Abstract
Background
Cardiotoxic and other side effects limit the usefulness of treatments for cancer.
Methods
This article is based on pertinent articles that were retrieved by a selective search in PubMed and other databases, and on the guidelines of the European Society of Cardiology, the Association of Scientific Medical Societies in Germany, and the European Society of Medical Oncology.
Results
Prospective studies have shown that some treatments for cancer are cardiotoxic. The heart damage that they cause can manifest itself as arrhythmia, arterial hypertension, thromboembolism, angina pectoris, myocardial infarction, or heart failure. It has been observed that potentially lethal complications can arise as late as 40 years after treatment of the original cancer. The anthracycline drug doxorubicin, given in a dose of 500 mg/m2 of body surface area, has been found to cause cardiac complications in 4–36% of the patients treated with it. Trastuzumab and epirubicin cause dose-limiting cardiac events in 1.7–5% of patients, depending on the dosage. Paclitaxel causes bradycardia, intracardiac conduction block, or arrhythmia in 0.5% of patients. 18% of patients treated with sunitimib or sorafenib have clinical manifestations relating to the heart (angina pectoris, dyspnea). 5-fluorouracil can cause angina pectoris at the beginning of treatment and rarely causes myocardial infarction. Cardiac radiation therapy, a form of treatment practiced in earlier decades, can cause cardiac complications 20 years after the event. The opportunity to prevent cardiac complications of anthracycline drugs with dexrazoxane is decidedly limited, but initial studies have shown that treatment with beta-blockers and ACE inhibitors lessens the likelihood of cardiotoxic side effects. When cardiac complications arise, the generally applicable rules for the treatment of each type of cardiac problem should be followed. The oncological treatment protocol should be adjusted or switched to one that is less damaging to the heart.
Conclusion
Treating physicians need to be thoroughly acquainted with the cardiotoxic effects of anti-cancer drugs so that they can diagnose them early on and avoid jeopardizing the overall success of treatment.
The number and variety of treatment options for cancer patients have increased significantly in recent years. More specific treatment approaches are possible thanks to newly introduced, targeted agents. However, side effects such as cardiotoxicity can restrict the use of some therapies (Table 1) (1, 2).
Table 1. Associations between oncological treatments and manifestations of cardiotoxicity (according to 5, 39, 40, e1–e8, e20, e21).
| Drug | Restricted left ventricular function % |
Cardiac ischemia % |
Association with arterial hypertension % |
Association with thromboembolism % |
|---|---|---|---|---|
| Anthracyclines Doxorubicin Epirubicin Idarubicin Liposomal anthracyclines |
3 to 26 0.9 to 3.3 5 to 18 2 |
– – – – |
– – – – |
– – – – |
| Alkylating agents Cyclophosphamide Ifosfamide Cisplatin |
7 to 28 17 – |
– – - |
– – - |
– – 8.5 |
| Antimetabolites Clofarabine Capecitabine 5-Fluoruracil |
27 – – |
– 3 to 9 1 to 68 |
– – - |
– – – |
| Antimicrotubule agents Docetaxel Paclitaxel |
2.3 to 8 – |
1.7 1 to 5 |
– – |
– – |
| Monoclonal antibodies Bevacizumab Trastuzumab |
1.7 to 3 2 to 28 |
0.6 to 1.5 – |
4 to 35 – |
12 – |
| Proteasome inhibitors Bortezomib |
2 to 5 |
– |
– |
– |
| Small-molecule tyrosine kinase inhibitors Dasatinib Imatinib Lapatinib Sunitinib Erlotinib Sorafenib |
2 to 4 0.5 to 1.7 1.5 to 2.2 2.7 to 11 – – |
– – – – 2.3 2.7 to 3 |
– – – 5 to 47 – 17 to 43 |
– – – – 3.9 to 11 – |
| Immunomodulators/piperidinediones Lenalidomide Thalidomide |
– – |
– – |
– – |
3 to 75 1 to 58 |
| Radiotherapy | >4 | 1 to 13 | – | – |
A distinction is made between acute cardiotoxicities, such as cardiac arrhythmia during anthracycline infusions, and chronic cardiotoxicities, such as restricted left ventricular pump function with clinical symptoms of cardiac insufficiency even decades after the end of treatment. Chronic cardiotoxicity has serious consequences (2). Late cardiotoxic complications have been observed in 8.3% of patients 30 years after anthracy-cline use (3, 4). The 2.2% to 13% mortality rate following high-dose 5-fluorouracil treatment also demonstrates how dangerous such side effects can be (5).
The precise mechanism of cardiotoxicity has been most thoroughly researched in the case of anthracy-clines. The molecular basis of this is binding to topoisomerase-2β (6). Combination therapies (e.g. anthracycline + trastuzumab) can increase cardiotoxicity (2, 6– 8). Genetic factors seem to increase the probability of cardiotoxic side effects (9).
This article reports on cardiotoxicity, a significant side effect that frequently limits oncological treatments. It focuses on anthracyclines, the monoclonal antibody trastuzumab, and radiotherapy. One section also addresses the subject of cardiotoxicity in the treatment of children with malignant diseases.
Diagnosis
Cardiac dysfunction during oncological treatment can manifest in various different ways. Cardiac arrhythmias, ECG alterations, pericarditis–myocarditis syndrome, and other manifestations have been described as early forms (8). The late form of cardiomyopathy can be detected on the basis of a reduction in left ventricular ejection fraction (LVEF) with resulting systolic heart failure. Like other forms of heart failure, it becomes clinically manifest as reduced performance, shortness of breath, weight gain, and edema (8). Diagnosis is established on the basis of clinical history and physical examination and confirmed using the gold standard of transthoracic echocardiogram (TTE). Potentially, TTE should be performed before every cardiotoxic chemotherapy (8, 10). Risk-adjusted monitoring should be performed during treatment.
Sensitive, reproducible ways to diagnose cardiotoxicity associated with tumor treatment are scintigraphy and, as a last resort, endomyocardial biopsy. Neither of these procedures is part of routine practice, as scintig-raphy is associated with a significant radiation burden and endomyocardial biopsy with the danger of local complications and a potentially fatal pericardial effusion. Endomyocardial biopsy is indicated only in exceptional cases of progressive disease forms, to verify diagnosis. The role of magnetic resonance imaging of the heart (cardiac MRI) as a noninvasive method has yet to be established (8, 10).
In recent years, various authors have investigated the value of serum markers in diagnosing cardiotoxicity. The main serum markers are the natriuretic peptides BNP and NT-proBNP and the cardiac structural protein troponin (8, 10). These are established as markers for the diagnosis and prognosis of patients with heart disease. As a predictive marker for the assessment of cardiomyopathy induced by tumor treatments, they are controversial and should not be used to rule out or diagnose cardiotoxicity (11– 17).
In everyday clinical practice, all patients should be monitored for potentially cardiotoxic complications in routine medical examinations even years and decades after oncological treatment; by far the most significant such complication is the manifestation of cardiac insufficiency following anthracycline treatment, with symptoms such as reduced performance, dyspnea, or edema (13, 14).
The cardiotoxicity of specific tumor treatments
Anthracyclines
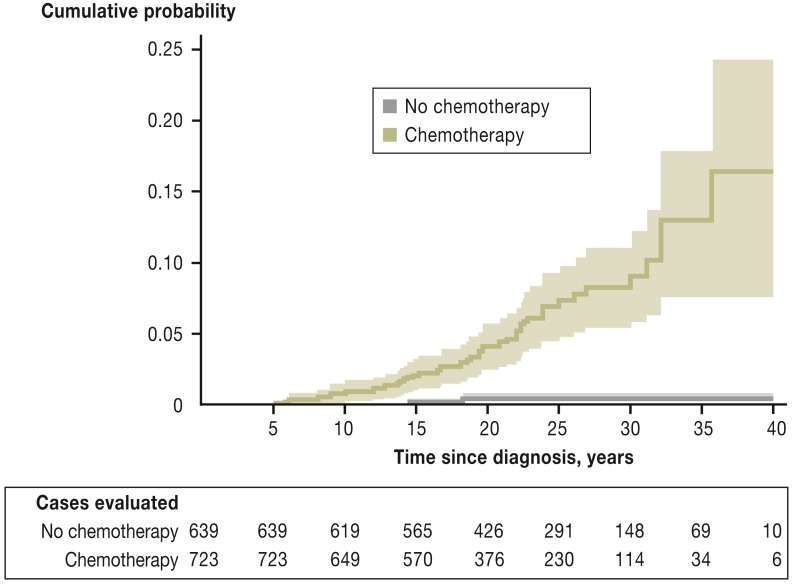
Anthracyclines such as doxorubicin and epirubicin are frequently used, highly effective agents in the treatment of malignant diseases. Cardiotoxic side effects are their main side effects. The most dangerous of these is congestive cardiomyopathy with reduced LVEF, which can develop as much as 40 years after anthracycline treatment in childhood (e.g. for acute lymphoblastic leukemia) (Figure 1) (14, 18, 19). Its precise probability in individual cases is difficult to estimate; for doxorubicin at a cumulative dose of 500 mg/m2, for example, the proportion of patients who suffer cardiac complications has been described as between 4% and 36% (17).
Figure 1.
Cumulative incidence of cardiac events (acute cardiac insufficiency, cardiac ischemia, heart valve diseases, arrhythmia, and/or pericarditis) in long-term survivors of childhood cancer (according to [e19]: van der Pal H. et al.: High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 2012; 30: 1429–37. Reproduced with the kind permission of the American Society of Clinical Oncology [ASCO]; the authors, editors, and ASCO are not responsible for errors or omissions in translations).
Risk factors are age, pre-existing cardiac damage, radiation in the region of the heart, but in particular cumulative anthracycline dose (17).
Anthracyclines should be used only after thorough clinical examination, including ECG and echocardiography with measurement of LVEF (17). For liposomal doxorubicin insufficient data is available on many diseases, but its use is justified for certain indications (18).
Trastuzumab
Trastuzumab, an antibody, was developed as an inhibitor of the tyrosine kinase-associated HER2 receptor (20). The first trial to demonstrate the clinical efficacy of trastuzumab was the 1996 Phase II trial conducted by Baselga et al. (21). Since then, trastuzumab treatment has become an established therapy for HER2-positive breast cancer. However, the licensing trial of trastuzumab in patients with metastatic breast cancer found an unacceptably high rate of cardiac events (NYHA [New York Heart Association] Class III and IV heart failure) for the very combination of trastuzumab and an anthracycline that was most effective: these occurred in 27% of cases, versus 8% for the combination including paclitaxel (19). In order to quantify the cardiotoxicity of trastuzumab and anthracycline combination therapy, the German HERCULES Trial investigated treatment with epirubicin (a less cardiotoxic epimer of doxorubicin), cyclophosphamide, and trastuzumab. Cardiac function was examined prospectively using a series of echocardiograms. The results of the trial showed a significantly lower rate of dose-limiting cardiac events after five years: 1.7% (at 60 mg/m2) versus 5% (at 90 mg/m2 epirubicin) (22).
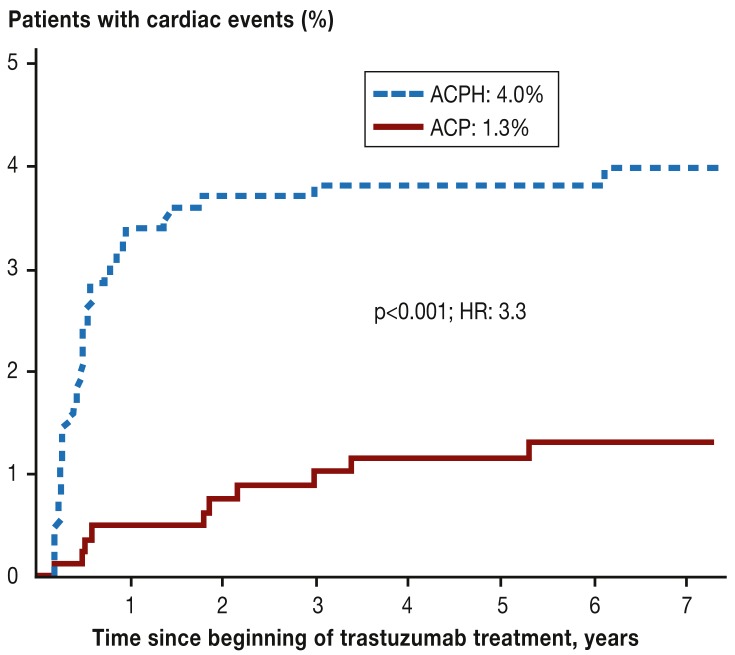
In cases of HER2 overexpression, trastuzumab can reduce the mortality rate by one-third when compared to adjuvant chemotherapy alone (relative risk [RR]: 0.66; 95% confidence interval; 0.57 to 0.77; p<0.00001); for node-positive disease, this is an absolute rate of 8.8% fewer deaths within 10 years. The risk of serious cardiac events, however, increases between three- and five-fold (to 4%) (Figure 2) (23– 27). These particularly affect patients with pre-existing cardiac risk.
Figure 2.
Cumulative incidence of cardiac events (acute cardiac insufficiency, cardiac death) in the National Surgical Adjuvant Breast and Bowel Project Trial B-31 (NSABP B-31).
ACP: Doxorubicin, cyclophosphamide, paclitaxel; ACPH: Doxorubicin, cyclophosphamide, paclitaxel, trastuzumab; HR, hazard ratio; (according to [24,]: Romond EH et al.: Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel [ACP] with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012; 30: 3792–9. Reproduced with the kind permission of the American Society of Clinical Oncology [ASCO]. The authors, editors, and ASCO are not responsible for errors or omissions in translations)
In contrast with anthracycline-induced cardiomy-opathy, trastuzumab-induced cardiomyopathy appears to be reversible; there is no further increase in risk after the end of treatment (Figure 2) (24).
In view of breast cancer’s high survival rates, in the future it will be important to test the indications for anthracyclines with and without trastuzumab against other combination therapies that may be less cardiotoxic, to determine factors that predict response to anthracyclines and potential cardiotoxicities, and to use this information to put together more tailor-made treatments.
Taxanes, 5-fluorouracil, and signal transduction inhibitors
Cardiac toxicity caused by paclitaxel takes the form of sub-acute or acute bradycardia, heart block, and atrial or ventricular arrhythmias (28). Its incidence is 0.5% (28). Paclitaxel itself does not induce congestive heart failure (26). However, paclitaxel combined with anthracyclines does foster anthracycline-associated cardiotoxicity. Interaction leads to reduced anthracycline elimination, resulting in higher plasma levels (29).
In 5-fluorouracil treatment, cardiac symptoms generally occur during the initial hours following the start of therapy. The most frequent symptom is reversible, typical angina pectoris, but myocardial infarctions have also been described (30). Complex arrhythmias are rarer. The clinical symptoms of ECG alterations can last more than 10 days. Transient regional or diffuse disruptions to left ventricular contractility are seen on echocardiograms (31). Coronary vasospasms are discussed as the basic pathological mechanism of 5-fluo-rouracil-induced cardiotoxicity (32). In cases of pre-existing coronary heart disease, the risk of clinically manifest cardiac side effects is increased by a factor of 6.83 (33). The cardiac side effects of the orally available prodrug capecitabine are similar to those of parenteral 5-fluorouracil (34).
Vascular endothelial growth factor (VEGF) and its signal cascades probably play an important role in myocardial response to acute and chronic ischemias (32). For bevacizumab, a monoclonal anti-VEGF antibody, myocardial dysfunction is described, in addition to arterial hypertension and arterial thromboembolic events, in approximately 1.6% of cases (35, 36).
As multikinase inhibitors, sunitinib and sorafenib also inhibit the VEGF receptor (37). In a prospective study involving 74 patients who received either sorafenib or sunitinib, a cardiac event was observed in 34% of patients, and clinical symptoms (angina pectoris, dyspnea) in 18%. In 12% of patients, echocardiography revealed a significant reduction in left ventricular ejection fraction (LVEF) or regional contractile dysfunction. ECG alterations were recorded in 16% of patients (38).
Radiotherapy
The risk of cardiotoxicity following radiotherapy to the thorax using a modern procedure is considered to be low, but it is relevant in the case of the treatment of long-term survivors who received radiotherapy in the past, administered via methods that are now obsolete (39). The high cure rate of Hodgkin’s lymphoma and breast cancer in particular means that comprehensive data on long-term risks of cardiac morbidity and mortality is available (39).
Following mantle field irradiation for Hodgkin’s lymphoma at typical doses of between 30 and 42 Gy, a two-fold increase in the relative risk of ischemic heart diseases (RR: 1.9) was reported after an average follow-up time of 11.2 years. The main reason for this figure is the number of patients with additional cardiovascular risk factors (40).
Cardiac MRI in 20-year survivors of mantle field irradiation at a median dose of 40 Gy and superhigh doses around cardiac structures detected hemodynamically relevant valve damage in 42% of cases, perfusion deficits in 68%, and evidence of prior infarctions in 29% (e1). Although no evidence was found of a direct relationship between reconstructed radiation dose and specific abnormal findings (e2), doses of around 40 Gy (heart valves), 35 Gy (pericardium, myocardium), and 30 Gy (coronary arteries) are seen as critical. In combinations including anthracyclines, the critical dose thresholds are lower (e3).
In pediatric oncology in particular, radiation doses have already been massively reduced. In long-term follow-up care of 1132 children from consecutive German–Austrian treatment studies on Hodgkin’s lymphoma with a median follow-up time of 15.1 years, heart disease could be detected in 4.4% of cases, and in two-thirds of these the abnormality was damage to heart valves. No comparison with an untreated control group was made (e4). At a dose of around 20 Gy, the current standard in pediatric oncology, only one case was observed. The future risk for patients receiving treatment today can therefore be estimated as considerably lower (e4).
In a recently published large case-control study, the risk of ischemic heart disease increased in proportion to the mean radiation dose received by the heart up to 20 years after radiotherapy; however, the observation period in the study lasted from 1958 to 2001 (e5). Overall, following radiotherapy planned using CT and administered via a linear accelerator for breast cancer, which has been standard practice since the 1990s, significant cardiotoxicities can no longer be detected (e6, e7). However, the risk inherent in combined radiotherapy and potentially cardiotoxic cytostatic agents or antibodies cannot yet be conclusively assessed, due to the long follow-up periods required. Every effort should therefore always be made, when planning radiotherapy, to reduce the dose received by cardiac structures.
Pediatric oncology
Cardiotoxic side effects are particularly important in pediatric oncology, as nowadays 80% of children who receive oncological treatment are still alive 15 years later (German Childhood Cancer Registry: Annual Report 2012). Anthracycline-induced cardiotoxicity is particularly relevant to the treatment of children with malignant diseases, partly because in the past 40% to 50% of these children were treated with anthracyclines, and this figure has now risen to 60% (e8). The cumulative incidences reported in patients who are children or were treated with anthracyclines in childhood vary widely; this reflects the many different risk factors for cardiotoxicity and varying study design: the probability of cardiac insufficiency has been reported as being between 0 and 16%, and the risk of subclinical cardiotoxicity between 0 and 57% (e9).
The known risk factors for anthracycline-induced cardiotoxicity in childhood and adolescence are shown in Table 2.
Table 2. Significant known risk factors for anthracycline-induced clinical heart failure in children and adolescents (4, e19, e20, e22).
| Risk factors | Absolute risk | Relative risk | 95% CI |
|---|---|---|---|
| Cardiac irradiation | Cardiac irradiation: 27.3% No cardiac irradiation: 2.5% |
11.1 | 3.7 to 33.5 |
| Age | <15 years: 1.6% = 15 years: 0.69% |
2.3 | 1.4 to 4 |
| Cumulative anthracycline dose | ≥500 mg/m²: 2.4% <500 mg/m²: 0.9% |
2.6 | 1.1 to 6 |
| Sex | Female: 1.5% Male: 0.7% |
2.1 | 1.3 to 3.5 |
95% CI: 95% confidence interval
Unfortunately, insufficient data is available on the effects of different anthracyclines on the risk of cardiotoxicity in children and adolescents (e10, e11). There is also a lack of data on the effect of protective substances (e9)—with the exception of dexrazoxane, which is now contraindicated for children and adolescents because of an increased risk of secondary malignancy, among other reasons (dexrazoxane product characteristics).
This makes genuine prevention impossible for pediatric patients. It is therefore all the more important to provide these patients with adequate follow-up care and, where necessary, to begin treatment early. Further pediatric studies investigating the issue of cardiotoxicity would also be worthwhile.
Monitoring, prophylaxis, and treatment
Monitoring
The following measures can be used to identify cardiotoxic side effects:
Medical history (risk factors and cardiac symptoms such as dyspnea or reduced performance)
Clinical examination (for edema)
ECG
Echocardiogram as gold standard.
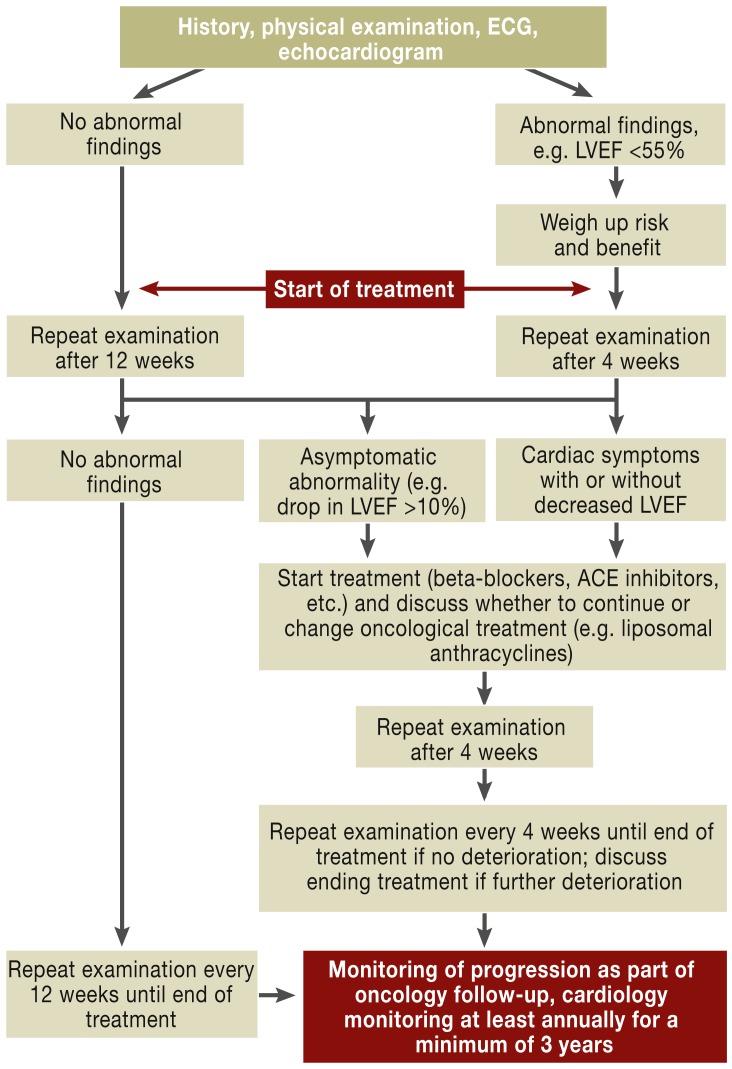
On an echocardiogram, restriction of left ventricular ejection fraction below the normal value of 55% or more than 10% below a previous finding and evidence of regional wall motility dysfunction should be regarded as abnormal (15, 16). Frequent monitoring via echocardiogram is recommended before, during, and after potentially cardiotoxic treatment, at intervals appropriate to the severity and progression of the disease (e12). A suggestion for diagnosis and treatment is provided in Figure 3 (according toe13).
Figure 3.
Suggested diagnosis and treatment procedure for cardiotoxic treatment (adapted according to [e13]). LVEF: left ventricular ejection fraction
Prophylaxis/cardioprotective agents
In the literature, oxidative stress was seen as the most likely cause of anthracycline-induced cardiomyopathy. However, antioxidants and free-radical interceptors demonstrated no effect (2).
Dexrazoxane is discussed as a possible option. Dexrazoxane is an intracellular iron chelator which is authorized for the prevention of anthracycline-induced cardiomyopathy in breast cancer patients. One of its potential side effects is cardiac arrhythmia such as tachy-cardia (dexrazoxane product characteristics). A 2011 Cochrane analysis showed that in randomized trials, dexrazoxane is suitable for the prevention of cardiotoxicity in adults with breast cancer or soft-tissue sarcoma (relative risk reduction: 0.29; 95% confidence interval: 0.20 to 0.41), with no evidence of lower treatment efficacy or higher risk of secondary tumors (e9).
A further review article showed a three-fold increase in the incidence of neoplasia, severe myelosuppression, and infections in children following dexrazoxane treatment (e14). According to its German product characteristics, dexrazoxane is contraindicated for patients under the age of 18 (dexrazoxane product characteristics).
Treatment
If a patient suffers the most serious type of cardiotoxicity, symptomatic left ventricular systolic heart failure, oncological treatment must be adjusted and the use of less cardiotoxic treatment regimens (e.g. liposomal anthracyclines or anthracycline-free regimens) should be discussed. In addition, patients should be treated in line with the general rules for the treatment of systolic heart failure. ACE inhibitors (angiotensin receptor blockers if these are not tolerated) and beta-blockers are the most important pharmacological treatments. The aims are to achieve blood pressure less than 140/90 mm Hg and heart rate less than 70 b.p.m. with an increase to the maximum tolerated dose (e15). One potentially interesting option is the angiotensin receptor blocker telmisartan, which led to reversibility of myocardial dysfunction in a fairly small randomized trial (e16).
The following agents may also be used: aldosterone antagonists, digitalis glycosides, diuretics, and ivabradine (e15– e20).
General treatment measures stated in guidelines include fluid restriction (approx. 1.5 L/day), physical activity, a healthy diet (e.g. restricted salt intake), and others (e14– e18).
Other causes that lead to restricted left ventricular ejection fraction, such as coronary heart disease or myocarditis, must also be considered during differential diagnosis. If necessary, they must be included in the diagnosis procedure (left heart catheterization, cardiac MRI) (e18).
In a pilot study involving 90 patients with leukemia or other blood cell malignancies who received prophylactic treatment consisting of enalapril/carvedilol or placebo, a combined endpoint consisting of deterioration of left ventricular function, cardiac insufficiency, and total mortality at six months was significantly reduced by administering enalapril/ carvedilol (6.7% for active treatment versus 22% for placebo) (e17).
Key Messages.
In recent years the mortality rates of malignant tumors have been successfully reduced using multimodality therapies.
Cardiotoxicity as a side effect of chemotherapy agents and targeted drugs (e.g. trastuzumab) can be treatment-limiting.
With current, modern methods of radiotherapy, the only cases in which radiogenic cardiotoxicity remains an issue are pulmonary and mediastinal tumors.
The gold standard for the diagnosis of cardiotoxicity is echocardiography. The value of biomarkers is disputed.
Tailor-made treatment protocols using less cardiotoxic substances (liposomal anthracyclines) reduce the risk. Dexrazoxane (in anthracycline treatment or anthracy-cline-free regimens only) may be used in patients with advanced or metastatic breast cancer if its risks and benefits are considered thoroughly.
Cancer drugs may need to be adjusted during treatment. General rules on the treatment of systolic heart failure must be followed.
Footnotes
Conflict of interest statement
Prof. Schlitt has received consultancy fees (Advisory Board) from Boehringer Ingelheim. He has received lecture fees and reimbursement of conference fees and travel expenses from Sanofi-Aventis, Servier, Boehringer Ingelheim, and Bayer AG. He has also received trial funding (third-party funds) from GSK, Sanofi-Aventis, Mitsubishi, Endotis, Bayer AG, Boehringer Ingelheim, Novartis, Actelion, and BMS.
Prof. Thomssen has received consultancy fees (Advisory Board) from Roche, Pfizer, AstraZeneca, Novartis, Celgene, and Genomic Health. He has received reimbursement of conference fees, travel expenses, and lecture fees from Roche, Pfizer, Novartis, Celgene, and Genomic Health.
Prof. Vordermark has received lecture fees as a speaker from Roche, Bristol-Myers Squibb, Lilly, and Astra Zeneca.
Dr. Jordan has received lecture fees as a speaker from MSD, Amgen, Helsinn, Riemser, and TRM.
Dr. Schwamborn and Prof. Langer declare that no conflict of interest exists.
References
- 1.Haberland J, Bertz J, Wolf U. Krebs in Deutschland 2005-2006. Häufigkeiten und Trends. Robert Koch-Institut. Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. 2010 [Google Scholar]
- 2.Kruger A, Wojnowski L. Cardiotoxicity of anthracyclines—an unsolved problem. Dtsch Arztebl. 2006;103(37):A2393–A-2397. [Google Scholar]
- 3.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 5.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the mo-lecular basis of doxorubicin-induced cardiotoxicitiy. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 7.Ochsle K, Bokemeyer C. Adäquate Begleitmaßnahmen bei Tumortherapie mit Anthrazyklinen. Im Focus Onkologie. 2007;4:55–59. [Google Scholar]
- 8.Oechsle K, Bokemeyer C. Kardiotoxizitäten bei Chemo- und Radiotherapie. Onkologe. 2009;15:157–162. [Google Scholar]
- 9.El-Tokhy MA, Hussein NA, Bedewy AM, Barakat MR. XPD gene polymorphisms and the effects of induction chemotherapy in cytogenetically normal de novo acute myeloid leukemia patients. Hematology. 2013 doi: 10.1179/1607845413Y.0000000144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Germanakis I, Anagnostatou N, Kalmanti M. Troponins and natriuretic peptides in the monitoring of anthracycline cardiotoxicity. Pediatr Blood Cancer. 2008;51:327–333. doi: 10.1002/pbc.21633. [DOI] [PubMed] [Google Scholar]
- 11.Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004;6:257–260. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Sandri MT, Cardinale D, Zorzino L, et al. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin Chem. 2003;49:248–252. doi: 10.1373/49.2.248. [DOI] [PubMed] [Google Scholar]
- 13.Paulides M, Wojnowski L. Chemotherapeutics-induced heart failure. Med Klin (Munich) 2007;102:574–578. doi: 10.1007/s00063-007-1071-y. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Sallan SE. Cardiovascular abnormalities in long-term survivors of childhood malignancy. J Clin Oncol. 1993;11:1199–1203. doi: 10.1200/JCO.1993.11.7.1199. [DOI] [PubMed] [Google Scholar]
- 15.Tissières P, Beghetti M. Biomarker in der pädiatrischen Kardiologie. Paediatrica. 2008;19:19–20. [Google Scholar]
- 16.Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10:391–599. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 17.Bovelli D, Plataniotis G, Roila F. ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21:277–282. doi: 10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 18.Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Journal of Clinical Oncology. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 22.Untch M, Muscholl M, Tjulandin S, et al. First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol. 2010;28:1473–1480. doi: 10.1200/JCO.2009.21.9709. [DOI] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;21:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 24.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slamon D, Eiermann W, Robert N, et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 27.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD006243.pub2. CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbuck SG, Strauss H, Rowinsky E, et al. A reassesment of cardiac toxicity associated with Taxol. J Natl Cancer Inst Monogr. 1993;15:117–130. [PubMed] [Google Scholar]
- 29.Holmes FA, Madden T, Newman RA, et al. Sequence-dependent alteration of doxorubicin pharmacokinetic by paclitaxel in a phase I study of paclitaxel and doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1996;14:2713–2721. doi: 10.1200/JCO.1996.14.10.2713. [DOI] [PubMed] [Google Scholar]
- 30.Maurer U, Härle M, Jungius KP, et al. 5-Fluorouracil: cause of a fatal myocardial infarction during combined radiochemotherapy. Strahlenther Onkol. 1996;172:257–260. [PubMed] [Google Scholar]
- 31.Patel B, Kloner RA, Ensly, et al. 5-Fluorouracil cardiotoxicity: left ventricular dysfunction and effect of coronary vasodilatators. Am J Med Sci. 1987;294:238–243. doi: 10.1097/00000441-198710000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Burger AJ, Mannino S. 5-Fluorouracil induced coronary vaso-spasm. Am Heart J. 1987;114:433–436. doi: 10.1016/0002-8703(87)90517-5. [DOI] [PubMed] [Google Scholar]
- 33.Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy. 1997;17:729–736. [PubMed] [Google Scholar]
- 34.Frickhofen N, Beck FJ, Jung B, et al. Capecitabine can induce acute coronary syndrome similar to 5-fluorouracil. Ann Oncol. 2002;13:797–801. doi: 10.1093/annonc/mdf035. [DOI] [PubMed] [Google Scholar]
- 35.Zhu X, Dahut WL, Parik CR. Risk of proteinuria and hypertension with bevacizumab, a monoclonal antibody against vascular endothelial growth factor: a systemic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Hawkes EA, Okines AF, Plummer C, Cunningham D. Cardiotoxicity in patients treated with bevacizumab is potentially reversible. J Clin Oncol. 2011;29:e560–e562. doi: 10.1200/JCO.2011.35.5008. [DOI] [PubMed] [Google Scholar]
- 37.Berardi R, Caramanti M, Savini A, et al. State of the art for cardiotoxicity due to chemotherapy and to targeted therapies: A literature review. Crit Rev Oncol Hematol. 2013;88:75–86. doi: 10.1016/j.critrevonc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Schmiedinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sutininib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;2:248–256. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 39.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 40.Glanzman C, Kaufmann P, Jenni R, Hess OM, Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1998;46:51–62. doi: 10.1016/s0167-8140(97)00125-4. [DOI] [PubMed] [Google Scholar]
- e1.Machann W, Beer M, Breunig M, et al. Cardiac magnetic resonance imaging findings in 20-year survivors of mediastinal radiotherapy for Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2011;79:1117–1123. doi: 10.1016/j.ijrobp.2009.12.054. [DOI] [PubMed] [Google Scholar]
- e2.Vordermark D, Seufert I, Schwab F, et al. 3-D reconstruction of anterior mantle-field techniques in Hodgkin’s disease survivors: doses to cardiac structures. Radiat Oncol. 2006;1 doi: 10.1186/1748-717X-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- e4.Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- e5.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- e6.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Patt DA, Goodwin JS, Kuo YF, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- e8.Bennet AM, Blom-Goldman U, Brønnum D, et al. Prospective longitudinal evaluation of doxorubicin-induced cardiomyopathy in sarcoma patients: a report of the late effects surveillance system (LESS) Pediatr Blood Cancer. 2006;46:489–495. doi: 10.1002/pbc.20492. [DOI] [PubMed] [Google Scholar]
- e9.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;6 doi: 10.1002/14651858.CD003917.pub4. CD003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.van Dalen EC, Michiels EM, Caron HN, et al. Different anthracy-cline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD005006.pub2. CD005006. [DOI] [PubMed] [Google Scholar]
- e11.Sieswerda E, Kremer LC, Caron HN, et al. The use of liposomal anthracycline analogues for childhood malignancies: A systematic review. Eur J Cancer. 2011;47:2000–2008. doi: 10.1016/j.ejca.2011.03.024. [DOI] [PubMed] [Google Scholar]
- e12.Buck T, Breithardt OA, Faber L, et al. Manual zur Indikation und Durchführung der Echokardiographie. Clin Res Cardiol Suppl. 2009;4(Suppl1):3–51. doi: 10.1007/s00392-009-0097-y. [DOI] [PubMed] [Google Scholar]
- e13.Todaro MC, Oreto L, Qamar R, Paterick TE, Carerj S, Khandheria BK. Cardiooncology: State of the heart. Int J Cardiol. 2013;168:680–687. doi: 10.1016/j.ijcard.2013.03.133. [DOI] [PubMed] [Google Scholar]
- e14.Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol. 2004;130:1–7. doi: 10.1007/s00432-003-0498-7. [DOI] [PubMed] [Google Scholar]
- e15.Bundesärztekammer, Kassenärztliche Bundesvereinigung, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. http://www.versorgungsleitlinien.de/themen/herzinsuffizienz/pdf/nvl-hi-lang-7.pdf. Programm für nationale Versorgungsleitlinien. Nationale Versorgungsleitlinie Chronische Herzinsuffizienz. (last accessed on 17 February 2014) [Google Scholar]
- e16.Mantovani C, Madeddu A, Piras, et al. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and myocardial dysfunction. Eur J Cancer. 2011;47 doi: 10.3892/etm.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e17.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial. J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- e18.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- e19.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- e20.Bu’Lock FA, Mott MG, Oakhill A, Martin RP. Left ventricular diastolic function after anthracycline chemotherapy in childhood: relation with systolic function, symptoms, and pathophysiology. Br Heart J. 1995;73:340–350. doi: 10.1136/hrt.73.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Early Breast Cancer Trialists’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- e22.von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]