Abstract
Vascularized composite allograft (VCA) transplantation can restore form and function following severe craniofacial injuries, extremity amputations or massive tissue loss. The induction of transplant tolerance would eliminate the need for long-term immunosuppression, realigning the risk–benefit ratio for these life-enhancing procedures. Skin, a critical component of VCA, has consistently presented the most stringent challenge to transplant tolerance. Here, we demonstrate, in a clinically relevant miniature swine model, induction of immunologic tolerance of VCAs across MHC barriers by induction of stable hematopoietic mixed chimerism. Recipient conditioning consisted of T cell depletion with CD3-immunotoxin, and 100 cGy total body irradiation prior to hematopoietic cell transplantation (HCT) and a 45-day course of cyclosporine A. VCA transplantation was performed either simultaneously to induction of mixed chimerism or into established mixed chimeras 85–150 days later. Following withdrawal of immunosuppression both VCAs transplanted into stable chimeras (n =4), and those transplanted at the time of HCT (n =2) accepted all components, including skin, without evidence of rejection to the experimental end point 115–504 days posttransplant. These data demonstrate that tolerance across MHC mismatches can be induced in a clinically relevant VCA model, providing proof of concept for long-term immunosuppression-free survival.
Keywords: Miniature swine, mixed chimerism, skin transplant tolerance, vascularized composite allograft
Introduction
Vascularized composite allograft (VCA) transplantation is an established treatment in the management of disfiguring injury, amputation and massive tissue loss. Although most frequently associated with the face and upper extremities, any somatic unit composed of multiple tissues, transplanted in a primarily vascularized manner, may be considered a VCA. The latest report of the International Registry on Hand and Composite Tissue Transplantation indicates that these procedures, which have to date also included transplantation of abdominal wall, larynx and lower extremity, offer patients significant improvement over conventional reconstructive surgery and/or prostheses with respect to functional outcomes, patient satisfaction and quality of life (1–5).
However, in contrast to solid organ transplants, VCAs are not acutely life preserving, and in this context, the necessity for life-long immunosuppressive therapy and its associated risks, present a considerable ethical dilemma limiting broader availability (6). Follow-up data now extend over 10 years, with encouraging functional outcomes (7,8). However, VCA recipients have been subject to a burden of immunosuppression comparable to that of solid organ transplantation, including metabolic complications, cytomegalovirus infection and neoplasia (1,9), and have experienced a high incidence of acute rejection episodes, the long-term sequelae of which, in terms of chronic rejection, may not yet have become evident.
The induction of robust, life-long transplant tolerance, defined as acceptance of tissues or organs transplanted between nonidentical individuals without evidence of rejection in the absence of long-term immunosuppressive therapy, holds the potential to facilitate transplantation without the risks associated with current regimens. Therefore, such tolerance would be of particular significance in the field of vascularized composite allotransplantation.
While tolerance of transplanted organs and tissues has been demonstrated in numerous murine models (10), only induction of multilineage hematopoietic mixed chimerism has led to durable tolerance in large animal models (11,12) or in clinical trials (13,14). In the successful clinical protocols, both transient (14) and stable (13) mixed chimerism have been effective for induction of tolerance for kidney transplants.
In comparison to the relatively tolerogenic nature of the kidney, skin has long been recognized as particularly immunogenic, and historically skin transplantation was of interest primarily as a stringent test of experimental tolerance protocols (15). Reliable induction of VCA tolerance, particularly the skin component, by mixed chimerism in large animal models has proved challenging, requiring either MHC matching or co-transplantation of donor mesenchymal stem cells to mitigate otherwise fatal graft versus host disease (GvHD) (16,17). The result has frequently been split tolerance, a condition where musculoskeletal elements of the graft are accepted, but skin is ultimately rejected (18,19).
We have previously reported induction of stable multi-lineage mixed chimerism across MHC barriers in Massachusetts General Hospital (MGH) miniature swine without GvHD using a nonmyeloablative conditioning regimen and transplantation of cytokine-mobilized donor hematopoietic stem cells (20,21). Furthermore, murine studies have demonstrated rapid establishment of donor-specific unresponsiveness early post-hematopoietic cell transplantation (HCT) allowing for indefinite acceptance of various transplanted tissues including skin grafts, when placed at time of mixed chimerism induction (22), leading us to hypothesize that contemporaneous HCT and VCA would result in establishment of both chimerism and VCA tolerance.
Here, we report, for the first time in an MHC-mismatched (single haplotype full mismatch, i.e. Class I plus Class II) large animal model, induction of VCA tolerance, both when placed at the time of HCT and when transplanted into stable chimeras. Induction of VCA tolerance by simultaneous HCT is an important proof of principle for development of clinical tolerance protocols, as in contrast to kidneys, only deceased donors are applicable to hand and face transplantation. Furthermore, the complete acceptance of VCAs transplanted into established mixed chimeras following cessation of all immunosuppression demonstrates the robustness and broad spectrum of tolerance to donor tissue-specific antigens in this model.
In addition, no evidence for either classical anergy or regulation of anti-donor responses by T regulatory cells (Tregs) could be demonstrated in vitro. These data demonstrate that mixed chimerism is sufficient for induction of VCA tolerance, including skin tolerance, and support development of clinically applicable protocols for VCA tolerance.
Materials and Methods
Animals
Haploidentical donor/recipient combinations were selected from the MGH miniature swine herd (23). Swine leukocyte antigenac (SLAac) animals positive for the allelic hematopoietic marker pig allelic antigen (PAA) were selected as donors (24). Recipients were SLAad PAA negative. MGH miniature swine are bred in a specific pathogen-free facility with defined MHC while maintaining minor antigen variation. Animals were housed at the Transplantation Biology Research Center in accordance with the Guide for the Care and Use of Laboratory Animals. All experiments were conducted with the approval of the Institutional Animal Care and Use Committee of the MGH.
Hematopoietic cell transplantation
Stem cell collection and recipient conditioning were performed according to previously established protocols (20). Donor animals received a 7-day course of porcine interleukin-3 (pIL-3) and stem cell factor (pSCF), injected intramuscularly at a dose of 100 μg/kg to 30 kg bodyweight, 50 μg/kg for each additional kilogram. Mobilized hematopoietic stem cells were collected from peripheral blood by apheresis over 3 days, commencing after the fifth dose of pIL-3/pSCF. Recipients underwent a reduced intensity conditioning regimen, referred to as ITC, consisting of 100 cGy total body irradiation on day –2, partial T cell depletion with CD3-immunotoxin, 50 μg/kg IV, either pCD3-CRM9 on day –2 (25) or recombinant CD3-immunotoxin (pCD3-DT390) twice daily from day –4 to –1 (26), and a 45-day course of cyclosporine A (target trough 400–800 ng/mL day 0–30 then taper to discontinuation). Unmodified cytokine-mobilized peripheral blood mononuclear cells were transplanted over 2 or 3 days following conditioning (days 0–2) as required to achieve the target dose of 15 × 109 cells/kg (Figure 1).
Figure 1. Reduced intensity conditioning regimen for induction of mixed chimerism and VCA tolerance.
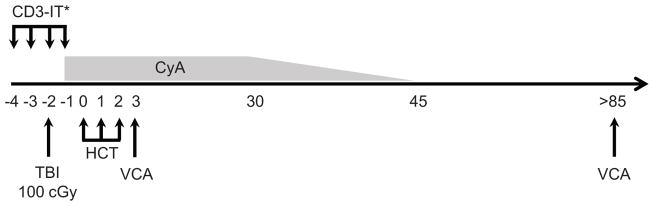
Recipients underwent 100 cGy TBI and T cell depletion with CD3-Immunotoxin (CD3-IT) prior to hematopoietic cell transplantation (HCT) with 15 × 109 cells/kg in two to three doses over days 0–2 as required to achieve target cell number. Cyclosporine A (CyA) was administered commencing on days −1 to 30 (target trough 400–800 ng/mL) followed by a taper to discontinuation on day 45. VCA transplantation was performed into stable chimeras between 85 and 150 days post-HCT, or simultaneous to induction of mixed chimerism, within 56 h of the first infusion of donor hematopoietic cells. *CD3-IT was administered either as the conjugate pCD3-CRM9 as a single 50 μg/kg dose on day −2 (animals 17468 and 17469) or as eight 50 μg/kg doses of recombinant pCD3-DT390, twice a day from day −4 to −1 (animals 20311, 20313, 20680 and 20681). TBI, total body irradiation; VCA, vascularized composite allograft.
VCA transplantation
A fasciocutaneous vascularized composite allotransplant model was used (27). Donor animals were placed under general anesthesia, positioned supine with the hind limbs extended and prepared for surgery. A skin island of approximately 10 × 18 cm was elevated, with the underlying subcutaneous tissue and fascia, on a vascular pedicle comprising the medial saphenous artery and veins to their junctions with the superficial femoral vessels, which were recovered to a length of 2 cm to facilitate anastomosis. Flaps were recovered and flushed with 100 U/mL heparin sulfate in 0.9% normal saline. Defects were prepared in either the neck or lower lateral abdominal wall of recipients and donor vessels anastomosed to the carotid artery/internal jugular vein, or femoral artery/vein using standard microsurgical techniques. Warm ischemia time was between 50 and 90 min. VCA transplantation was performed into stable mixed chimeras 85–150 days post-HCT, or simultaneously to induction of mixed chimerism within 56 h of the first infusion of donor hematopoietic cells.
Chimerism and engraftment
Peripheral blood chimerism was monitored by flow cytometry using monoclonal antibodies to PAA (1038H-10-9) (24) and lineage markers to CD1 (76-7-4) (28), CD3ε (898H2-6-15) (29), CD4 (74-12-4) (28), CD8a (76-2-11) (30), CD16 (G7) (31), CD25 (231.3B2) (32), CD172 (74-22-15A) (28), FoxP3 (FJK-16s; eBioscience, San Diego, CA). Samples were acquired on a FACScan or FACSCalibur (Becton Dickinson, Franklin Lakes, NJ), and data analyzed in FlowJo (TreeStar, Inc., Ashland, OR). Tissue chimerism in bone marrow and thymus was similarly assessed pretransplant and at day 50 and 100 posttransplant, following preparation of single cell suspensions from biopsy specimens. Recipient bone marrow was plated in CFU assay and colonies screened for presence of donor MHC by PCR amplification of MHC Class Ic, followed by Southern blot confirmation according to previously described methods (29,33).
In vitro functional assays
Mixed lymphocyte reaction (MLR) and cell-mediated lymphocytotoxicity (CML) assays were performed as previously described (34,35). Briefly, for MLR, peripheral blood mononuclear cells were isolated from recipient, donor or donor MHC-matched and third-party animals. Recipient (responder) cells were co-cultured in triplicate with irradiated self, donor and third-party stimulators for 5 days, pulsed with tritiated (3H) thymidine and, after a further 5-h incubation, incorporated tritium measured by beta-counter. Data are expressed for each stimulator as mean counts per minute (CPM) with standard deviation. The role of CD25+ cells in MLR was assessed by depletion of CD25+ cells from responder populations by magnetic activated cell sorting (MACS). Peripheral blood mononuclear cells were stained using biotinylated anti-swine α-CD25 Bio (231.3B2), followed by streptavidin phycoerythin exposure and depleted using α-PE beads and LD columns (Miltenyi Biotech, Cambridge, MA) prior to plating in MLR. Exogenous IL-2 MLRs were performed as previously described, but with addition of 5 U/mL porcine IL-2 per well. Thymidine uptake was measured as for standard MLR, and data expressed as the delta-CPM in comparison to anti-self responses. CML assays were performed by placing responder populations in co-culture with irradiated stimulators for 5 days, followed by re-plating with Chromium-51 labeled target cells at effector:target ratios of 100:1, 50:1, 25:1 and 12.5:1. Target cell lysis was measured by quantification of Chromium-51 in culture supernatant by gamma counter.
Histopathology and immunohistochemistry
Biopsy samples were immediately placed in formalin and processed by routine histologic methods, followed by hematoxylin and eosin staining and assessment by a board certified pathologist. Immunohistochemistry was performed on formalin fixed, paraffin embedded specimens following antigen recovery with Diva or Borg decloaking solution (Biocare Medical, Concord, CA). Adjacent 10-μm sections were stained for CD3+ (rabbit anti-human CD3 [DAKO, Carpinteria, CA]; biotinylated goat anti-rabbit IgG [Vector Labs, Burlingame, CA]; streptavidin [Biogenex, Fremont, CA]; DAB [DAKO]), or FoxP3+ (rat anti-FoxP3 [eBioscience]; rat-on-mouse horseradish peroxidase probe/polymer [Biocare, Concord, CA]; AEC [Biocare]). CD3+ and FoxP3+ cells in the dermis and epidermis were quantified from images captured at 400× magnification using a DP25 camera and BX53 microscope (Olympus, Center Valley, PA).
Results
Nonmyeloablative conditioning and HCT achieve stable multilineage mixed chimerism in miniature swine
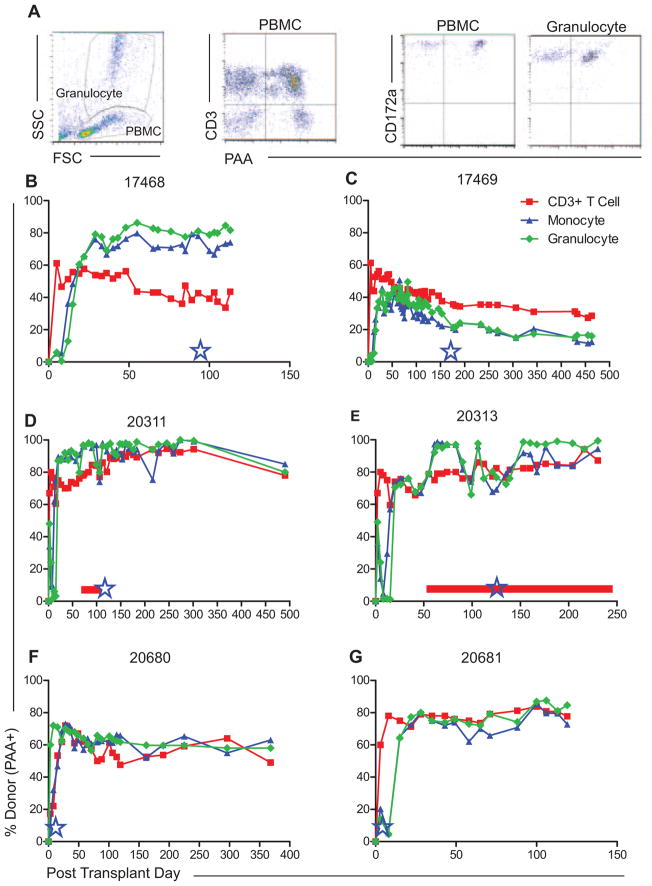
Stable multilineage hematopoietic mixed chimerism was established in MGH miniature swine by haploidentical HCT following nonmyeloablative conditioning with a regimen comprised of 100 cGy total body irradiation, CD3 targeted diphtheria immunotoxin and a 45-day course of cyclosporine A, as previously described (Figure 1) (20). The 15 × 109 cytokine-mobilized peripheral blood mononuclear cells per kilogram recipient weight were collected from SLAac donors by apheresis following mobilization with pIL-3 and pSCF and administered intravenously to SLAad recipients. Long-term, stable multilineage mixed chimerism (between 20% and 100% donor) was established in six of six animals as assessed by the flow cytometric detection of donor-derived CD3+ T cell (including CD4+CD25+FoxP3+ Treg), B cell, NK cell, monocyte, and granulocyte populations in peripheral blood, through the use of a donor-specific marker, PAA (24) (Figure 2A–G). Donor-type cells were also identified by flow cytometry of cell suspensions from biopsy specimens of bone marrow and thymus collected on weeks 7 and 14 post-HCT in all animals (Figure S1). Engraftment of donor hematopoietic stem cells was confirmed in all recipients over 100 days post-HCT by detection of donor-derived colony forming units cultured from recipient bone marrow biopsies by polymerase chain reaction amplification and Southern blot of SLA Class Ic (data not shown). Control animals which underwent conditioning and VCA without HCT (20312, 20989), or VCA alone with neither conditioning nor HCT (17519, 17520) failed to demonstrate detectable chimerism (data not shown). All animals included in this study are summarized in Table 1.
Figure 2. Induction of multilineage mixed chimerism across MHC barriers.
(A) Representative peripheral blood chimerism flow cytometry data. Chimerism was calculated as percentage donor-type (PAA positive) cells in CD3+T cell, monocyte and granulocyte lineages. Monocytes and granulocytes were defined as CD16+CD172a+ populations within the PBMC and granulocyte gates, respectively. (B–G) Multilineage mixed chimerism was followed longitudinally in recipients of haploidentical HCT and VCA. VCA transplantation was performed in delayed fashion (days 85–150 post-HCT) or contemporaneous to HCT and is denoted by blue stars. Cutaneous GvHD was diagnosed in animals 20311 (D) and 20313 (E) as denoted by horizontal red bars. Two control animals underwent conditioning and VCA at day 100 (20312) and day 3 (20989), respectively, and never demonstrated detectable mixed chimerism (data not shown). HCT, hematopoietic cell transplantation; PAA, pig allelic antigen; PBMC, peripheral blood mononuclear cell; VCA, vascularized composite allograft.
Table 1.
Summary of animals included in this study
| Animal number | Conditioning | HCT | VCA timing1 | Stable mixed chimerism2 | VCA survival (days)3 | Skin graft survival4
|
Additional comments | |
|---|---|---|---|---|---|---|---|---|
| Donor | Third party | |||||||
| 17468 | Yes | Yes | Delayed | Yes | >46 | – | – | Central line complications, euthanasia day 46 |
| 17469 | Yes | Yes | Delayed | Yes | >400 | >275 | – | – |
| 20311 | Yes | Yes | Delayed | Yes | >504 | >338 | 24/24 | Cutaneous GvHD stage 1 diagnosed day 50. CyA days 76–106. Complete resolution, no recurrence |
| 20313 | Yes | Yes | Delayed | Yes | >115 | – | – | Cutaneous GvHD stage 1 day 45, progressive to stage 3 by day 70. Relapsing-remitting course treated with CyA days 86–110, Methylprednisolone days 71–114, 130–150, 160–179 |
| 20312 | Yes | No | Delayed | No | 9 | – | – | – |
| 20680 | Yes | Yes | Simultaneous | Yes | >486 | >335 | 42/56 | – |
| 20681 | Yes | Yes | Simultaneous | Yes | >139 | – | – | – |
| 20989 | Yes | No | Simultaneous | Yes | 79 | – | – | – |
| 17519 | No | No | N/A | No | 6 | – | – | – |
| 17520 | No | No | N/A | No | 6 | – | – | – |
CyA, cyclosporine A; GvHD, graft versus host disease; HCT, hematopoietic cell transplantation; VCA, vascularized composite allograft.
VCA transplantation was either delayed (85–150 days post-HCT) or simultaneous to induction of mixed chimerism (within 56 h of first infusion of donor hematopoietic cells). Control animals 17519 and 17520 received VCA with neither conditioning nor HCT.
Chimerism levels for individual chimeric animals are shown in Figure 2.
>denotes rejection-free VCA survival at termination of experiment.
>denotes rejection-free skin graft survival at termination of experiment. Animals 20311 and 20680 each received two third-party grafts, survival for each is presented.
GvHD is a major complication of allogeneic HCT with a reported incidence of approximately 60% in some series (36). Two animals in the current series developed GvHD, which in both cases was limited to the skin. Stage 1 cutaneous GvHD was diagnosed in 20311 on posttransplant day 50. The disease was not progressive, and resolved completely by day 106 following treatment for 30 days with cyclosporine A (target trough level in blood 200–400 ng/mL). GvHD was diagnosed in 20313 on day 45, and progressed steadily to stage 3 by day 70. Cyclosporine A alone proved insufficient to induce remission, but rapid resolution followed an intravenous corticosteroid pulse. However, following taper and discontinuation of steroids, the disease recurred and thereafter followed a relapsing-remitting course, necessitating several further courses of treatment. In both animals, the VCA remained free from GvHD, the skin rash clearly demarcating at the border of host and transplanted skin (Figure S2).
Stable mixed chimeras are tolerant of all components of VCAs without requirement of immunosuppression at time of transplant
Following confirmation of hematopoietic stem cell engraftment and specific unresponsiveness to donor antigens by in vitro assays (see below) between 85 and 150 days post-HCT, four animals (17468, 17469, 20311 and 20313) received VCAs from their original HCT donors. We sought to assess the robustness of donor-specific unresponsiveness by placing VCAs from the original HCT donors following completion of the induction regimen and cessation of all immunosuppression. Three recipients accepted VCAs indefinitely with neither clinical (Figure 3A and C) nor histological (Figure 3B and D) evidence of rejection at any point. Animal 17468 required euthanasia on postoperative day 46 due to complications related to a central line, although the VCA was free from histological evidence of rejection at postmortem examination. The remaining experimental animals accepted VCAs with neither gross nor histological evidence of rejection to the study end point between 115 and 504 days posttransplant. Tolerance was confirmed in two animals (17469 and 20311) by the indefinite acceptance of donor split thickness skin grafts placed over 100 days post-VCA. Furthermore, 20311 subsequently received two third-party skin grafts, with donor-matched (minor antigen mismatched) and single haplotype mismatched (SLAaa) skin, to test tolerance. These grafts rejected completely within 24 days (Figure S3). Control animal 20312, which underwent conditioning but did not receive HCT, also received a VCA greater than 100 days following conditioning, at which point it demonstrated normal anti-donor responses in MLR (Figure 5F) and CML assays (Figure S4). Acute rejection, with complete necrosis by posttransplant day 9 was observed (Figure 3E and F). Two additional controls (17519 and 17520) which received haplomatched VCAs with neither conditioning nor HCT acutely rejected their grafts by day 6 (data not shown). VCA transplantation in both 20311 and 20313 was performed while evidence of cutaneous GvHD was still present; the disease did not appear to affect the transplanted tissue, either clinically or on histology.
Figure 3. Established mixed chimeras accepted VCAs from the original HCT donor between 85 and 150 days later without additional immunosuppression.
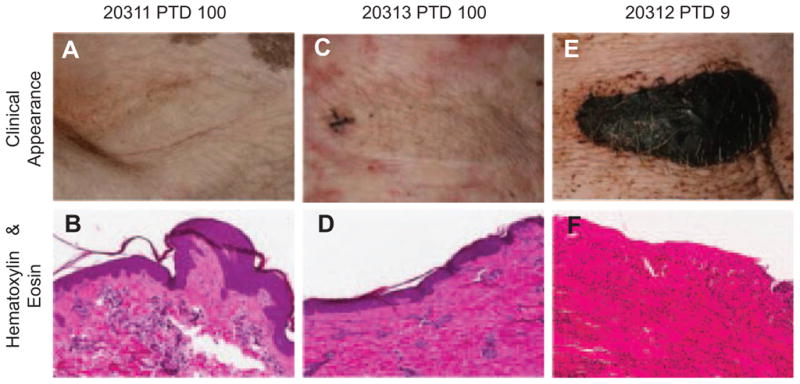
Representative clinical images and hematoxylin/eosin stained sections showing complete absence of signs of rejection at day 100 in (A, B) 20311 and (C, D) 20313. A control animal (20312) undergoing conditioning, but not HCT, prior to VCA 100 days later acutely rejected VCA with 9 days with eschar formation (E) and necrosis (F). Two additional control animals (17519, 17520) underwent VCA alone, without conditioning and rejected by day 6 (data not shown). HCT, hematopoietic cell transplantation; VCA, vascularized composite allograft.
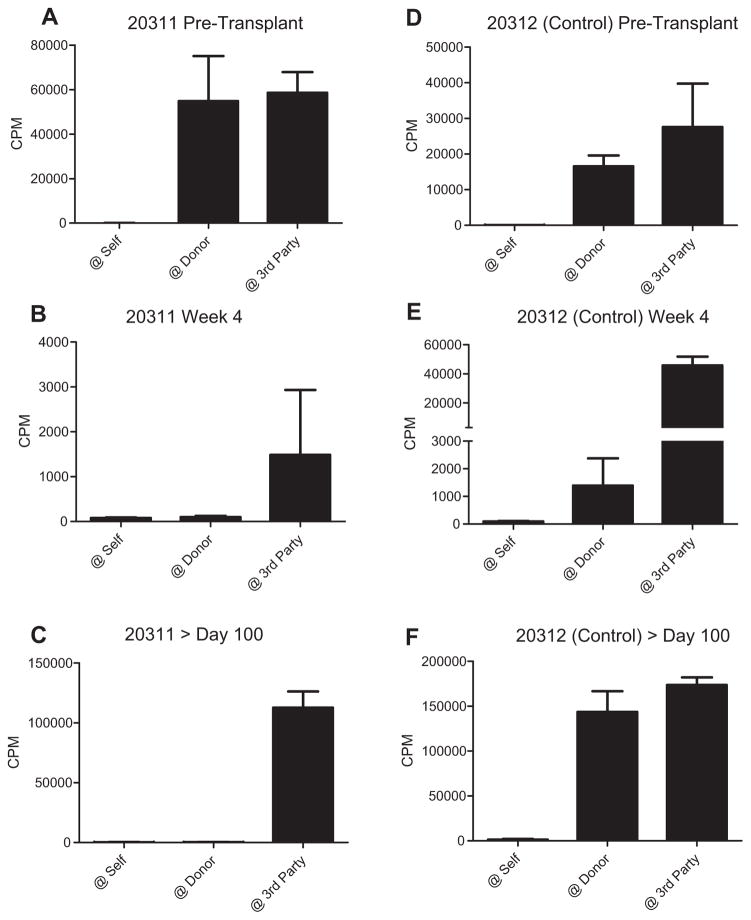
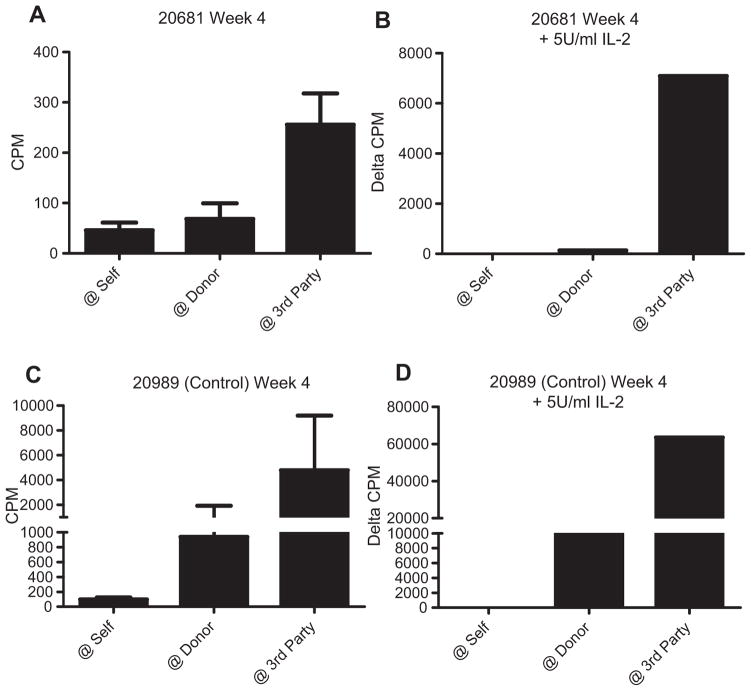
Figure 5. In vitro evidence of donor-specific unresponsiveness in mixed chimeras prior to VCA transplantation.
(A–C) Mixed lymphocyte reactions (MLRs) were performed 2 and 4 weeks post-HCT, and on an approximately 50-day schedule thereafter. Representative data from 20311 are shown (A) pretransplant, demonstrating normal anti-donor responses, (B) at week 4, and (C) >100 days post-HCT demonstrating specific absence of proliferative response to donor antigen. (D–F) Control animal (20312), which underwent conditioning but did not receive HCT, never demonstrated donor-specific unresponsiveness. CPM, counts per minute; HCT, hematopoietic cell transplantation; VCA, vascularized composite allograft.
Permanent acceptance of VCAs placed at the time of HCT
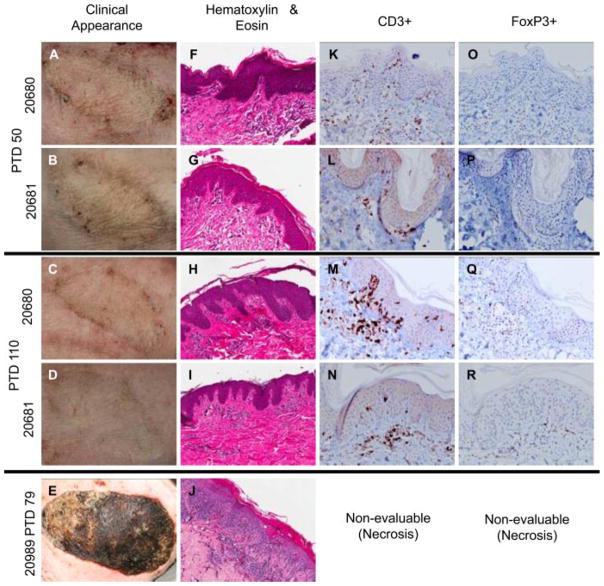
While acceptance of VCAs placed in established mixed chimeras is an impressive display of robust tolerance, the clinical applicability of preestablishing chimerism is limited in hand or face transplantation. Therefore, we performed simultaneous VCA and HCT transplantation in two recipients (20680 and 20681). Both of these animals developed durable multilineage mixed chimerism (Figure 2F and G), and, like established mixed chimeras, accepted VCAs for the duration of follow up (139 and 486 days), with no gross evidence of rejection at any time (Figure 4A–D). Tolerance was tested in animal 20680 by application of donor and third-party (donor-matched and SLAaa) skin grafts. Consistent with observations in 20311, the donor graft survived indefinitely, while third-party grafts rejected on days 42 (donor-matched) and 56 (SLAaa), respectively (Figure S3). In these two animals, histology of day 50 VCA biopsy specimens revealed perivascular inflammatory infiltrates with or without extension into the epidermis, consistent with Banff stages 1 and 2 acute cellular rejection, respectively (Figure 4F and G). However, in both animals, these changes resolved completely by the subsequent biopsy (day 110 post-VCA), and did not recur (Figure 4H and I). In contrast, a control animal (20989) which underwent conditioning and early VCA without HCT never developed donor-specific unresponsiveness in vitro, demonstrated clinical and histological signs of rejection as early as week 2, and experienced complete rejection of VCA by day 79 (Figure 4E and J).
Figure 4. Induction of vascularized composite allograft tolerance achieved with contemporaneous HCT.
(A–D) Recipients of HCT and VCA displayed no clinical signs of VCA rejection at any time point, although Banff stage 1/2 rejection was diagnosed on biopsy at day 50 (F, G), this resolved by the subsequent biopsy following day 100 (H, I) and did not recur. In contrast, signs of rejection could be identified as early as day 14 in a VCA recipient which did not receive HCT (20989), while still under immunosuppression, progressing to complete rejection and necrosis by day 79 (E, J). Immunohistochemical analysis at day 50 revealed the presence of CD3+ infiltrates (K, L), which persisted beyond day 100 (M, N) but which were not associated with histological evidence of tissue damage. (O–R) FoxP3+ cells could be identified within these foci of CD3+ cells at all time points tested. Immunohistochemical staining of the rejected VCA in control animal 20989 was nonevaluable due to necrosis. HCT, hematopoietic cell transplantation; VCA, vascularized composite allograft.
Immunohistochemical staining confirmed the presence of CD3+ infiltrating cells at day 50 (Figure 4K and L). Interestingly, despite the absence of detectable evidence of rejection at subsequent time points, clusters of dermal CD3+ cells persisted (Figure 4M and N). Further analysis of these focal areas of infiltration identified the presence of FoxP3+ cells (Figure 4O–R), suggesting that mechanisms for inducing and/or maintaining VCA tolerance in this model may include a local regulatory component.
At experimental end points, samples from all VCAs were submitted for final histological evaluation. No changes consistent with chronic rejection were identified in any case (Figure S5).
Mixed chimeras demonstrate donor-specific unresponsiveness by in vitro assays early post-HCT
In vitro evidence of donor-specific unresponsiveness was detected early (2 weeks) post-HCT, as demonstrated by absence of a proliferative response to donor antigens in MLR while maintaining responses to third-party antigens. Conditioning uniformly resulted in loss of normal responses (Figure 5A) and global nonresponsiveness at week 1 (data not shown), but HCT recipients progressively regained anti-third-party responses between weeks 2 and 4 (Figure 5B). In contrast, control animals treated with the same conditioning regimen but not undergoing HCT, maintained both anti-donor and anti-third-party responses following recovery from the transient period of global unresponsiveness posttransplant (Figure 5D and E). In chimeras, the lack of anti-donor responses in vitro was maintained long-term following cessation of calcineurin inhibition at the end of the induction regimen, and was observed both as a specific lack of proliferation against donor antigen in MLR (Figure 5C) and absence of donor target cell lysis in CML assays (Figure S4). Similarly, no evidence could be detected for generation of anti-donor antibody at any time post-HCT or VCA (data not shown).
Donor-specific unresponsiveness in vitro is not reversed by depletion of CD25+ cells or addition of IL-2
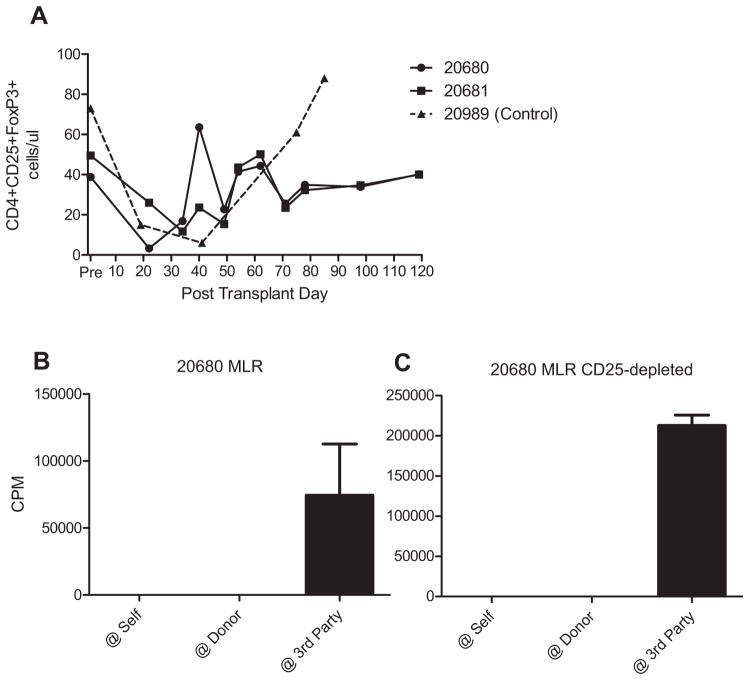
The establishment of stable mixed chimerism and the acceptance of all components of VCAs suggested that multiple mechanisms for induction and maintenance of tolerance might be occurring. To investigate the potential contribution of CD4+CD25+FoxP3+ Tregs in this model, we followed peripheral blood levels of CD4+CD25+FoxP3+ cells by flow cytometry in recipients of HCT and early VCA. No significant increase in levels of FoxP3 expression, either as a percentage of the CD4+ cell population or in absolute cell numbers, was identified during induction of chimerism and tolerance in comparison to pretransplant levels, nor was there a substantial difference in expression between the chimeras and the conditioned control (Figure 6A).
Figure 6. Lack of evidence for systemic regulation by Tregs in maintenance of VCA tolerance.
(A) Absolute numbers of CD4+CD25+FoxP3+ cells in recipient peripheral blood were followed by flow cytometry. No significant difference was detected between systemic Treg levels in chimeric recipients and nonchimeric control. To investigate the functional role of CD25+ cells, these were depleted, by magnetic activated cell sorting, from responder populations prior to setting up MLR. (B, C) Depletion of CD25+ cells failed to restore anti-donor responses in MLR. CPMs to self and donor were all less than 100. Representative data from animal 20680 on POD 178 are shown. CPMs, counts per minute; MLR, mixed lymphocyte reaction assay; Treg, T regulatory cell; VCA, vascularized composite allograft.
While CD4+CD25+FoxP3+cells were not observed to differ substantially in peripheral blood populations following HCT, they may still have been functional to suppress anti-donor responses. To assess suppression of anti-donor responses in vitro, we performed MLRs following depletion of CD25+ cells by MACS. Consistent with findings in the delayed VCA model, recipients of simultaneous HCT and VCA demonstrated specific unresponsiveness to donor in peripheral blood responder populations (Figure 6B). Depletion of CD25+ cells, which effectively removed >98% FoxP3+ cells (data not shown) failed to restore anti-donor responses in MLR (Figure 6C), suggesting that regulation by CD4+CD25+FoxP3+ Tregs, as measured in peripheral blood, does not play a dominant role at the systemic level in this model, at least not at the time points tested.
In addition to regulation, cellular anergy has been implicated in mechanisms of allograft tolerance. Classical anergy is described as a state of cellular unresponsiveness reversible by re-stimulation with antigen in the presence of exogenous IL-2 (37). MLRs with and without addition of 5 U/mL exogenous porcine IL-2 were performed to investigate the potential contribution of anergy. As was observed in animals receiving delayed VCAs, recipients of combined HCT and VCA demonstrated generalized hyporesponsiveness early post–T cell depletion and transplant, with significantly reduced responses to all stimulators, but relative preservation of third-party responses, in MLR when compared to the pretransplant state (Figure 7A). Addition of 5 U/mL porcine IL-2 to MLR increased the overall proliferation to all stimulators, but did not result in restoration of anti-donor responsiveness beyond self. Rather, donor-specific unresponsiveness was confirmed, with relative amplification of the response to third party in comparison to the response to either donor or self (Figure 7B). Similar results were seen following addition of 10 and 20 U/mL as well as at all later time points over 100 days posttransplant (data not shown). In contrast, the conditioned control animal 20989 maintained both anti-donor and anti-third-party responses both with and without IL-2 (Figure 7C and D). This finding suggests that cellular anergy, as defined by IL-2 reversibility in vitro, may not contribute appreciably to early (week 4) induction of VCA tolerance in this model.
Figure 7. Lack of evidence for IL-2 reversible anergy as the dominant mechanism of tolerance following HCT and VCA.
(A) Recipients of HCT and VCA together developed donor-specific unresponsiveness in vitro as early as 2–4 weeks posttransplant. Representative data from 20681 at week 4 are shown. (B) Addition of exogenous porcine IL-2 to MLR failed to restore anti-donor responses. (C, D) The conditioned control animal 20989 maintained anti-donor and anti-third party both with and without IL-2. Addition of IL-2 promotes a general increase in proliferation in MLR; therefore, proliferation in IL-2 assays is expressed as the delta-counts per minute (CPM) in comparison to the anti-self response. HCT, hematopoietic cell transplantation; MLR, mixed lymphocyte reaction assay; VCA, vascularized composite allograft.
Discussion
We believe this report to be the first for induction of tolerance of all components of VCAs, including skin, and durable mixed chimerism following nonmyeloablative conditioning and HCT across MHC barriers in a large animal model. Furthermore, demonstrating that tolerance can be induced by establishment of mixed chimerism simultaneous to VCA is an important step toward a clinically applicable protocol.
The potent immunogenicity of skin has been recognized since the early experiments of Medawar and Gibson (38,39), and was subsequently defined in comparison to other tissues and organs by Murray (15) and others (40). More recently in VCA transplantation experiments, rejection of skin or epidermis alone has been a common result (19), even in MHC-matched combinations (18).
Advances in nonmyeloablative conditioning and HCT have made establishment of mixed chimerism across MHC barriers a reality in a large animal, porcine model (21). GvHD is a well-recognized risk of HCT, but would clearly be unacceptable in the context of a clinical tolerance protocol for a non-life-sustaining transplant. The development of GvHD by two animals in this study was associated with very high levels of chimerism and stands in marked contrast to the remaining animals in this study, and nine animals previously transplanted on this protocol in which only one case of transient, stage 1 GvHD was observed (20). Studies investigating factors controlling GvHD in this model are ongoing.
Studies in murine models have delineated the mechanisms responsible for induction and maintenance of donor-specific unresponsiveness following HCT. These studies have suggested a predominant role for central deletion of alloreactive T cells in maintenance of tolerance in engrafted mixed chimeras, with other mechanisms demonstrated to play a role in the induction phase (41,42). The indefinite acceptance of VCAs placed after establishment of stable mixed chimerism in the absence of any additional necessary immunosuppression demonstrates that robust tolerance can be established to nonhematopoietic antigens found in the VCA. In an attempt to assess the mechanisms involved in this porcine model, we have found no evidence that T cell anergy, as defined by cellular unresponsiveness reversible by exogenous IL-2, or regulation by CD4+CD25+ Tregs played a predominant role in maintenance of tolerance at a systemic level. While these represent preliminary inquiries, more detailed work in isolating and analyzing the response of specific subsets of leukocytes is currently under way, as is development of approaches to assess deletion of donor-reactive T cells comparable to models used in rodent systems. These findings are not only of significance for the development of clinically applicable strategies for induction of VCA tolerance, but also offer insights into the mechanisms contributing to VCA tolerance in a relevant preclinical model.
While assays of peripheral blood leukocyte function provide insight into the systemic immune status of transplant recipients, it is likely that local factors, operational within the VCA itself, may contribute to its fate. The presence of focal areas of CD3+ cell infiltration within tolerant VCAs in this study, in the absence of either clinical or histological evidence of rejection, is consistent with recent descriptions of an extensive network of memory CD3+ cells in normal murine and human skin (43,44). These findings suggest that the difficulty of inducing tolerance of transplanted skin may reflect lack of appropriate signaling between donor and recipient skin immune system elements, rather than due to the recognition of skin-specific antigens. This would be consistent with the recent demonstration that Langerhans cells contribute to maintenance of skin immune homeostasis by activation of resident Tregs (45). The identification of FoxP3+ cells within tolerant VCAs is consistent with previous findings in MHC-matched canine chimeras, and with observations in biopsy specimens from hand transplant recipients (16,46). The presence of these cells may suggest contribution from a local regulatory mechanism to VCA tolerance, occurring within the graft itself, but not evident at a systemic level as assayed in peripheral blood. However, such local regulation has not been demonstrated and further experiments will investigate the function of putative Tregs isolated from VCAs. In our model of HCT and VCA transplantation between haploidentical individuals, donor and recipient share one MHC haplotype for both Class I and Class II. It is conceivable that the mechanism of tolerance in this model may involve haplotype sharing, and therefore may not translate across full MHC barriers. Future experiments will evaluate this protocol in the context of fully MHC disparate donor–recipient pairs.
In summary, we have demonstrated that tolerance of VCAs may be achieved in a large animal model by contemporaneous establishment of multilineage mixed chimerism. Further, we have demonstrated no evidence for either T cell anergy or regulation by Tregs as the predominant mechanism of systemic tolerance in this model at the time points tested. However, the persistence of CD3+FoxP3+ cells in tolerant VCAs may indicate that regulation at a local level within the transplanted tissue plays a role. Further work will be required to define more precisely the systemic mechanisms operational during induction of VCA tolerance, to investigate the role of the skin immune system in skin tolerance and rejection, and to progress from this experimental model toward protocols ready for translation to clinical trials.
Supplementary Material
Figure S1: Detection of chimerism in bone marrow and thymus of mixed chimeras. (A) Representative flow cytometry data demonstrating presence of donor-type (PAA positive) cells in recipient bone marrow greater than 100 days posttransplant. (B) Chimerism was also detected in thymic tissue, where donor-type cells were found to contribute to both single-positive and double-positive thymocyte populations. Representative data, animal 20680.
Figure S2: VCA sparing in cutaneous GvHD. (A) Animal 20313, VCA and surrounding skin 50 days post-VCA transplant. The GvHD skin rash was observed to demarcate clearly at the VCA border. (B) Histological assessment of host skin confirmed this diagnosis, while (C) no signs consistent with rejection or GvHD were identified on VCA biopsy.
Figure S3: Recipients tolerant of VCA accept donor split thickness skin grafts indefinitely and reject third-party skin grafts. (A) 20311 donor graft POD 102. (B) 20311 donor-matched graft POD 24. (C) 20311 single haplotype disparate graft POD 24. (D) 20680 donor graft POD 135. (E) 20680 donor-matched graft POD 42. (F) 20680 single haplotype SLAaa graft POD 56. In both animals, donor skin grafts were placed over 150 days after VCA, third-party grafts were placed after interval of at least 80 days.
Figure S4: Mixed chimeras demonstrate specific unresponsiveness to donor in CML. (A) CML data from animal 20311 demonstrating normal anti-donor and anti-third-party responses prior to conditioning and transplant. (B) Comparable lytic activity was observed prior to conditioning in control animal 20312 which underwent conditioning without HCT and subsequently received a VCA greater than 100 days later. (C) Following HCT chimeric animals demonstrated specific unresponsiveness to donor antigens in CML. Representative data from 20311 over 100 days posttransplant are shown. (D) In contrast, in the absence of HCT, anti-donor responses are maintained. Data shown for conditioned control animal 20312 greater than 100 days postconditioning. Percentage-specific lysis against self targets <5% in all assays.
Figure S5: Lack of evidence for chronic pathological changes in VCAs accepted long term. All VCAs were monitored regularly for histological evidence of acute or chronic rejection throughout experimental follow up. No changes consistent with chronic rejection were identified in any specimen. Representative images from terminal specimens are presented. (A) Dilated apocrine unit without rejection, 20311, 504 days posttransplant (×20). (B) 20313, 115 days posttransplant. (C) Artery with no evidence of allograft vasculopathy, 20680, 486 days posttransplant (×20). (D) 20681, 139 days posttransplant (×15).
Acknowledgments
The authors wish to thank Jim Winter, Betsy Neylon and Taylor Glor for anesthesia and operating room support; Edward Harrington, Ashley Gusha and Eric Batista for expert animal care and laboratory technical assistance; Nicole Brousaides for histology assistance; Dr. Robert Hawley, Dr. Isabel Hanekamp and Dr. Larry Turka for internal review of the manuscript and Rebecca A. Wark for administrative support. We acknowledge support from CO6RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine. Experimental funding was provided by NIH NCI P01CA111519, NIH NIAID RO1A1084657, the Musculoskeletal Transplant Foundation and Melina Nakos Foundation. DAL received funding from an AST-Genentech Basic Science Research Fellowship Award.
Abbreviations
- CML
cell-mediated lymphocytotoxicity
- CMV
cytomegalovirus
- GvHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- MACS
magnetic activated cell sorting
- MGH
Massachusetts General Hospital
- MLR
mixed lymphocyte reaction
- PAA
pig allelic antigen
- pIL-3
porcine interleukin-3
- pSCF
porcine stem cell factor
- SLA
swine leukocyte antigen
- Treg
T regulatory cell
- VCA
vascularized composite allograft
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Petruzzo P, Lanzetta M, Dubernard JM, et al. The international registry on hand and composite tissue transplantation. Transplantation. 2010;90:1590–1594. doi: 10.1097/TP.0b013e3181ff1472. [DOI] [PubMed] [Google Scholar]
- 2.Fattah A, Cypel T, Donner EJ, Wang F, Alman BA, Zuker RM. The first successful lower extremity transplantation: 6-Year follow-up and implications for cortical plasticity. Am J Transplant. 2011;11:2762–2767. doi: 10.1111/j.1600-6143.2011.03782.x. [DOI] [PubMed] [Google Scholar]
- 3.Strome M, Stein J, Esclamado R, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344:1676–1679. doi: 10.1056/NEJM200105313442204. [DOI] [PubMed] [Google Scholar]
- 4.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173–2176. doi: 10.1016/S0140-6736(03)13769-5. [DOI] [PubMed] [Google Scholar]
- 5.Petruzzo P, Dubernard JM. The international registry on hand and composite tissue allotransplantation. Clin Transpl. 2011:247–253. [PubMed] [Google Scholar]
- 6.Morris P, Bradley A, Doyal L, et al. Face transplantation: A review of the technical, immunological, psychological and clinical issues with recommendations for good practice. Transplantation. 2007;83:109–128. doi: 10.1097/01.tp.0000254201.89012.ae. [DOI] [PubMed] [Google Scholar]
- 7.Petruzzo P, Kanitakis J, Badet L, et al. Long-term follow-up in composite tissue allotransplantation: In-depth study of five (hand and face) recipients. Am J Transplant. 2011;11:808–816. doi: 10.1111/j.1600-6143.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman CL, Ouseph R, Blair B, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12:1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 9.Gordon CR, Avery RK, Abouhassan W, Siemionow M. Cytomegalovirus and other infectious issues related to face transplantation: Specific considerations, lessons learned, and future recommendations. Plast Reconstr Surg. 2011;127:1515–1523. doi: 10.1097/PRS.0b013e318208d03c. [DOI] [PubMed] [Google Scholar]
- 10.Sachs DH. Tolerance: Of mice and men. J Clin Invest. 2003;111:1819–1821. doi: 10.1172/JCI18926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 12.Horner BM, Cina RA, Wikiel KJ, et al. Predictors of organ allograft tolerance following hematopoietic cell transplantation. Am J Transplant. 2006;6:2894–2902. doi: 10.1111/j.1600-6143.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- 13.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray JE. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast Reconstr Surg. 1971;47:425–431. doi: 10.1097/00006534-197105000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Mathes DW, Hwang B, Graves SS, et al. Tolerance to vascularized composite allografts in canine mixed hematopoietic chimeras. Transplantation. 2011;92:1301–1308. doi: 10.1097/TP.0b013e318237d6d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo YR, Goto S, Shih HS, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769–1777. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 18.Mathes DW, Randolph MA, Solari MG, et al. Split tolerance to a composite tissue allograft in a swine model. Transplantation. 2003;75:25–31. doi: 10.1097/00007890-200301150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514–521. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 20.Cina RA, Wikiel KJ, Lee PW, et al. Stable multilineage chimerism without graft versus host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation. 2006;81:1677–1685. doi: 10.1097/01.tp.0000226061.59196.84. [DOI] [PubMed] [Google Scholar]
- 21.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 23.Lunney JK, Sachs DH. Transplantation in miniature swine. V. Characterization of Ia antigens. J Immunol. 1979;122:623–627. [PubMed] [Google Scholar]
- 24.Fuchimoto Y, Huang CA, Shimizu A, Seebach J, Arn JS, Sachs DH. An allelic non-histocompatibility antigen with wide tissue distribution as a marker for chimerism in pigs. Tissue Antigens. 1999;54:43–52. doi: 10.1034/j.1399-0039.1999.540105.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang CA, Yamada K, Murphy MC, et al. In vivo T cell depletion in miniature swine using the swine CD3 immunotoxin, pCD3-CRM9. Transplantation. 1999;68:855–860. doi: 10.1097/00007890-199909270-00019. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Duran-Struuck R, Crepeau R, et al. Development of a diphtheria toxin based antiporcine CD3 recombinant immunotoxin. Bioconjug Chem. 2011;22:2014–2020. doi: 10.1021/bc200230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horner BM, Randolph MA, Duran-Struuck R, et al. Induction of tolerance to an allogeneic skin flap transplant in a preclinical large animal model. Transplant Proc. 2009;41:539–541. doi: 10.1016/j.transproceed.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- 29.Huang CA, Lorf T, Arn JS, Koo GC, Blake T, Sachs DH. Characterization of a monoclonal anti-porcine CD3 antibody. Xenotransplant. 1999;5:201–212. doi: 10.1034/j.1399-3089.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 30.Jonjic S, Koszinowski UH. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J Immunol. 1984;133:647–652. [PubMed] [Google Scholar]
- 31.Halloran PJ, Sweeney SE, Strohmeier CM, Kim YB. Molecular cloning and identification of the porcine cytolytic trigger molecule G7 as a Fc gamma RIII alpha (CD16) homologue. J Immunol. 1994;153:2631–2641. [PubMed] [Google Scholar]
- 32.Denham S, Shimizu M, Bianchi AT, Zwart RJ, Carr MM, Parkhouse RM. Monoclonal antibodies recognising differentiation antigens on porcine B cells. Vet Immunol Immunopathol. 1994;43:259–267. doi: 10.1016/0165-2427(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 33.Lima B, Gleit ZL, Cameron AM, et al. Engraftment of quiescent progenitors and conversion to full chimerism following non-myelosuppressive conditioning and hematopoietic cell transplantation in miniature swine. Biol Blood Marrow Transplant. 2003;9:571–582. doi: 10.1016/s1083-8791(03)00227-1. [DOI] [PubMed] [Google Scholar]
- 34.Thistlethwaite JR, Jr, Auchincloss H, Jr, Pescovitz MD, Sachs DH. Immunologic characterization of MHC recombinant swine: Role of Class I and II antigens in in vitro immune responses. J Immunogenet. 1984;11:9–19. doi: 10.1111/j.1744-313x.1984.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 35.Leight GS, Sachs DH, Rosenberg SA. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977;23:271–276. [PubMed] [Google Scholar]
- 36.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson T, Medawar PB. The fate of skin homografts in man. J Anat. 1943;77:299–310. [PMC free article] [PubMed] [Google Scholar]
- 39.Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- 40.Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401–411. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Wekerle T, Blaha P, Koporc Z, Bigenzahn S, Pusch M, Muehlbacher F. Mechanisms of tolerance induction through the transplantation of donor hematopoietic stem cells: Central versus peripheral tolerance. Transplantation. 2003;75:21S–25S. doi: 10.1097/01.TP.0000067947.90241.66. [DOI] [PubMed] [Google Scholar]
- 42.Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23:165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T (RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eljaafari A, Badet L, Kanitakis J, et al. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttrans-plantation. Transplantation. 2006;82:1764–1768. doi: 10.1097/01.tp.0000250937.46187.ca. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Detection of chimerism in bone marrow and thymus of mixed chimeras. (A) Representative flow cytometry data demonstrating presence of donor-type (PAA positive) cells in recipient bone marrow greater than 100 days posttransplant. (B) Chimerism was also detected in thymic tissue, where donor-type cells were found to contribute to both single-positive and double-positive thymocyte populations. Representative data, animal 20680.
Figure S2: VCA sparing in cutaneous GvHD. (A) Animal 20313, VCA and surrounding skin 50 days post-VCA transplant. The GvHD skin rash was observed to demarcate clearly at the VCA border. (B) Histological assessment of host skin confirmed this diagnosis, while (C) no signs consistent with rejection or GvHD were identified on VCA biopsy.
Figure S3: Recipients tolerant of VCA accept donor split thickness skin grafts indefinitely and reject third-party skin grafts. (A) 20311 donor graft POD 102. (B) 20311 donor-matched graft POD 24. (C) 20311 single haplotype disparate graft POD 24. (D) 20680 donor graft POD 135. (E) 20680 donor-matched graft POD 42. (F) 20680 single haplotype SLAaa graft POD 56. In both animals, donor skin grafts were placed over 150 days after VCA, third-party grafts were placed after interval of at least 80 days.
Figure S4: Mixed chimeras demonstrate specific unresponsiveness to donor in CML. (A) CML data from animal 20311 demonstrating normal anti-donor and anti-third-party responses prior to conditioning and transplant. (B) Comparable lytic activity was observed prior to conditioning in control animal 20312 which underwent conditioning without HCT and subsequently received a VCA greater than 100 days later. (C) Following HCT chimeric animals demonstrated specific unresponsiveness to donor antigens in CML. Representative data from 20311 over 100 days posttransplant are shown. (D) In contrast, in the absence of HCT, anti-donor responses are maintained. Data shown for conditioned control animal 20312 greater than 100 days postconditioning. Percentage-specific lysis against self targets <5% in all assays.
Figure S5: Lack of evidence for chronic pathological changes in VCAs accepted long term. All VCAs were monitored regularly for histological evidence of acute or chronic rejection throughout experimental follow up. No changes consistent with chronic rejection were identified in any specimen. Representative images from terminal specimens are presented. (A) Dilated apocrine unit without rejection, 20311, 504 days posttransplant (×20). (B) 20313, 115 days posttransplant. (C) Artery with no evidence of allograft vasculopathy, 20680, 486 days posttransplant (×20). (D) 20681, 139 days posttransplant (×15).