Abstract
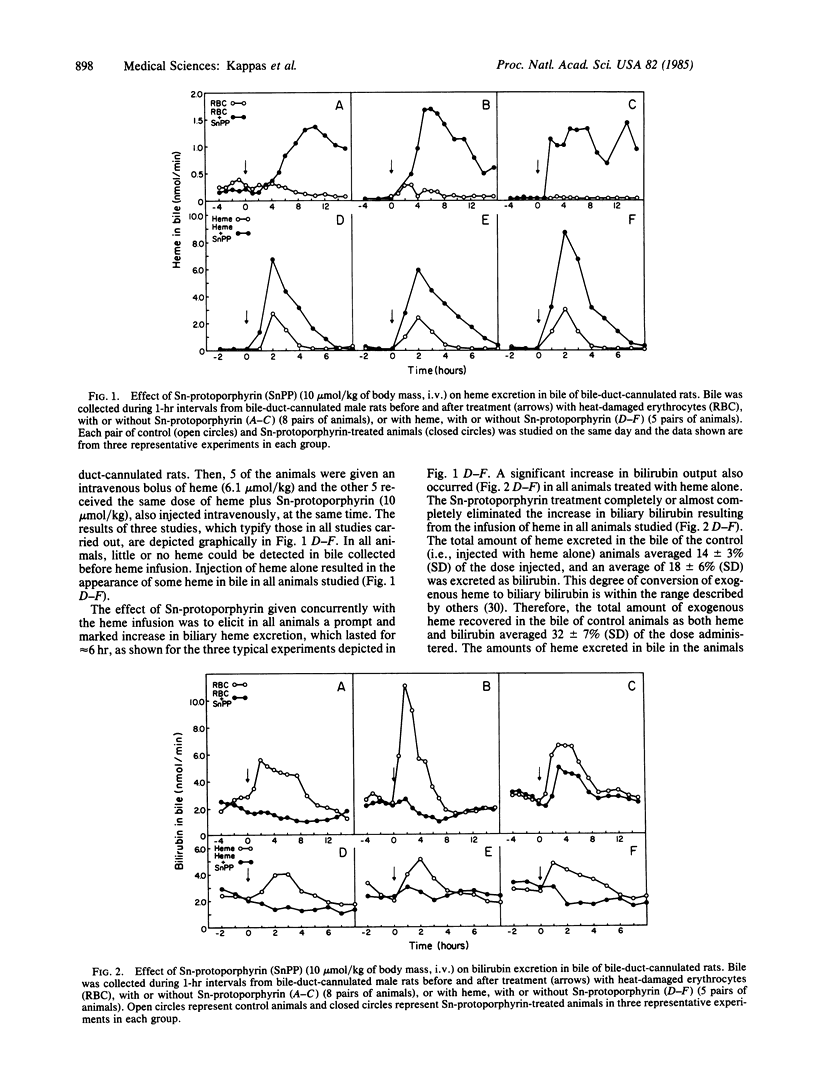
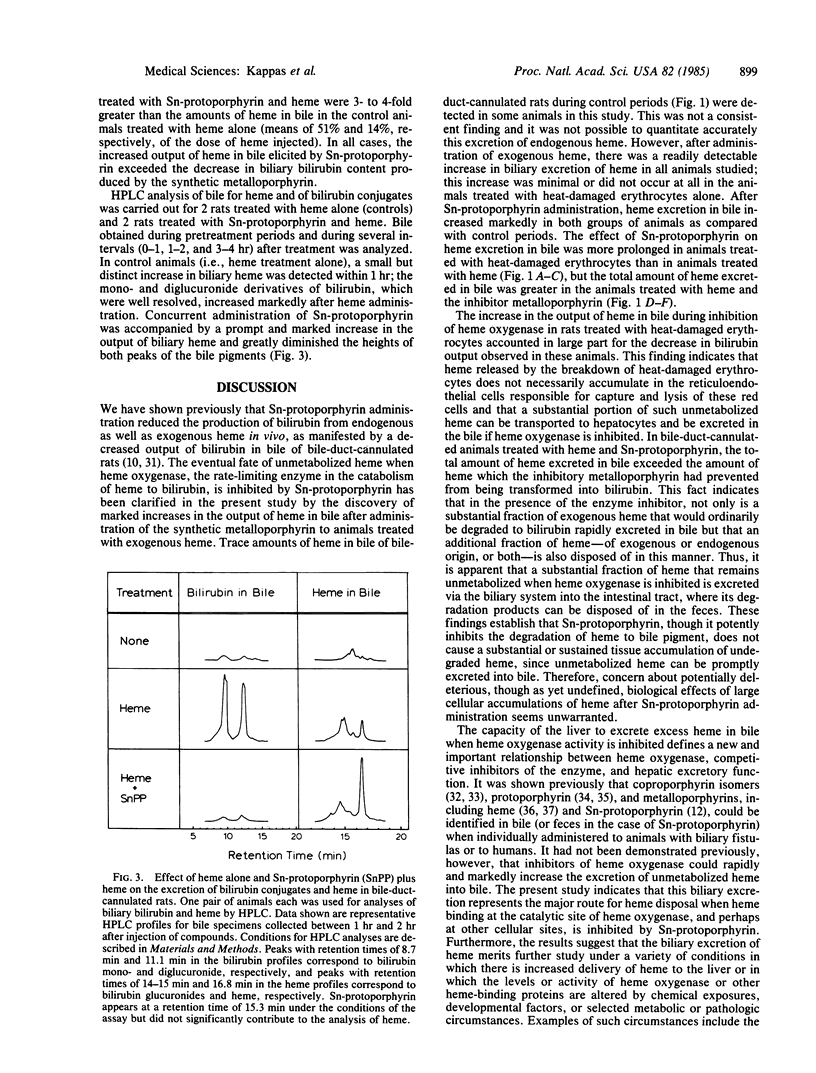
TinIV-protoporphyrin IX (Sn-protoporphyrin) potently inhibits heme degradation to bilirubin in vitro and in vivo, and it completely suppresses neonatal hyperbilirubinemia in experimental animals, including primates. It also reduces plasma bilirubin levels in certain naturally occurring or induced forms of jaundice in animals and man. We have examined in this study the fate of that fraction of heme whose degradation to bile pigment is inhibited in vivo by administration of this heme oxygenase (EC 1.14.99.3) inhibitor. In bile-duct-cannulated rats, infused exogenous heme is rapidly converted to biliary bilirubin; a small amount of the infused heme is excreted into bile as well. Sn-protoporphyrin, administered with the exogenous heme, markedly increased (3- to 4-fold) the amount of heme excreted into bile and greatly diminished biliary output of bilirubin. The increase in biliary heme output exceeded the decrease in bilirubin excretion elicited by the inhibitor metalloporphyrin. In the same experimental model, Sn-protoporphyrin substantially decreased the conversion of heme, derived from heat-damaged erythrocytes, to biliary bilirubin. This decrease in biliary bilirubin output was accounted for entirely by a prompt and marked increase in biliary excretion of unmetabolized heme. The enhanced biliary excretion of unmetabolized heme following administration of Sn-protoporphyrin is a newly defined and biologically important response associated with use of this synthetic heme analogue. The features of the action of this compound in vivo--suppression of formation of the potentially neurotoxic metabolite, bilirubin; enhancement of disposal of the untransformed substrate (heme) of the enzyme that it inhibits; and its own elimination without metabolic alteration--define some of the characteristics of a therapeutically useful chemical.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Simionatto C. S., Drummond G. S., Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984 Feb;228(2):327–333. [PubMed] [Google Scholar]
- Avner D. L., Berenson M. M. Effect of choleretics on canalicular transport of protoporphyrin in the rat liver. Am J Physiol. 1982 Apr;242(4):G347–G353. doi: 10.1152/ajpgi.1982.242.4.G347. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Hemoglobin and erythrocyte catabolism in rat liver: the separate roles of parenchymal and sinusoidal cells. Blood. 1972 Dec;40(6):812–822. [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L., Schmid R. Liver sinusoidal cells. Identification of a subpopulation for erythrocyte catabolism. J Cell Biol. 1972 Jul;54(1):107–119. doi: 10.1083/jcb.54.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal S. G., Ruebner B. H., Ikeda R. M., Hanson F. W., Bergstrom D. E. Protohemin in bile during primate development. Experientia. 1977 May 15;33(5):592–593. doi: 10.1007/BF01946515. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Collins A., Doherty J., Bossenmaier I., Cardinal R., Watson C. J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2725–2729. doi: 10.1073/pnas.68.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Wu G., Shouval R., Arias I. M. Bilirubin mono- and diglucuronide formation by human liver in vitro: assay by high-pressure liquid chromatography. Hepatology. 1981 Nov-Dec;1(6):622–627. doi: 10.1002/hep.1840010610. [DOI] [PubMed] [Google Scholar]
- Cornelius C. E., Rodgers P. A. Prevention of neonatal hyperbilirubinemia in rhesus monkeys by tin-protoporphyrin. Pediatr Res. 1984 Aug;18(8):728–730. doi: 10.1203/00006450-198408000-00010. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. An experimental model of postnatal jaundice in the suckling rat. Suppression of induced hyperbilirubinemia by Sn-protoporphyrin. J Clin Invest. 1984 Jul;74(1):142–149. doi: 10.1172/JCI111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science. 1982 Sep 24;217(4566):1250–1252. doi: 10.1126/science.6896768. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Suppression of hyperbilirubinemia in the rat neonate by chromium-protoporphyrin. Interactions of metalloporphyrins with microsomal heme oxygenase of human spleen. J Exp Med. 1982 Dec 1;156(6):1878–1883. doi: 10.1084/jem.156.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS I. M., MCALISTER J. M., PRANKERD T. A. The relationship of abnormal red cells to the normal spleen. Clin Sci. 1957 May;16(2):223–230. [PubMed] [Google Scholar]
- Healey J. F., Bonkowsky H. L., Sinclair P. R., Sinclair J. F. Conversion of 5-aminolaevulinate into haem by liver homogenates. Comparison of rat and chick embryo. Biochem J. 1981 Sep 15;198(3):595–604. doi: 10.1042/bj1980595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko C., Cook J. D., Finch C. A. Storage iron kinetics. II. The uptake of hemoglobin iron by hepatic parenchymal cells. J Lab Clin Med. 1972 Nov;80(5):624–634. [PubMed] [Google Scholar]
- Ishii D. N., Maniatis G. M. Haemin promotes rapid neurite outgrowth in cultured mouse neuroblastoma cells. Nature. 1978 Jul 27;274(5669):372–374. doi: 10.1038/274372a0. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., FILES N. M., BARNETT S. B., MACDONALD R. A. PROLIFERATIVE RESPONSE OF THE SPLEEN AND LIVER TO HEMOLYSIS. J Exp Med. 1965 Aug 1;122:299–326. doi: 10.1084/jem.122.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N., Javitt N., Kappas A. Coproporphyrin I and 3 excretion in bile and urine. J Clin Invest. 1972 Nov;51(11):2895–2899. doi: 10.1172/JCI107113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Drummond G. S., Simionatto C. S., Anderson K. E. Control of heme oxygenase and plasma levels of bilirubin by a synthetic heme analogue, tin-protoporphyrin. Hepatology. 1984 Mar-Apr;4(2):336–341. doi: 10.1002/hep.1840040227. [DOI] [PubMed] [Google Scholar]
- Liem H. H., Miyai K., Muller-Eberhard U. Effect of porphyrinogenic agents on protein synthesis and bilirubin formation by the isolated perfused rat liver. Biochim Biophys Acta. 1977 Jan 24;496(1):52–64. doi: 10.1016/0304-4165(77)90114-3. [DOI] [PubMed] [Google Scholar]
- McCormack L. R., Liem H. H., Strum W. B., Grundy S. M., Muller-Eberhard U. Effects of haem infusion on biliary secretion of porphyrins, haem and bilirubin in man. Eur J Clin Invest. 1982 Jun;12(3):257–262. doi: 10.1111/j.1365-2362.1982.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U., Bosman C., Liem H. H. Tissue localization of the heme-hemopexin complex in the rabbit and the rat as studied by light microscopy with the use of radioisotopes. J Lab Clin Med. 1970 Sep;76(3):426–431. [PubMed] [Google Scholar]
- Petryka Z. J., Pierach C. A., Smith A., Goertz M. N., Edwards P. S. Billiary excretion of exogenous hematin in rats. Life Sci. 1977 Oct 1;21(7):1015–1020. doi: 10.1016/0024-3205(77)90269-7. [DOI] [PubMed] [Google Scholar]
- Roth M. Dosage fluorimétrique de la bilirubine. Clin Chim Acta. 1967 Sep;17(3):487–492. doi: 10.1016/0009-8981(67)90225-2. [DOI] [PubMed] [Google Scholar]
- SANO S., RIMINGTON C. Excretion of various porphyrins and their corresponding porphyrinogens by rabbits after intravenous injection. Biochem J. 1963 Feb;86:203–212. doi: 10.1042/bj0860203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER A. L., SCHMID R. THE CONVERSION OF HEMATIN TO BILE PIGMENT IN THE RAT. J Lab Clin Med. 1965 May;65:817–824. [PubMed] [Google Scholar]
- Sassa S., Drummond G. S., Bernstein S. E., Kappas A. Tin-protoporphyrin suppression of hyperbilirubinemia in mutant mice with severe hemolytic anemia. Blood. 1983 May;61(5):1011–1013. [PubMed] [Google Scholar]
- Simionatto C. S., Anderson K. E., Sassa S., Drummond G. S., Kappas A. Fluorometric measurement of tin-protoporphyrin in biological samples. Anal Biochem. 1984 Aug 15;141(1):213–219. doi: 10.1016/0003-2697(84)90448-2. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Uesugi T., Adachi S., Kamisaka K. Separation of bilirubin isomers and their conjugates by high-performance reversed-phase liquid chromatography. J Chromatogr. 1983 Oct 14;277:308–313. doi: 10.1016/s0378-4347(00)84850-1. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Dhar G. J., Bossenmaier I., Cardinal R., Petryka Z. J. Effect of hematin in acute porphyric relapse. Ann Intern Med. 1973 Jul;79(1):80–83. doi: 10.7326/0003-4819-79-1-80. [DOI] [PubMed] [Google Scholar]
- Whetsell W. O., Jr, Sassa S., Kappas A. Porphyrin-heme biosynthesis in organotypic cultures of mouse dorsal root ganglia. Effects of heme and lead on porphyrin synthesis and peripheral myelin. J Clin Invest. 1984 Aug;74(2):600–607. doi: 10.1172/JCI111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]



