower concentration of Cu2+
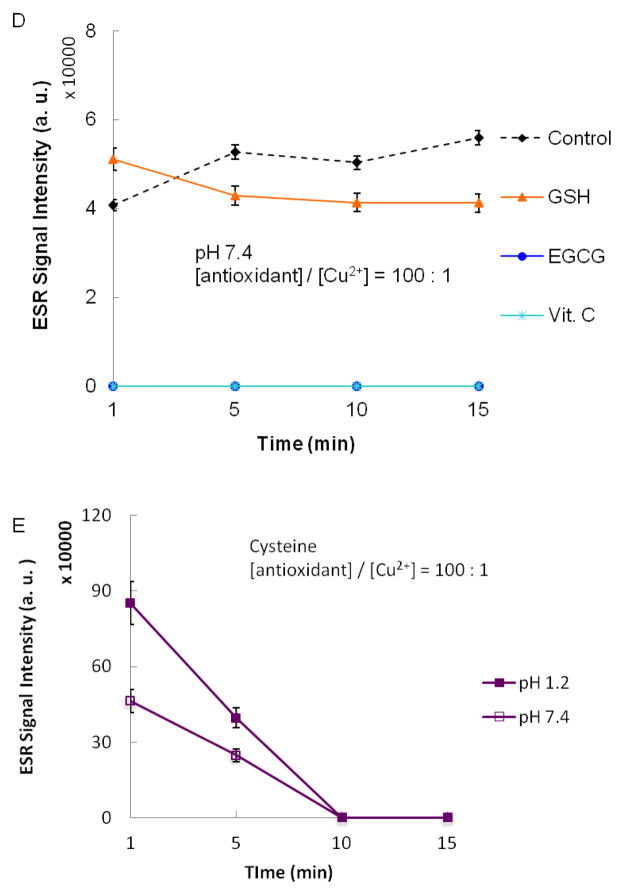
Figure 2 shows the results obtained when a 100 to 1 molar ratio of antioxidant (10 mM) to CuSO4 (0.1 mM) was used. At pH 1.2, Vit. C and GSH inhibited the formation of the spin adduct, DMPO-OH, measured 1 min after addition of H2O2 (1mM) (Figures 2A and 2B). Addition of EGCG had little effect on formation of DMPO-OH (Figure 2A). Antioxidant property in samples having pH 7.4 was also observed for Vit. C, GSH and EGCG (Figures 2C and 2D). A pH dependent variation in the antioxidant properties of GSH and EGCG was observed. A larger antioxidant effect was observed for GSH at pH 1.2 than at pH 7.4. In contrast, EGCG elicited a greater antioxidant effect at pH 7.4 than at pH 1.2. In general, the antioxidant property we observed may arise in two distinct ways. First, the antioxidant may reduce the formation of hydroxyl radicals by limiting the redox cycling of Cu2+. This effect has been reported for GSH radical.56 Alternatively, if the concentration of an antioxidant is sufficiently high, the antioxidant can intercept hydroxyl radicals before they reach other targets of oxidative damage (e.g. DMPO under our experimental conditions). In some instances both modes of antioxidant property may be important. The concentration dependence for the pro-oxidant and antioxidant properties we observed may give some indication of the mechanism of antioxidant properties for GSH, Vit. C and EGCG in the experimental system we have used. For GSH, we observed a low level of pro-oxidant activity when the molar ratio of GSH to Cu2+ was 10 and modest antioxidant property when the molar ratio was 100. The observed low level of pro-oxidant activity would suggest that GSH limits redox cycling of Cu2+. Stabilization of Cu+ by GSH, as reported by Hanna and Mason,56 may be the mechanism underlying this limitation of redox cycling. At higher concentrations, both stabilization of Cu+ and scavenging free hydroxyl radicals may contribute to the antioxidant property of GSH. In contrast, the pro-oxidant activity observed for Vit. C and EGCG (Figure 1) suggests that Vit. C and EGCG do not limit hydroxyl formation. Their antioxidant properties, observed when the molar ratio of antioxidant to Cu2+ was 100, would appear to derive from interception of hydroxyl radicals and prevention of the formation of DMPO-OH.
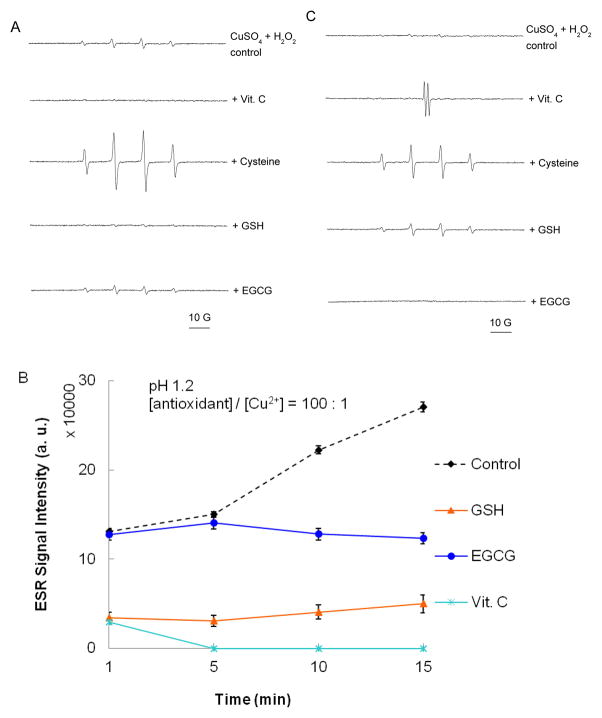
Figure 2. Activity of antioxidants at a higher concentration relative to copper (100:1).
Samples contained 50 mM DMPO, 0.1 mM CuSO4, 10 mM antioxidant, and 1 mM H2O2. Reactions were carried out in buffered solutions with (A) pH 1.2 and (C) pH 7.4. ESR spectra were recorded after 1 min sample mixing. (B) and (D) show the effect of time on the progress of the reaction at pH 1.2 and 7.4 respectively. Figure 2E depicts the time dependence of the ESR signal for samples containing 10 mM cysteine at pH 1.2 and pH 7.4.ESR instrument settings were: 20 mW microwave power, 1 G field modulation, and 100 G scan range. Measurements were taken at room temperature (27 °C). The time dependence of the ESR signal intensity was obtained by measuring the peak to peak height of the second line of ESR spectrum of the hydroxyl radical adduct with DMPO. Error bars represent ± SD.
Cysteine was unique among the antioxidants studied because it showed pro-oxidant activity under all the described experimental conditions. Figures 2A and 2C show that addition of cysteine (10 mM) to samples containing Cu2+ (0.1 mM) and H2O2 (1 mM) at pH 1.2 and 7.4, respectively, results in a dramatic increase in the intensity of the ESR signal for DMPO-OH. Therefore, we observed cysteine’s pro-oxidant activity when it was present both at a 10 to 1 (Figure 1) and 100 to 1 molar ratio to Cu2+. Heinecke et al.64 have reported similar results for Cu2+-dependent oxidation of low density lipoproteins. They observed that, unlike other antioxidants which exhibit pro-oxidant activity at low concentrations and antioxidant property at high concentrations, cysteine maintains its pro-oxidant activity over a wide concentration range. Figure 2E shows the time dependence for the ESR intensity for samples containing 10 mM cysteine at pH 1.2 and pH 7.4. The highest ESR intensity was observed 1 min after addition of H2O2 indicating rapid formation of hydroxyl radical. This observation is consistent with that of Patterson et al.65. These investigators found that within 2 min after mixing Cu2+ (10 μM) and cysteine (100 μM), approximately 75% of Cu2+ is reduced to Cu+ followed by minimal reduction thereafter. Similar results have been reported by Pecci et al.66 in experiments involving 0.2 mM Cu2+ and 2 mM cysteine. In the presence of H2O2, one would therefore expect concomitant rapid formation of the hydroxyl radical through a Fenton-like mechanism. These results demonstrate that cysteine has pro-oxidant activity over a wider range of concentrations than Vit. C or EGCG.
The effect of Cu2+ on the reducing agents in a form of copper-albumin complex
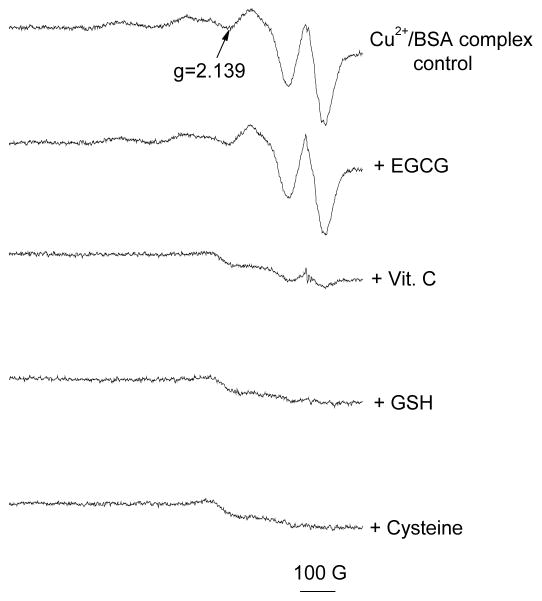
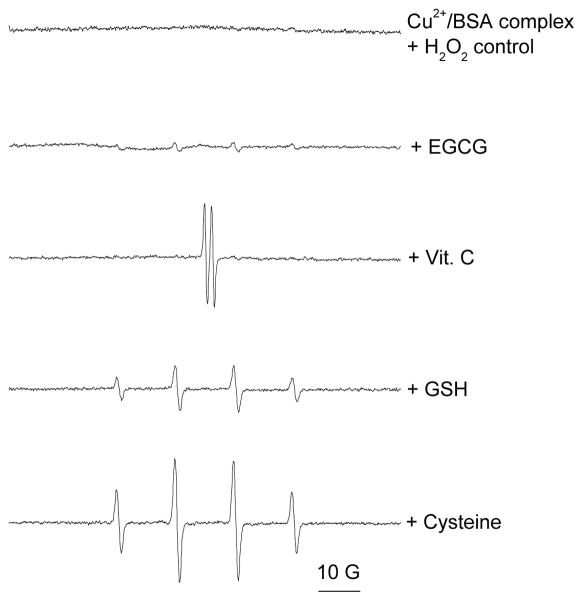
It has been reported that 85–95% of copper in serum is tightly bound to ceruloplasmin, and is unlikely to undergo Fenton-like reactions.32,67 The remaining copper is loosely bound to proteins and small molecules and may undergo redox cycling producing ROS. To better understand the reactivity of this Cu2+ loosely bound to BSA (Figure 3 and 4) we studied aqueous solutions of copper-albumin complex (final concentration 0.25 mM copper to 0.375 mM BSA). The ESR spectrum (Figure 3) obtained for this complex reflects slow tumbling of BSA resulting in a spectrum for Cu2+ intermediate between four lines for characteristic for Cu2+ bound to a fast tumbling site and the completely immobilized cation.68 When 5 mM EGCG was added to the solution, no change in the spectrum was observed (Figure 3). However, addition of equimolar concentrations of Vit. C, GSH, or cysteine caused a significant decrease in signal intensity, suggesting the reduction of the paramagnetic Cu2+ to the diamagnetic Cu+, which is ESR silent. Only a small residual signal was observed after addition of these antioxidants. After addition of Vit. C, a trace free radical signal attributable to an ascorbyl radical was observed (data not shown). To further confirm that the changes observed in the ESR spectra resulted from reduction of Cu2+ to Cu+, and to determine whether the Cu+-albumin complex thus formed could participate in redox reactions, 1 mM hydrogen peroxide and 50 mM DMPO were added and the production of free radicals was monitored. The Cu+-albumin complex did not generate any radicals. Addition of GSH and cysteine resulted in increased production of hydroxyl radicals, whilst addition of Vit. C generated only the ascorbyl radical (Figure 4). These results are consistent with the observations of Ozawa et al.42 that the Cu2+-albumin complex reacts with cysteine, in the presence of H2O2, to yield hydroxyl radical. As expected from our previous observation that EGCG did not reduce Cu2+ when it is bound to albumin (Figure 3), EGCG also had no significant effect on radical production by the albumin-bound complex in the presence of H2O2. Our findings indicate that in the presence of some antioxidants, copper sequestered to albumin may still generate radicals at physiologic pH through the reduction of Cu2+ to Cu+. These results also suggest that the physiological effects of dietary supplements, which increase levels of blood borne copper and antioxidants, warrant further investigation.
Figure 3. Effect of antioxidant on Cu2+/albumin complex.
ESR spectra of Cu2+-albumin complex without (control) antioxidants in aqueous solutions (pH 7.4). Final concentrations were 0.25 mM Cu2+, 0.375 mM BSA albumin and 10 mM antioxidant. ESR instrument settings were: 20 mW microwave power, 6.79 G field modulation, and 1000 G scan range. The measurements were taken at room temperature (27 °C). Error bars represent ± SD.
Figure 4. Radical generation by Cu2+-albumin complex on addition of antioxidants.
Samples contained 50 mM DMPO, 0.25 mM Cu2+, 0.375 mM BSA albumin, 1 mM H2O2 and 10 mM antioxidant in 50 mM PBS (pH 7.4). ESR instrument settings were 20 mW microwave power, 1 G field modulation, and 100 G scan range. All measurements were taken at room temperature (27 °C).
Our concept of the physiological role of antioxidants has evolved throughout the last decades of research. Presently, it is clear that antioxidants have broad physiological activities which include intercepting free radicals and direct activation of gene expression through antioxidant response elements.69 Our study has investigated another role of antioxidants, their pro-oxidant activity. We have found that pro-oxidant activity can be observed over a wide range of pH, from the pH in the stomach to that in tissues and cells. Our results also indicate that for some antioxidants, such as Vit. C, the pro-oxidant activity associated with redox cycling is evident when the ratio of antioxidant to metal ion is relatively low. For other antioxidants, such as cysteine, the pro-oxidant activity can be observed over a wide range of concentrations. Therefore, one should be cautious in making generalizations about the crossover from antioxidant to pro-oxidant activity for antioxidant ingredients used in dietary supplements. Currently most experimental and clinical studies are designed to evaluate the risks and benefits of individual ingredients in dietary supplements. Our results and those of other investigators indicate that there is also a need to investigate interactions between ingredients found in dietary supplements. The results we have obtained will hopefully help frame questions about risks associated with combining antioxidants and transition metals in dietary supplements, and encourage further research to answer some of these questions.
Acknowledgments
The authors gratefully acknowledge helpful comments on the manuscript provided by Dr. John Callahan, Office of Regulatory Science, CFSAN, FDA.
ABBREVIATIONS USED
- BSA
bovine serum albumin
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- EGCG
(−)-epigallocatechin gallate
- ESR
electron spin resonance
- GSH
L-glutathione
- Vit. C
L-ascorbic acid
- ROS
reactive oxygen species
Footnotes
This article is not an official guidance or policy statement of U.S. Food and Drug Administration (FDA). No official support or endorsement by the U.S. FDA is intended or should be inferred.
References
- 1.Davies KJA. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Stadtman ER, Berlett BS. Reactive-oxygen mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 5.Droge W. Free Radicals in the Physiological Control of Cell Function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 6.Rajindar SS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radical Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 7.Sachidanandame K, Fagan SC, Ergul A. Oxidative stress and cardiovascular disease: antioxidants and unresolved issues. Cardio Vasc Drug Rev. 2005;23:115–132. doi: 10.1111/j.1527-3466.2005.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 8.Bodamyali T, Stevens CR, Blake DR, Winyard PG. Reactive oxygen/nitrogen species and acute inflammation: a physiological process. In: Winyard PG, Blake DR, Evans CH, editors. Free Radicals and Inflammation. 1. Birkhauser; Basel, Switzerland: 2000. pp. 11–19. [Google Scholar]
- 9.Butterfield DA. 1. Alzheimer’s β-amyloid peptide and free radical oxidative stress. In: Gilbert DL, Colton CA, editors. Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach. Kluwer Academic Publishers; New York: 1999. pp. 609–638. [Google Scholar]
- 10.Cohen G. Oxidative stress and Parkinson’s disease. In: Gilbert DL, Colton CA, editors. Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach. 1. Kluwer Academic Publishers; New York: 1999. pp. 593–608. [Google Scholar]
- 11.Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 12.Kawanishi S, Hiraku Y, Murata M, Oikawa S. The role of metals in site-specific DNA damage with reference to carcinogensis. Free Radical Biol Med. 2002;32:822–832. doi: 10.1016/s0891-5849(02)00779-7. [DOI] [PubMed] [Google Scholar]
- 13.Poli G, Leonarduzzi U, Biasi F, Chiarpotto E. Oxidative stress and cell signaling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 14.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 16.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Crawford DR. Regulation of mammalian gene expression by reactive oxygen species. In: Gilbert DL, Colton CA, editors. Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach. 1. Kluwer Academic Publishers; New York: 1999. pp. 155–171. [Google Scholar]
- 18.Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radical Biol Med. 2004;37:582–593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 2. Oxford University Press; New York: 1989. The chemistry of oxygen radicals and other oxygen- derived species; pp. 22–81. [Google Scholar]
- 20.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate, and free radicals: combinations to avoid. Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 21.Lubec G. The hydroxyl radical: from chemistry to human disease. J Invest Med. 1996;44:324–346. [PubMed] [Google Scholar]
- 22.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 23.Prior RL, Wu XL, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 24.Hossain MA, Asada K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J Biol Chem. 1985;260:12920–12926. [PubMed] [Google Scholar]
- 25.Freedman JH, Rosa M, Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- 26.Leonard S, Gannett PM, Rojanasakul Y, Schwegler-Berry D, Castranova V, Vallyathan V, Shi X. Cobalt-mediated generation of reactive oxygen species and its possible mechanisms. Inorg Biochem. 1998;70:239–244. doi: 10.1016/s0162-0134(98)10022-3. [DOI] [PubMed] [Google Scholar]
- 27.Kadiiska MB, Hanna PM, Hernandez L, Mason RP. In vivo evidence of hydroxyl radical formation after acute copper and ascorbic acid intake: electron spin resonance spin-trapping investigation. Mol Pharmacol. 1992;42:723–729. [PubMed] [Google Scholar]
- 28.Kadiiska MB, Mason RP. In vivo copper-mediated free radical production: an ESR spin-trapping study. Spectrochim Acta A. 2002;58:1227–1239. doi: 10.1016/s1386-1425(01)00713-2. [DOI] [PubMed] [Google Scholar]
- 29.Slivka A, Kang JO, Cohen G. Hydroxyl radicals and the toxicity of oral iron. Biochem Pharmacol. 1986;35:553–556. doi: 10.1016/0006-2952(86)90346-1. [DOI] [PubMed] [Google Scholar]
- 30.Naito Y, Yoshikawa T, Yoneta T, Yagi N, Matsuyama K, Arai M, Tanigawa T, Kondo M. A new gastric-ulcer model in rats produced by ferrous iron and ascorbic-acid injection. Digestion. 1995:472–478. doi: 10.1159/000201278. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 32.Brewer GJ. Risks of copper and iron toxicity during aging in humans. Chem Res Toxicol. 2010;23:319–326. doi: 10.1021/tx900338d. [DOI] [PubMed] [Google Scholar]
- 33.Urbański NK, Beręsewicz A. Generation of ·OH initiated by interaction of Fe+2 and Cu+ with dioxygen; comparison with the Fenton chemistry. Acta Biochim Polonica. 2000;47:951–961. [PubMed] [Google Scholar]
- 34.Daniel KG, Harbach RH, Guida WC, Dou QP. Copper storage diseases: Menkes, Wilson’s, and cancer. Front Biosci. 2004;9:2652–2662. doi: 10.2741/1424. [DOI] [PubMed] [Google Scholar]
- 35.Olivares M, Pizarro F, Speisky H, Lonnerdal V, Uauy R. Copper in infant nutrition: safety of World Health Organization provisional guideline value for copper content of drinking water. J Pediatr Gastroenterol Nutr. 1998;26:251–257. doi: 10.1097/00005176-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer risk in relation to serum copper levels. Cancer Res. 1989;49:4353–4356. [PubMed] [Google Scholar]
- 37.Wu TJ, Sempos CT, Freudenheim JL, Muti P, Smith E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol. 2004;14:195–201. doi: 10.1016/S1047-2797(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 38.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 39.Brewer GJ, Askari F, Dick RB, Sitterly J, Fink JK, Carlson M, Kluin KJ, Lorincz MT. The treatment of Wilson’s disease with tetrathiomolybdate: V. Control of free copper by TM and a comparison with trientine. Transl Res. 2009;154:70–77. doi: 10.1016/j.trsl.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med (Maywood) 2007a;232:323–335. [PubMed] [Google Scholar]
- 41.Brewer GJ. Elevated levels of dietary copper may accelerate cognitive decline and hasten the onset of Alzheimer disease. Nutr M D. 2007b;33:1–4. [Google Scholar]
- 42.Ozawa T, Ueda J, Hanaki A. Copper (II)-albumin complex can activate hydrogen peroxide in the presence of biological reductants: first ESR evidence for the formation of hydroxyl radicals. Biochem Mol Biol Int. 1993;29:247–253. [PubMed] [Google Scholar]
- 43.Wardman P, Candeias LP. Fenton chemistry: an introduction. Radiat Chem. 1996;145:523–531. [PubMed] [Google Scholar]
- 44.Moore J, Yin JJ, Yu L. Novel fluorometric assay for hydroxyl radical scavenging capacity (HOSC) estimation. J Agric Food Chem. 2006;54:617–626. doi: 10.1021/jf052555p. [DOI] [PubMed] [Google Scholar]
- 45.Taqui Khan MM, Martell AE. Metal ion and metal chelate catalyzed oxidation of ascorbic acid by molecular oxygen. I. Cupric and ferric ion catalyzed oxidation. J Am Chem Soc. 1967;89:4176–4185. doi: 10.1021/ja00992a036. [DOI] [PubMed] [Google Scholar]
- 46.Kachur AV, Koch CJ, Biaglow JE. Mechanism of copper-catalyzed autooxidation of cysteine. Free Radic Res. 1999;31:23–34. doi: 10.1080/10715769900300571. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Yin J, Shen S. Effects of epi-gallocatechin gallate on PC-3 cell cytoplasmic membrane in the presence of Cu2+ Fd Chem. 2006;95:108–115. [Google Scholar]
- 48.Millero FJ, Sharma VK, Karn B. The rate of reduction of copper(II) with hydrogen peroxide in seawater. Marine Chem. 1991;36:71–83. [Google Scholar]
- 49.Nakajima A, Ueda Y. Relationship between copper biosorption and microbial inhibition of hydroxyl radical formation in a copper(II)-hydrogen peroxide system. World J Microbiol Biotech. 2008;24:1253–1257. [Google Scholar]
- 50.Lohmann W, Holz D. Structure of ascorbic acid and its biological function I. ESR determination of the ascorbyl radical in biological samples and in model systems. Biophys Struct Mech. 1984;10:197–204. [PubMed] [Google Scholar]
- 51.Laroff GP, Fessenden RW, Schuler RH. The electron spin resonance spectra of radical intermediates in the oxidation of ascorbic acid and related substances. J Am Chem Soc. 1972;94:9062–9073. doi: 10.1021/ja00781a013. [DOI] [PubMed] [Google Scholar]
- 52.Bielski BHH, Allen AO, Schwarz HA. Mechanism of disproportionation of ascorbate radicals. J Am Chem Soc. 1981;103:3516–3518. [Google Scholar]
- 53.Kachur AV, Koch CJ, Biaglow JE. Mechanism of copper-catalyzed oxidation of glutathione. Free Radic Res. 1998;28:259–269. doi: 10.3109/10715769809069278. [DOI] [PubMed] [Google Scholar]
- 54.Spear N, Aust SD. Hydroxylation of deoxyguanosine in DNA by copper and thiols. Arch Biochem Biophys. 1995;317:142–148. doi: 10.1006/abbi.1995.1146. [DOI] [PubMed] [Google Scholar]
- 55.Held KD, Biaglow JE. Mechanisms for the oxygen radical-mediated toxicity of various thiol-containing compounds in cultured mammalian cells. Radiat Res. 1994;139:15–23. [PubMed] [Google Scholar]
- 56.Hanna PM, Mason RP. Direct evidence for inhibition of free radical formation from Cu(I) and hydrogen peroxide by glutathione and other potential ligands using EPR spin-trapping technique. Arch Biochem Biophys. 1992;295:205–213. doi: 10.1016/0003-9861(92)90507-s. [DOI] [PubMed] [Google Scholar]
- 57.Vulpe CD, Packman S. Cellular copper transport. Ann Rev Nutr. 1995;15:293–322. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka H, Senba Y, Saita K, Kimura T, Hayakawa F. Spin-trapping study on the hydroxyl radical formed from a tea catechin-Cu(II) system. Biosci Biotechnol Biochem. 2001;65:1697–1706. doi: 10.1271/bbb.65.1697. [DOI] [PubMed] [Google Scholar]
- 59.Hayakawa F, Ishizu Y, Hoshino N, Yamaji A, Ando T, Kimura T. Prooxidative activities of tea catechins in the presence of Cu2+ 2004;68:1825–1830. doi: 10.1271/bbb.68.1825. [DOI] [PubMed] [Google Scholar]
- 60.Perron NR, García CR, Pinzón JR, Chaur MN, Brumaghim JL. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J Inorg Biochem. 2011;105:745–753. doi: 10.1016/j.jinorgbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Sun LM, Zhang CL, Li P. Characterization, antimicrobial activity, and mechanism of a high-performance (−)-epigallocatechin-3-gallate (EGCG)-CuII/polyvinyl alcohol (PVA) nanofibrous membrane. J Agric Food Chem. 2011;59:5087–5092. doi: 10.1021/jf200580t. [DOI] [PubMed] [Google Scholar]
- 63.Mochizuki M, Yamazaki S, Kano K, Ikeda T. Kinetic analysis and mechanistic aspects of autooxidation of catechins. Biochim Biophys Acta. 2002;1569:35–44. doi: 10.1016/s0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
- 64.Heinecke JW, Kawamura M, Suzuki L, Chait A. Oxidation of low density lipoprotein by thiols: superoxide-dependent and –independent mechanisms. J Lipid Res. 1993;34:2051–2061. [PubMed] [Google Scholar]
- 65.Patterson RA, Lamb DJ, Leake DS. Mechanisms by which cysteine can inhibit or promote the oxidation of low density lipoprotein by copper. Atherosclerosis. 2003;169:87–94. doi: 10.1016/s0021-9150(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 66.Pecci L, Montefoschi G, Musci G, Cavallini D. Novel findings on the copper catalysed oxidation of cysteine. Amino Acids. 1997;13:355–367. [Google Scholar]
- 67.Healy J, Tipton K. Ceruloplasmin and what it might do. J Neural Transm. 2007;114:777–781. doi: 10.1007/s00702-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 68.Rakhit G, Antholine WE, Froncisz W, Hyde JS, Pilbrow JR, Sinclair GR, Sarkar B. Direct evidence of nitrogen coupling in the copper(II) complex of bovine serum albumin by S-band electron spin resonance technique. J Inorg Biochem. 1985;25:217–224. doi: 10.1016/0162-0134(85)80015-5. [DOI] [PubMed] [Google Scholar]
- 69.Finley JW, Kong AN, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem. 2011;59:6837–6846. doi: 10.1021/jf2013875. [DOI] [PubMed] [Google Scholar]