Abstract
Background
Stress is a key precipitant to discontinuing naltrexone and relapsing to opiate abuse. Alpha-2 adrenergic agonists like guanfacine may reduce stress induced craving and have reduced opiate relapse in small clinical trials.
Methods
This randomized, double blind double dummy placebo-controlled 6-month trial tested oral naltrexone with or without guanfacine for reducing stress and preventing opiate relapse. We randomized 301 patients to: naltrexone 50 mg/day + guanfacine 1 mg/day (n = 75) (N/G), naltrexone + guanfacine placebo (N/P) (n = 76), naltrexone placebo + guanfacine (n = 75) (P/G), and double placebo (n = 75) (P/P).
Results
Among the 75 patients in each group the percentage still retained on naltrexone treatment at six months was: N/G 26.7%, N/P 19.7% (p = 0.258 to N/G), P/G 6.7% (p < 0.05 to both N groups), and P/P 10.7% (p = 0.013 to N + G). Guanfacine reduced the severity of stress particularly at weeks 10 and 18. Adverse events (AE) were infrequent (4.7%) without group differences, with most common AEs: headache, poor appetite, insomnia, and dizziness.
Conclusions
Adding guanfacine to naltrexone did not improve treatment retention or opiate free urines, but it reduced both stress and craving at later time points in treatment, which may be related to stress-induced craving and the animal model of incubation of reinstatement. During treatment, HIV risk, anxiety, and depression reduced among all patients in treatment, regardless of group.
Keywords: Naltrexone, Guanfacine, Opiate dependence, Stress, Clinical trial
1. Introduction
Naltrexone blocks the euphoric effects of opioids by competitive antagonism at the μ-opioid receptor (Kleber, 2007). It is a non-addictive alternative to agonist maintenance treatments such as methadone and buprenorphine. However, the poor compliance and high drop-out rates make naltrexone relatively poorly effective in heroin dependence management. Reasons for compliance problems are that opioid-dependent subjects experience stress and protracted withdrawal symptoms in early recovery, which may increase craving and relapse susceptibility (Hyman et al., 2009). Another possible contributor to the poor adherence associated with this form of treatment is that opioid abusers are characterized by impulsive behavior, which may also increase drug craving and provoke relapse. Since naltrexone does not influence protracted withdrawal and stress in opioid-dependent patients, adding an additional treatment to reduce these two factors may improve treatment outcomes.
Centrally acting alpha-2 adrenergic agonists such as clonidine, lofexidine and guanfacine reduce symptoms of opiate withdrawal (Lobmaier et al., 2010). These medications reduce noradrenergic neuronal firing and noradrenaline turnover, which might work not only for acute opiate withdrawal but also for protracted withdrawal. However, use of clonidine is associated with significant adverse events such as hypotension (Gish et al., 2010). Several studies have demonstrated the lofexidine and guanfacine have greater selectivity for alpha-2 receptors and have fewer side effects compared to clonidine (Gish et al., 2010; Bukstein and Head, 2012). Moreover guanfacine has been shown to improve working memory in healthy volunteers (Sofuoglu et al., 2013) and sustained attention in adolescents with ADHD (Bukstein and Head, 2012). This action might help to reduce impulsivity, which is a common reason for relapse in opiate-dependent subjects.
Previous studies have shown that stress plays a key role in drug craving and addiction relapse, and several classes of agents including benzodiazepines, serotonin reuptake inhibitors and alpha-2 adrenergic agonists may reduce this stress (Hyman et al., 2007). Hyman et al. have demonstrated that opioid dependent patients experience higher levels of stress than normal healthy control subjects and have maladaptive responses to stress (Hyman et al., 2009). Benzodiazepines are one potential agent to combine with naltrexone in order to address this stress, but their significant rate of dependence in this population and the exacerbation of opiate withdrawal in benzodiazepine dependent individuals have discouraged such studies (De Wet et al., 2004; SAMHSA, 2011). We had previously examined the serotonin reuptake inhibitor fluoxetine with naltrexone and shown that it had no efficacy beyond the naltrexone in reducing relapse to opiate abuse (Krupitsky et al., 2006). Sinha conducted one of the first human studies to assess the alpha-2 adrenergic receptor agonist lofexidine as an anti-stress and relapse prevention treatment. Her 18 opioid-dependent patients were treated with oral naltrexone and lofexidine for 4 weeks and found higher opioid abstinence rates and reduced stress-related craving among naltrexone-lofexidine patients compared to placebo (Sinha et al., 2007). Despite the small sample size, this finding provided support for future studies of alpha-2 adrenoreceptor agonists for stress protection and prevention of relapse. This current study is a randomized, double blind, double dummy, placebo-controlled randomized clinical trial to follow up these preliminary findings and evaluate the efficacy of oral naltrexone with or without guanfacine for preventing relapse to opiate addiction and reducing perceived stress as a precipitant of opiate use, HIV risk behaviors, and psychiatric symptoms.
2. Materials and methods
2.1. Study design
This was a double-blind, double-dummy, placebo-controlled trial in which patients were randomized to four groups: naltrexone 50 mg/day and guanfacine 1 mg/day (N/G); naltrexone 50 mg/day and guanfacine placebo (N/P); naltrexone placebo and guanfacine 1 mg/day (P/G); and naltrexone placebo and guanfacine placebo (P/P). The study lasted 24 weeks on medications.
2.2. Participants
Males and females were eligible if they had: a primary diagnosis of opioid dependence according to SCID interview and DSM-IV that had been present for at least a year; education at the high school level or above; abstinence from heroin and other substances of abuse including alcohol for at least one week; a negative urine opiate drug screen and alcohol breath test; at least one relative willing to participate in treatment and monitor medication adherence and assist in follow-up; a stable address within the St. Petersburg/Leningrad Region; a home telephone number at which he/she could be reached; if female, a negative pregnancy test and willingness to use adequate contraception; demonstrated ability to give informed consent; no regular use of psychotropic medication; and 18–50 years of age (American Psychiatric Association, 2000).
Patients were excluded if they had clinically significant cognitive impairment; schizophrenia; major depression, bipolar or seizure disorder; advanced neurological, cardiovascular, renal, or hepatic disease; active tuberculosis or current febrile illness; a significant laboratory abnormality such as severe anemia, unstable diabetes, or liver function tests > 3× above normal; legal charges with impending incarceration; current participation in another treatment study; or concurrent treatment in another substance abuse program.
2.3. Treatment sites and recruitment
The study was done in the outpatient unit at the Laboratory of Clinical Pharmacology of Addictions, St. Petersburg Pavlov State Medical University, and in the outpatient research unit of the Leningrad Regional Addiction Hospital. Each of these two units participated in the earlier naltrexone studies (Krupitsky et al., 2004, 2006, 2010, 2011, 2012). The Leningrad Regional Addiction Hospital is located just outside St. Petersburg and serves the Leningrad Region; the Laboratory of Clinical Pharmacology of Addictions is located in St. Petersburg and recruited patients from St.-Petersburg City Addiction Hospital, which serves the city of St.-Petersburg. Research assistants at each site reviewed medical records while patients were on inpatient units, explained the study to those who appeared to meet admission criteria, and obtained informed consent. Patients who consented were instructed to report to one of the two outpatient research units on the day of hospital discharge where they were given additional evaluations to confirm eligibility. About 80% of participants were referred from inpatient programs at these two sites. District psychiatrists who detoxified patients at other inpatient or outpatient sites and knew about the study referred the others. For these patients, informed consent and evaluations to confirm eligibility were obtained at the outpatient study units. Those found not eligible were referred to the usual community based treatments.
2.4. Medications
Zambon Group (Italy) provided naltrexone and EGIS (Hungary) provided guanfacine. Pharmacy staff at Pavlov State Medical University prepared naltrexone and naltrexone placebo in identically appearing capsules containing a 50 mg riboflavin marker; guanfacine (1 mg) and guanfacine placebo were similarly prepared but without riboflavin.
2.5. Induction on study medications
After successfully completing screening and baseline assessments, urine was checked for opioids prior to naltrexone induction and patients were given a naloxone challenge (0.8 mg, I.M.) and observed for 2 h to check for signs and symptoms of withdrawal. Patients who experienced withdrawal after the naloxone challenge were asked to refrain from heroin use and return in 1–2 days to have the procedure repeated. Failure to pass induction on three occasions disqualified a patient from participation.
Patients were initially given a one-month supply of study medication so it would be available if they were unable to keep the next appointment. At each subsequent biweekly visit patients were given a two-week supply of medication, which was continued for six months if the patient remained in treatment.
2.6. Drug counseling
Individual drug counseling was provided bi-weekly for six months, as in the prior studies. More frequent counseling was not practical since most patients had to travel 1–2 h or more on public transportation for appointments. Therapists were trained in drug counseling prior to beginning the study according to a manual written for the NIDA cocaine/psychotherapy study (Mercer and Woody, 1998) that was modified for opioid dependence and translated into Russian. The modifications involved a de-emphasis on participation in self-help groups because they are not widely used in Russia, an emphasis on medication adherence, and helping the patient deal with persistent opioid withdrawal symptoms. Therapists were experienced master’s level psychologists or psychiatrists trained in the treatment of substance use disorders in Russia and supervised biweekly by Dr. Krupitsky. No effort was made to tape record therapy sessions or rate adherence to the manual; it was simply used as a treatment guide. Patients received basic HIV risk reduction information in the course of treatment prior to beginning the study including how the virus is transmitted and how to prevent infection by using sterile injection equipment, not sharing injection equipment, and using condoms.
2.7. Assessment of primary and secondary outcomes
Patients were counted as early terminators if they missed more than two consecutive appointments (did not show for one month), and considered to have relapsed if they reported systematic everyday heroin use, or had three consecutive opioid positive urine tests, or signs and symptoms of withdrawal. Patients who reported occasional (few times) heroin use, had less than three consecutive opioid positive urines and did not have signs and symptoms of withdrawal were not considered to have had a relapse. Parents or close relatives who were living with the patients sometimes came to appointments and were familiar with the abstinence/relapse status and thus able to provide additional information. Parents also were contacted by phone to obtain information on patients who were early terminators. For the 9 and 12-month follow-up we contacted the patient and family member for self-reported return to daily illicit opiate use, but did not obtain urine toxicology results. HIV risk, psychiatric symptoms and overall outcome were measured using standardized self-report, laboratory, and interviewer-administered measures; Pavlov staff trained clinical and research staff in study procedures prior to beginning the research.
2.8. Measures
Routine blood tests including a complete blood count, electrolytes, ALT/AST and urinalysis were done as part of the usual treatment patients received prior to entering the study and were available to research staff for assessing overall health. Baseline assessments added for the study included a drug use history and physical examination to confirm opioid dependence, urine testing, alcohol breath tests, Addiction Severity Index (ASI; McLellan et al., 1985), the Risk Assessment Battery (RAB; Navaline et al., 1994), Time Line Follow-Back (TLFB) for alcohol and other drugs (Sobell and Sobell, 1992), pregnancy test, a Visual Analog Scale of craving for heroin (VASC), Global Assessment of Function (GAF); DSM-IV (2000), Beck Depression Inventory (BDI; Beck et al., 1961), Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962), Spielberger State-Trait Anxiety Test (SSTAT; Spielberger et al., 1976), Perceived Stress Scale (PSS; Cohen et al., 1983), and a naloxone challenge. In the course of doing follow-up assessments we did not attempt to measure craving, depression, anxiety, and stress among subjects who were known to have relapsed since these symptoms are unstable in the context of active drug use. Biweekly follow-up assessments were urine and breathalyzer tests, the brief TLFB, the VASC, BDI, SSTAT, and PSS; the RAB and GAF were done at 3 and 6 months; the ASI was repeated at 6 months.
Naltrexone adherence was assessed in three ways: a count of remaining capsules at each appointment; visual inspection for the presence of riboflavin in the urine using ultraviolet (UV) light at the long wave setting (444 nm) in a room with low ambient light (O’Malley et al., 1992); and involvement of a significant other in treatment who was asked to supervise and report on medication adherence and relapse status at each study visit, either in person or by telephone. Patients were informed of the urine color change in the consent and told that it was harmless. The reimbursement for patients’ transportation and time (ruble equivalent of $10–20, depending on transportation cost) for each assessment was given to the patients at each visit.
2.9. Randomization and blinding
A biostatistician generated the allocation sequence and randomization was done using an SPSS-based software program. Medications were placed in numbered containers by pharmacy staff and transported to the two outpatient study sites where research assistants enrolled participants. Medications were dispensed double blind, double dummy at each bi-weekly counseling session and made available for six months. Research assistants, treating physicians, all other staff involved in the project and participants were blinded as to group assignment. Formal procedures were not done to assess the success of blinding. A master code was kept at Pavlov so the blind could be broken in case of emergency (which never happened).
2.10. Statistical methods
Data were double entered and checked for errors and analyzed using the Statistical Package for the Social Sciences (SPSS. Ver. 17). Survival analysis (Kaplan–Meier Survival Functions with Log Rank Mantel–Cox criteria for group comparison) was used to determine the primary outcome of retention defined as not missing two consecutive counseling sessions and not having relapsed (relapse defined as 3 consecutive opiate positive urines and/or self-reported return to using opiates daily). The proportion of non-survivors attributable to proven relapse was also determined. Secondary outcomes reported here are the cumulative percentages of opiate negative urines during the 24-week medication phase. Fisher exact test and calculation of Odds ratios with 95% confidence intervals were used for analysis of between group percentage differences.
With the substantial loss of subjects during this 6-month clinical trial, an intent to treat analysis was only possible for the relapse/treatment retention variable. The other variables such as stress over time involved too many missing values beyond the first few weeks of the study to perform an intent to treat analysis. Instead we examined the available subjects at each of four time points (weeks 6, 12, 18, and 24) for differences in these other measures across the four groups. Stress reactivity and other psychometrics (also considered as secondary outcomes) were analyzed using ANOVA repeated measures with the Tukey test for between groups’ post hoc comparisons. Safety assessments included Adverse Events using Fisher exact tests with Monte-Carlo modeling for more than 2 groups and liver enzyme results at 12 and 24 weeks.
The sample size provided 80% power to detect a difference of 20% or greater between the groups for the primary outcome assuming an alpha of 0.025 (2 contrasts) and a retention rate of approximately 60% in the N + G group, which meant that our statistical power for these other secondary outcomes was only sufficient to about the 6 week outcome, the median retention time for the four groups. For two-group comparisons such as between guanfacine vs. placebo (regardless of getting naltrexone or placebo naltrexone) the sample sizes were larger, and the power was 0.8 or greater even with sample retention to as little as 20% overall, which was the overall retention at week 18.
2.11. Human subjects
Approval for the study was obtained from the Human Subjects Committees at St. Petersburg Pavlov State Medical University and the Baylor College of Medicine. The consent form was written via a collaborative process in English and translated into Russian. A Russian-speaking faculty member at Baylor checked the Russian version for adherence to the English version as part of the Baylor human subjects’ approval. Written informed consent was obtained from each patient before enrollment. The consent and all evaluations were in Russian.
3. Results
3.1. Recruitment, demographic and clinical features
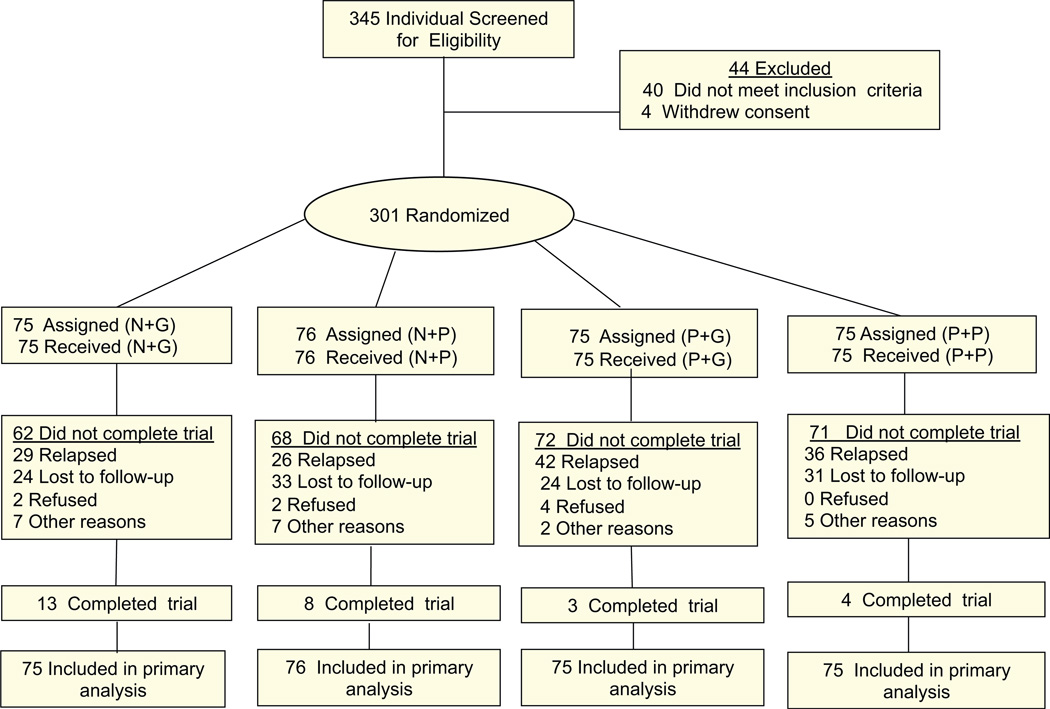
Patients were recruited over four years. 345 patients were checked for eligibility, 40 of them did not meet inclusion criteria and 4 withdrew consent. 301 patients gave informed consent and were randomized to one of the four groups (Fig. 1).
Fig. 1.
Study patient flow diagram showing subject retention and reasons for dropout over 24 week study, where “end of study” refers to week 24.
All patients were dependent on intravenous heroin; prescription opioids are highly restricted, expensive, and difficult to obtain in Russia. Patients’ mean (SD) age was 28.3 (4.4), most (N = 248; 82.4%) were male, average years dependent was 8.2 (4.3), and average number of previous treatments was 4.2 (4.0). Among 301 study patients, the baseline assessment showed that 146 (49.3%) were HIV positive; 285 (95.0%) positive for hepatitis C; and 68 (23.1%) for hepatitis B. Past 30-day self-reported substance use at baseline showed that 70 (23.3%) used marijuana; 31(10.3%) amphetamines; 32 (10.7%) sedatives, mostly benzodiazepines; and none reported using cocaine. Average alcohol use was 10.1 (19.3) grams/day. There were no significant baseline differences between groups in demographics or clinical variables (Table 1).
Table 1.
Baseline demographics and clinical characteristics.
| Medication group | N + G | N + P | P + G | P + P | All |
|---|---|---|---|---|---|
| No. patients | 75 | 76 | 75 | 75 | 301 |
| Age (years) (M ± SE) | 28.0 ± 0.52 | 28.5 ± 0.51 | 28.2 ± 0.52 | 28.5 ± 0.53 | 28.3 ± 0.26 |
| Sex | |||||
| Male n (%) | 63 (84.0%) | 64 (84.2%) | 61 (81.3%) | 60 (80.0%) | 248 (82.4%) |
| Female n (%) | 12 (16.0%) | 12 (15.8%) | 14 (18.7%) | 15 (20.0%) | 53 (17.6%) |
| Duration of heroin abuse (years) (M ± SE) | 7.7 ± 0.45 | 8.4 ± 0.49 | 8.3 ± 0.58 | 8.7 ± 0.55 | 8.3 ± 0.26 |
| Craving (sm) (M ± SE) | 3.3 ± 0.33 | 3.4 ± 0.31 | 3.1 ± 0.31 | 3.5 ± 0.31 | 3.3 ± 0.16 |
| Average daily dose of heroin (mg) (M ± SE) | 0.97 ± 0.103 | 1.11 ± 0.110 | 0.87 ± 0.067 | 0.94 ± 0.106 | 0.98 ± 0.049 |
| Use of amphetamines N (%) | 9 (12.0%) | 6 (7.9%) | 7 (9.5%) | 9 (12.0%) | 31 (10.3%) |
| Use of cocaine N (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Use marijuana N (%) | 13 (17.3%) | 15 (19.7%) | 17 (23.0%) | 25 (33.3%) | 70 (23.3%) |
| Use of sedatives (benzos) N (%) | 9 (12.0%) | 6 (7.9%) | 6 (8.1%) | 11 (14.7%) | 32 (10.7%) |
| Use of alcohol (grams of ethanol per day) | 10.8 ± 2.60 | 8.8 ± 1.59 | 11.0 ± 2.58 | 9.7 ± 2.03 | 10.1 ± 1.12 |
| Number of previous treatments (M ± SE) | 4.0 ± 0.46 | 4.4 ± 0.40 | 3.6 ± 0.41 | 4.7 ± 0.58 | 4.2 ± 0.23 |
| Employment N (%) | 31 (41.3%) | 32 (42.1%) | 33 (44.0%) | 33 (4 4.0%) | 129 (42.9%) |
| HIV positive N (%) | 34 (47.2%) | 30 (39.5%) | 43 (57.3%) | 39 (53.4%) | 146 (49.3%) |
| Hepatitis B N (%) | 14 (19.4%) | 16 (21.3%) | 24 (32.0%) | 14 (19.2%) | 68 (23.1%) |
| Hepatitis C N (%) | 72 (96.0%) | 72 (94.7%) | 70 (93.3%) | 71 (95.9%) | 285 (95.0%) |
| RAB drug risk (M ± SE) | 8.3 ± 0.55 | 9.1 ± 1.47 | 7.8 ± 0.60 | 8.6 ± 0.59 | 8.5 ± 0.45 |
| RAB sex risk (M ± SE) | 4.7 ± 0.30 | 4.4 ± 0.27 | 4.2 ± 0.30 | 5.1 ± 0.55 | 4.6 ± 0.19 |
| GAF (M ± SE) | 61.1 ± 0.86 | 64.0 ± 0.79 | 64.4 ± 0.83 | 61.8 ± 0.85 | 62.8 ± 0.42 |
| ASI medical problems (M ± SE) | 0.08 ± 0.017 | 0.14 ± 0.027 | 0.12 ± 0.023 | 0.11 ± 0.022 | 0.11 ± 0.011 |
| ASI work problems (M ± SE) | 0.76 ± 0.028 | 0.75 ± 0.027 | 0.75 ± 0.032 | 0.75 ± 0.032 | 0.75 ± 0.015 |
| ASI alcohol use problems (M ± SE) | 0.10 ± 0.014 | 0.12 ± 0.015 | 0.11 ± 0.014 | 0.11 ± 0.014 | 0.11 ± 0.007 |
| ASI drug use problems (M ± SE) | 0.28 ± 0.009 | 0.37 ± 0.080 | 0.28 ± 0.007 | 0.30 ± 0.012 | 0.31 ± 0.021 |
| ASI legal problems (M ± SE) | 0.09 ± 0.013 | 0.09 ± 0.017 | 0.07 ± 0.015 | 0.09 ± 0.017 | 0.08 ± 0.008 |
| ASI family problems (M ± SE) | 0.32 ± 0.025 | 0.33 ± 0.026 | 0.44 ± 0.114 | 0.34 ± 0.025 | 0.36 ± 0.031 |
| ASI psychiatric problems (M ± SE) | 0.21 ± 0.028 | 0.21 ± 0.025 | 0.18 ± 0.022 | 0.27 ± 0.043 | 0.22 ± 0.015 |
Note: NS between group differences.
3.2. Medication adherence
Urine tests were collected at the biweekly assessments for patients who remained in treatment, and the proportion of riboflavin positive urines varied between 75 and 100%, although the riboflavin only reflected use of naltrexone, not guanfacine. These data were consistent with pill counts and information from relatives, indicating that those who remained in treatment were taking the naltrexone or naltrexone placebo and the guanfacine by these reports.
3.3. Primary outcomes: treatment retention and relapse
Retention without relapse: at the end of six months, 20 (26.7%) patients in the N + G group and 15 (19.7%, p = 0.258 to N/G) in N + P group were retained in treatment compared to 5 (6.7%) in the P + G group (p = 0.002 to N + G group and p = 0.017 to N + P group, Fisher exact test) and 8 (10.7%) in the double placebo group (p = 0.013 to N + G group, Fisher exact test). There was no significant difference in retention at the end of treatment between the N + G group and N + P group. The relative risk (odds ratio 95% confidence intervals) at week 24 for the various comparisons above were as follows: ORP+P/P+G = 0.60 (95% CI: 0.19–1.92); ORP+P/N+P = 2.06 (95% CI: 0.82–5.20); ORP+P/N+G = 3.05 (95% CI: 1.25–7.45); ORP+G/N+G = 5.09 (95% CI: 1.80–14.43); ORP+G/N+P = 3.44 (95% CI: 1.18–10.02). The number of subjects at 6, 12, 18 and 24 weeks is given in Fig. 2 legend.
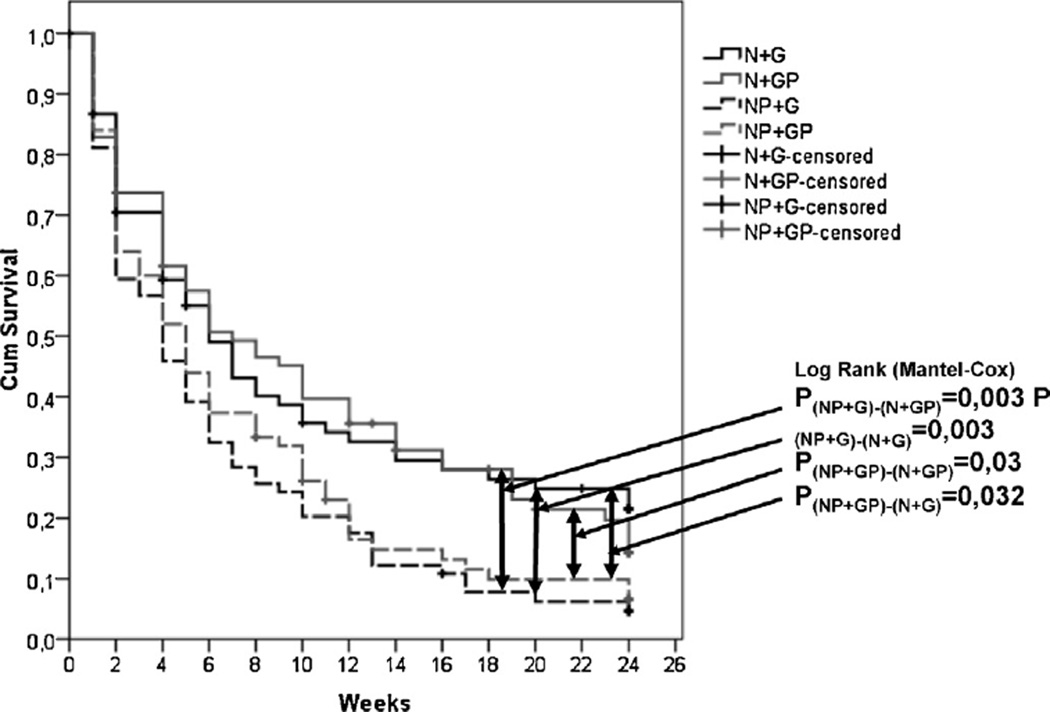
Fig. 2.
Kaplan–Meier survival: treatment dropout over 24 weeks with median retention time of: 6 weeks for NG and NP, 5 weeks for PP and 4 weeks for PG. The number of patients at 6, 12, 18 and 24 weeks for each group was: NG (38, 27, 23, 20); NP (39, 28, 23, 15); PG (25, 14, 7, 5); PP (28, 14, 10, 8).
Fig. 2 shows the Kaplan–Meier survival curves for these comparisons. Log Rank tests showed a significant overall effect for treatment group (Log Rank Stat = 14.1. df = 3, p = 0.003). There were significant differences between the N + G and P + G groups (Log Rank Stat = 8.6; df = 1; p = 0.003), between the N + G and P + P groups (Log Rank Stat = 4.6; df = 1; p = 0.032), between the N + P and P + G groups (Log Rank Stat. = 8.7; df = 1. p = 0.003) and between the N + P and P + P groups (Log Rank Stat. = 4.7; df = 1. p = 0.030).
3.4. Secondary outcomes
3.4.1. Opiate urine test results: missed urines were imputed as opiate positive
The cumulative percentage of opiate negative urines in the N + G group was 367/1064 (35.0%), significantly greater than the P + G group 255/1037 (24.6%; (OR = 1.6, 95% CI = 1.35–1.765, p < 0.001) and P + P group 268/1050 (26.9%; OR = 1.5, 95% CI = 1.22–1.76, p < 0.001). The cumulative proportion of opiate negative urines in the N + P group was greater than the P + P group (OR = 1.5, 95% CI = 1.22–1.76, p < 0.001) and P + G group (OR = 1.6, 95% CI = 1.33–1.93, p < 0.001). The results of self-reported opiate use were very similar to the urine drug test results
3.4.2. Relapse at follow-up
At 9 months: 101 out of 301 patients returned for follow-up assessments. Among these, 29 were in N + G (2/75 in remission); 25 in N + P (8/76 in remission, p = 0.098 to N + G group); 27 in P + G (3/75 in remission, p = 0.683 to N + G group); and 20 in P + P (4/75 in remission, p = 0.681 to N + G group).
At 12 months: 85 of the 301 patients were assessed and among these 26 N + G (3/75 in remission); 20 in N + P (5/76 in remission, p = 0.719 to N + G group); 23 in P + G (3/75 in remission, p = 1.00 to N + G group); and 16 in P + P (2/75 in remission, p = 0.683 to N + G group).
3.4.3. Psychometrics, adjustment, and behavioral outcome measures
3.4.3.1. Perceived Stress Scale (PSS) and craving
We used the Perceived Stress Scale (PSS) to examine whether individual differences in perceived stress and frequency and magnitude of stressful life events were reduced by guanfacine treatment because of its importance for vulnerability to opiate relapse. We also assessed craving as it might be related to stress and be relieved by guanfacine. Patients in all four groups had similar PSS and craving profiles at baseline. As shown in Table 2, there was a significant decrease of the PSS scores, but not craving over the course of treatment (F5,1095 = 21.2; p < 0.0001; ANOVA repeated measures) due to an increase in craving at week 24. The Tukey test for post hoc comparisons revealed significantly lower PSS scores for active guanfacine (N + G and P + G groups) than guanfacine-placebo group (N + P and double placebo) at 18 weeks (16 [SD = 5] vs 26 [SD = 4]) (p = 0.01) after randomization. Similarly, craving scores were significantly lower for the guanfacine groups at week 18 (0.3 [SD = 0.9] vs 0.9 [SD = 0.9]) (p < 0.05) and week 24 (2.3 [SD = 0.8] vs 3.5 [SD = 0.8]) (p < 0.05).
Table 2.
Change in Perceived Stress Scale (PSS) and heroin craving over 6 month clinical trial
| Scales | PSS | PSS | Differ | Craving | Craving | Differ |
|---|---|---|---|---|---|---|
| Medication group | Guanfacine | Placebo | G < P | Guanfacine | Placebo | G < P |
| Weeks of treatment | ||||||
| 0 | 28 [1] | 27 [1] | NS | 3.2 [0.3] | 3.4 [0.3] | NS |
| 6 | 19 [1] | 21 [1] | NS | 1.0 [0.4] | 1.3 [0.4] | NS |
| 12 | 19 [2] | 19 [2] | NS | 0.9 [0.6] | 0.8 [0.6] | NS |
| 18 | 16 [3] | 26 [3] | 0.01 | 0.3 [0.8] | 0.9 [0.8] | 0.05 |
| 24 | 14 [3] | 17 [3] | NS | 2.3 [0.9] | 3.5 [0.9] | 0.05 |
Note: NS = no between group differences
3.4.3.2. Depression, anxiety, overall adjustment, and HIV risk behavior
We did not find significant effects of depression, anxiety, overall adjustment (Global Assessment of Function Scale) or HIV risk behavior (alone or the interaction with medication group) on the treatment outcome. All psychiatric symptoms and overall adjustment gradually improved while HIV drug risky behavior declined over the course of treatment in all four groups with no differences between the groups in any of these measures during the course of treatment (among those patients retained in treatment). For the overall sample Beck depression dropped from 19 [SD = 1] to 6 [SD = 2] and HIV drug risk dropped from 8.1 [SD = 0.6] to 3 [SD = 1] at 12 weeks and to 5 [SD = 1] at 24 weeks.
3.5. Tolerability and safety
We found that a combination of naltrexone and guanfacine was safe and well tolerated: only 4.7% of patients reported any side effects. Side effects were generally mild with most come side effects – headache, poor appetite, insomnia, and dizziness. No differences between the groups were found.
At baseline the mean ALT varied between 1.3 and 1.7 (±0.6 mmol/L); AST ranged between 0.6 and 0.7 (±0.16 mmol/L) with no significant differences between groups. End of treatment measures were only available on patients who remained in treatment and did not relapse. For these patients the ALT varied from 0.8 to 1.2 (±0.15 mmol/L); AST varied from 0.4 to 0.6 (±0.07 mmol/L); with no significant differences across groups or from baseline to six months.
4. Discussion
This study was similar to our previous studies (Krupitsky et al., 2004, 2006) in showing that naltrexone is more effective than placebo for relapse prevention in opioid dependent patients. However, retention in treatment at the end of six months in this study was slightly lower than in the previous naltrexone studies, probably because patients of this study were older and had less family involvement in the control of their adherence to the study medication. These results are consistent with US studies where the importance of family involvement in naltrexone treatment was demonstrated (Carroll et al., 2001; Fals-Stewart and O’Farrell, 2003). Other contributors to medication adherence in Russia may have included that naltrexone was provided free, that we had high quality psychosocial treatment from experienced therapists, and a high level of community interest in a pharmacotherapy with naltrexone as the only effective pharmacotherapy currently available in Russia (Krupitsky et al., 2006). Long acting sustained release formulations of naltrexone (injectable and implantable) are the best possible way to improve adherence to naltrexone and enhance it’s efficacy in treatment of opiate dependence (Krupitsky and Blokhina, 2010; Krupitsky et al., 2010, 2011, 2012). The alternatives of methadone or buprenorphine clearly have better treatment retention and where available are more suitable for a general population of opiate dependent patients (Stotts et al., 2009). Whether this poor retention is related to biological factors is unknown, but blocking opioid receptors also possibly blocks the effects of endogenic opioids. The greater treatment retention of buprenorphine is particularly important in view of the very high percentages of infectious diseases (HIV 49%, HCV 95%, HBV 23%) among these patients. Clearly, treatment strategies that are focused on Harm Reduction, in order to reduce morbidity and mortality in this population are critical, as we have previously noted (Krupitsky et al., 2004, 2006).
Similar to results of Sinha et al. (2007), guanfacine in this study reduced perceived stress at later time points in this study, but it did not also significantly improve retention in naltrexone treatment (though the retention in naltrexone with guanfacine group was slightly better than in naltrexone with placebo). Moreover, this moderate stress reducing effect of guanfacine can be used in the treatment of opiate dependence to improve stress tolerance and potentially to reduce craving for opiates, as we saw at weeks 18 and 24 compared to placebo. A second peak for perceived stress at week 18 is consistent with the concept of incubation described in animal studies of stress induced reinstatement of opiate use after extinction (Shalev et al., 2001). Stress induced reinstatement of drug use after extinction gets more likely as the period since last use is extended for several weeks to months. Our increase in craving at week 24 even more than the difference from placebo at week 18 may have reflected this stress induced effect that was attenuated by guanfacine. Alpha-2 agents have attenuated these incubation phenomena, and the current human study may provide some data supporting this phenomena and its attenuation by these alpha-2 adrenergic agonists in humans. This attenuation might have been stronger using lofexidine, but we could not obtain approval for its use in Russia and used a relatively low dose of an alternative alpha 2 adrenergic agonist – guanfacine. Future studies in other countries might use lofexidine or consider a larger guanfacine dose such as 4–7 mg daily. Another limitation was that medication compliance using riboflavin was only possible for one of the two medications, and we chose the naltrexone as most critical medication. Although we showed good tolerability of guanfacine and its combination with naltrexone in opiate addicts, we relied on pill counts, self-reports and relatives’ observations for compliance with guanfacine. Finally, intent to treat analysis was not possible for the stress effect due to the substantial loss of subjects during this 6-month clinical trial. Instead we examined differences in this measure across the four groups using the available subjects at each of the 12 biweekly time points out to week 24.
Acknowledgement
The material in this manuscript is original, is not published elsewhere, and has not been submitted elsewhere for consideration.
Role of funding source
Funding for this study was provided by NIH Grant 5R01 DA018863-04; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
All authors must have materially participated in the research and/or manuscript preparation. All authors have reviewed and approved the final manuscript.
Conflict of interest
There is no conflict of interest for any participating author.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth edition. Text Revision. Washington, DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bukstein OG, Head J. Guanfacine ER for the treatment of adolescent attention-deficit/hyperactivity disorder. Expert Opin. Pharmacother. 2012;13:2207–2213. doi: 10.1517/14656566.2012.721778. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nick C, O’Connor PG, Egan DA, Frankforter TL. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence – efficacy of contingency management and significant other involvement. Arch. Gen. Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- De Wet C, Reed L, Glasper A, Moran P, Bear nJ, Gossop M. Benzodiazepine co-dependence exacerbates the opiate withdrawal syndrome. Drug Alcohol Depend. 2004;76:31–35. doi: 10.1016/j.drugalcdep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. J. Consult. Clin. Psychol. 2003;71:432–442. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- Gish EC, Miller JL, Honey BL, Johnson PN. Lofexidine, an {alpha}2-receptor agonist for opioid detoxification. Ann. Pharmacother. 2010;44:343–351. doi: 10.1345/aph.1M347. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp. Clin. Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S, Hong KI, Chaplin TM, Dabre Z, Comegys AD, Kimmerling A, Sinha R. A stress-coping profile of opioid dependent individuals entering naltrexone treatment: a comparison with healthy controls. Psychol. Addict. Behav. 2009;23:613–619. doi: 10.1037/a0017324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber H. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogs Clin. Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoi MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O’Brien CP, Woody GE. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J. Subst. Abuse Treat. 2004;26:285–294. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Zwartau EE, Masalov DV, Tsoy MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O’Brien CP, Woody GE. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J. Subst. Abuse Treat. 2006;31:319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Blokhina EA. Long-acting formulations of naltrexone for heroin dependence: a review. Curr. Opin. Psychiatry. 2010;23:210–214. doi: 10.1097/YCO.0b013e3283386578. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Woody G. Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr. Psychiatry Rep. 2010;12:448–453. doi: 10.1007/s11920-010-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes E, Ling W, Illeperuma A, Gastfriend D, Silverman B. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo controlled multicentre randomized trial. Lancet. 2011;337:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin V, Bushara N, Burakov A, Masalov D, Romanova T, Tyurina A, Palatkin V, Slavina T, Pecoraro A, Woody GE. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch. Gen. Psychiatry. 2012;69:973–981. doi: 10.1001/archgenpsychiatry.2012.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction—a clinical perspective. Eur. J. Clin. Pharmacol. 2010;66:537–545. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- Mercer D, Woody GE. The Penn-VA Addiction Counseling Manual. [accessed 30.09.05];1998 Available at http://www.nida.nih.gov ( http://archives.drugabuse.gov/txmanuals/IDCA/IDCA1.html)
- McLellan AT, Luborsky L, Cacciola J, Griffith J. New data from the Addiction Severity Index: reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro C, Tobin D, Metzger D, Alterman A. Preparations for AIDS vaccine trials. An automated version for the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res. Hum. Retroviruses. 1994;10(Suppl. 2):S281–S283. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe A, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch. Gen. Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psycholog. Rep. 1962;10:799–812. [Google Scholar]
- Shalev U, Morales M, Hope BT, Yap J, Shaham Y. Time dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl.) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl.) 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychological and Biological Methods. Totwa, NJ: Humana Press; 1992. [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Anton WD, Bedell J. The nature and treatment of text anxiety. In: Zuckerman M, Spielberger CD, editors. Emotion and Anxiety: New Concepts, Methods and Applications. Hillsdale, N.J.: Erlbaum; 1976. [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin. Pharmacother. 2009;10:1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. The TEDS Report: Substance Abuse Treatment Admissions for Abuse of Benzodiazepines. Rockville, MD: 2011. Jun 2, [Google Scholar]