Abstract
Myofibrillogenesis, the process of sarcomere formation, requires close interactions of sarcomeric proteins and various components of sarcomere structures. The myosin thick filaments and M-lines are two key components of the sarcomere. It has been suggested that myomesin proteins of M-lines interact with myosin and titin proteins and keep the thick and titin filaments in order. However, the function of myomesin in myofibrillogenesis and sarcomere organization remained largely enigmatic. No knockout or knockdown animal models have been reported to elucidate the role of myomesin in sarcomere organization in vivo. In this study, by using the gene-specific knockdown approach in zebrafish embryos, we carried out a loss-of-function analysis of myomesin-3 and slow myosin heavy chain 1 (smyhc1) expressed specifically in slow muscles. We demonstrated that knockdown of smyhc1 abolished the sarcomeric localization of myomesin-3 in slow muscles. In contrast, loss of myomesin-3 had no effect on the sarcomeric organization of thick and thin filaments as well as M- and Z-line structures. Together, these studies indicate that myosin thick filaments are required for M-line organization and M-line localization of myomesin-3. In contrast, myomesin-3 is dispensable for sarcomere organization in slow muscles.
Keywords: Myosin, Myomesin 3, M-line, Sarcomere
INTRODUCTION
Sarcomere, the basic contractile unit in skeletal and cardiac muscles, is one of the most highly ordered macromolecular assemblies in cells. Defective sarcomere organization in skeletal and cardiac muscles leads to muscular dystrophies and cardiomyopathies. Assembly of ordered sarcomere structures, also called myofibrillogenesis, requires the integration of hundreds of proteins in a sequential, regulated manner by specific protein-protein interactions that link various components of sarcomere structure together. The sarcomere is divided into four major compartments including the Z-line, M-line, thick and thin filaments. It has been well documented that the Z-line which anchors the actin filaments, and the M-line which is thought to cross-link the myosin filaments, play pivotal roles in sarcomere organization of thick and thin filaments (Agarkova and Perriard, 2005). Although the sarcomeric structure has been well characterized, the regulatory mechanisms of myofibrillogenesis that lead to the formation of this highly organized structure is, however, not yet completely understood.
The thick filaments in a sarcomere of skeletal muscles consist of several hundreds of myosin II molecules that contain two heavy chains with the globular head and long α-helical tail domains as well as two pairs of light chains called the essential and regulatory light chains. Myosin II molecules group together to form highly regular bipolar structures called bipolar thick filaments. The globular myosin heads are positioned on either side of the filament, and the tail regions are clustered in the middle. This geometry enables myosin II molecules in thick filaments to pull from either side, generating contractile forces. It has been reported that thick filament assembly and many aspects of myofibrillogenesis are independent of the myosin head and these processes are regulated by the myosin rod and tailpiece (Cripps et al., 1999).
The central portion of each bipolar thick filament is connected to its neighbors by proteins of the M-line. The M-line is a transverse structure in the center of the sarcomere which is thought to stabilize the thick filament lattice. The muscle-specific protein myomesin is a major candidate for the role of M-line bridges to regulate packing of thick filaments and to uniformly distribute the tension over the myosin filament lattice in the activated sarcomere (Agarkova et al., 2003). Three myomesins, namely myomesin-1, -2 and -3, have been identified in skeletal and cardiac muscles in vertebrates. Myomesin-1 is ubiquitously expressed in all types of striated muscles (Agarkova et al., 2004). Myomesin-2 (also known as M-protein), on the other hand, is predominantly expressed adult heart and fast-twitch skeletal muscles (Agarkova et al., 2004). In contrast, myomesin-3 is specifically expressed in slow muscles (Schoenauer et al., 2008). Although a proof for the essential function of myomesin awaits the generation of a knockout model, it has been shown that mice with a conditional knockout for M-band region of titin (including the myomesin binding site) resulted in muscle weakness and progressive sarcomere disassembly (Gotthardt et al., 2003; Musa et al., 2006). Moreover, it has been reported that downregulation of myomesin by siRNA interference disrupted M-band structure leading to sarcomere disorganization in cultured neonatal rat cardiomyocytes (Fukuzawa et al., 2008). A recent report showed that a myomesin mutation is associated with hypertrophic cardiomyopathy (Siegert et al., 2011). However, a direct genetic evidence of myomesin function in myofibrillogenesis and sarcomere organization is still lacking. A proof for the essential function of myomesin awaits the generation of a knockout or knockdown animal models.
In this study, we knocked down the expression of slow myosin heavy chain 1 (smyhc1) and myomesin-3 expressed in slow muscles of zebrafish embryos and compared the muscle phenotypes in the sarcomere organization. We demonstrated that knockdown of smyhc1 and myomesin-3 had a different effect on sarcomere organization. Thick filament formation and M-line localization of myomesin-3 were completely disrupted in slow muscles of smyhc1 knockdown zebrafish embryos. In contrast, loss of myomesin-3 had no effect on the sarcomeric organization. Normal organization of thick and thin filaments as well as Z-lines was observed in slow muscles of myomesin-3 knockdown embryos. Together, these studies suggest that myosin thick filaments are required for the organization of M-line structure and M-line localization of myomesin-3. In contrast, myomesin-3 is dispensable for M-line formation and sarcomere organization in slow muscles of zebrafish embryos.
Materials and methods
Zebrafish maintenance
Adult zebrafish were raised at the zebrafish facility of the Aquaculture Research Center, Institute of Marine and Environmental Technology, USA. The fish were maintained at 28°C with a photoperiod of 14 h light and 10 h dark, in 8-gallon aquaria supplied with freshwater and aeration. The slotu44c mutant zebrafish line was obtained from Tubingen Zebrafish Stock Center, USA (Granato et al., 1996). The slotu44c mutant carries a nonsense mutation in the heat shock protein 90α1 gene (hsp90α1) resulting in truncated molecules missing the C-terminal domain, which is important for both homo- and heterodimerization (Hawkins et al., 2008; Ali et al., 2006). The myomesin-RFP zebrafish line [myom3(mnGt0067)] was obtained from Steve Ekker’s laboratory at Mayo Clinic, USA. It carries a RFP gene trap insertion in the myomesin-3 gene (Clark et al., 2011). Additional information can be found at http://www.zfishbook.org/index.php?topic=GBT0067#. The Tg(ef1a:Smyd1b_tv1-GFP) transgenic fish were generated in our laboratory (Xu and Du, unpublished).
Synthesis of morpholino antisense oligos
Morpholino antisense oligos were synthesized by Gene Tools (Corvalis, OR, USA). The smhyc1-MO was targeted to the sequence flanking the ATG start codon. The Unc-45b and hsp90a1 morpholino oligos were targeted to the sequence flanking the ATG start codon. The control-MO was the standard control oligo purchased from Gene Tools.
smyhc1-MO: 5′-TCTAAAGTTTTACCCACTGCGGCAA-3′
Unc-45b ATG-MO: 5′-ATCTCCAATTTCTCCCATCGTCATT-3′
hsp90a1 ATG-MO: 5′-CGACTTCTCAGGCATCTTGCTGTGT-3′.
Microinjection in zebrafish embryos
Morpholino antisense oligos were dissolved in 1x Danieau buffer to a final concentration of 0.5 mmol/L or 1 mmol/L. Approximately 1–2 nL (5 ng or 10 ng) was injected into each zebrafish embryo at the 1 or 2 cell stages.
Analysis of smyhc1 expression in wild type or smyhc1-MO injected embryos by RT-PCR
Total RNA was extracted from 50 wild type and 50 smyhc1-MO injected zebrafish embryos at 24 hpf. Smyhc1 expression was determined by RT-PCR using primers myhc1-UTR and myhc1-E4-R derived from the sequence in 5′-UTR and exon-4, respectively. The PCR products were cloned into pGEM-T easy vector and sequenced.
myhc1-UTR: 5′–CAAGGTACAGAGGTCTGACAAACA-3′
myhc1-E4-R: 5′–CTATCTGACAGCATGTACTGGTA-3′
Analysis of myomesin-3 and myomesin-3- RFP expression by RT-PCR
Total RNA was extracted from 50 wild type and 50 myomesin-3 RFP homozygous zebrafish embryos at 48 hpf. Expression of myomesin-3 and myomesin-3-RFP transcripts was determined by RT-PCR using the following primers. The PCR products were cloned into pGEM-T easy vector and sequenced.
myom3rt-fp2: 5′-AGGCAGAAATCAGAGAGCATCTGG -3′
myom3rt-rp2: 5′-CTTCCTGGTCACCTTGCACTTCAT-3′
myom3rt-fp3: 5′-ATGAAGTGCAAGGTGACCAGGAAG-3′
myom3rt-rp4: 5′-GATATACACTGGACCAGCGGAGAG-3′
myom3-fp1: 5′-TCAGGACACACACAAGCGCTCGCTGGAT-3′
myom3-rp2: 5′-AGAGACTACATCTTTATTTATCAAGCAC-3′
mRP-fp1: 5′-GAACGGCCACGAGTTCGAGATCGAG-3′
mRP-rp2: 5′-CTCGTACTGTTCCACGATGGTGTAG-3′
Whole mount in situ hybridization
Whole mount in situ hybridization was carried out using digoxigenin-labeled antisense probe in the smyhc1 knockdown and control embryos at 24 hpf (Cordina et al., 2010). Plasmid pGEM-stnnc was digested with Spe I and transcribed with T7 RNA polymerase to synthesize the digoxigenin-labled antisense RNA probe.
Whole-mount immunostaining
Immunostaining was carried out using whole-mount zebrafish embryos (1-3 days post-fertilization) as previously described (Tan et al., 2006; Du et al., 2008). Briefly, zebrafish embryos were fixed in 4% paraformaldehyde (in PBS) for 1 h at room temperature. The fixed embryos were washed for 15 min 3 times in PBST. Three day old embryos were digested in 1 mg/mL collagenase for 75 min. Immunostaining was performed with the following primary antibodies: anti-α-actinin (clone EA-53, #A7811, Sigma,USA), anti-MyHC for slow muscles (F59, DSHB, USA), anti-myosin light chain for fast muscles (F310, DSHB), anti-MyHC (MF-20, DSHB), anti-myomesin (mMaC myomesin B4, DSHB), anti-α–actin (Ac1-20.4.2, Progen, Germany), and anti-Prox1 antibody (AB5475, Millipore, USA). Secondary antibodies were FITC or TRITC conjugates (Sigma).
RESULTS
1. Blocking splicing of smyhc1 intron 1 resulted in nuclear localization of smyhc1 mRNA transcripts
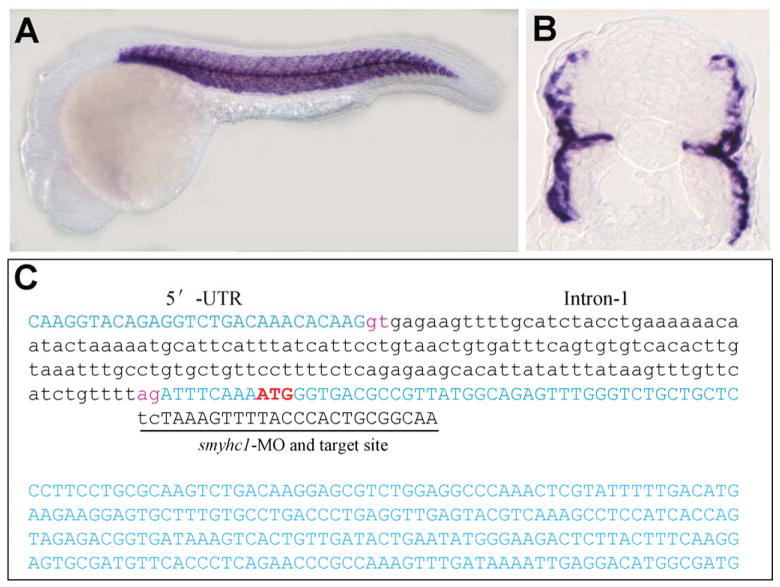
Zebrafish embryos contain two major types of skeletal muscles, slow muscle and fast muscle, based on the expression of myosin heavy chain (MyHC). Smyhc1 represents the primary MyHC expressed in slow muscles of zebrafish embryos (Fig. 1A and B) that can be labeled specifically with F59 monoclonal antibody (Devoto et al., 1996; Bryson-Richardson et al., 2005; Elworthy et al., 2008). We have previously shown that a smyhc1-specific morpholino (smyhc1-MO) targeted to the ATG start codon could knock down smyhc1 expression and resulted in disruption of thick and thin filament organization in zebrafish slow muscles (Cordina et al., 2010). Close examination of the smyhc1-MO sequence revealed that the last two nucleotides (….CT), in fact, could target to the splicing acceptor (…. AG) of intron 1 in the pre-mRNA (Fig. 1C). To determine whether smyhc1-MO could disrupt the smyhc1 pre-mRNA splicing, we amplified the smyhc1 transcripts from the smyhc1-MO injected embryos by RT-PCR (Fig. 2A). The data showed that unlike the uninjected control which showed only one PCR product of 542 bp, two PCR products were produced in smyhc1-MO injected embryos (Fig. 2B). One product of 542 bp (approximately 30%) was identical to that of the uninjected control. Another product (approximately 70%) was a longer product of 702 bp (Fig. 2B). Sequence analysis confirmed that the longer PCR product of 702 bp contained the unspliced intron 1 of 160 bp, confirming that the smyhc1-MO could block the splicing of smyhc1 pre-mRNA, and resulted in the generation of mis-spliced smyhc1 transcripts.
Fig. 1. Expression of smyhc1 in zebrafish slow muscles and target sequence of smyhc1-MO.
A and B: In situ hybridization shows the slow muscle specific expression of smyhc1 in wild type zebrafish embryos at 24 hpf. A, side view; B, view of cross section. C: DNA sequence shows the smyhc1-MO targeted site at the ATG start codon and the intron 1 splicing acceptor. The 5′-UTR, intron 1 and partial coding sequence at the 5′ end are shown.
Fig. 2. Defective splicing of smyhc1 pre-mRNA in smyhc1-MO injected zebrafish embryos.
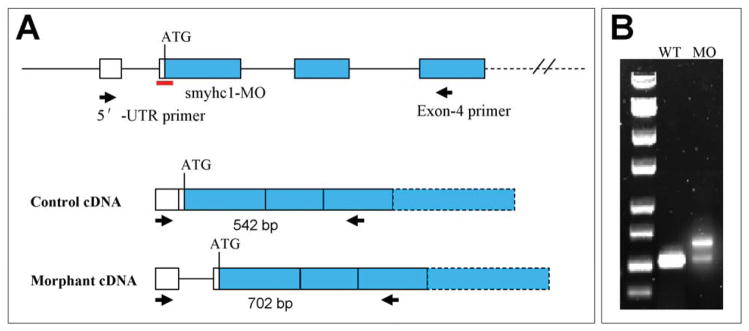
A: diagram shows the gene structure of smyhc1 at the 5′-UTR. The ATG start codon, MO target site, and PCR primers are indicated. B: RT-PCR analysis of smyhc1 expression in the control and smyhc1-MO injected embryos at 24 hpf. Compared with the control, two PCR products were amplified in the smyhc1-MO injected embryos.
RNA splicing is required for nuclear exportation. To determine whether blocking the splicing of intron 1 could interfere with the nuclear to cytoplasmic exportation of smyhc1 pre-mRNA, we performed in situ hybridization on smyhc1-MO injected embryos. The data showed that in contrast to the control embryos that had cytoplasmic localization of smyhc1 mRNAs (Fig. 3A and C), blocking intron 1 splicing indeed interfered with the nuclear to cytoplasmic exportation of smyhc1 pre-RNA (Fig. 3B and D). A clear nuclear localization of smyhc1 pre-mRNA transcripts was observed in slow muscles of smyhc1-MO injected embryos (Fig. 3B and D). The nuclear localization of smyhc1 RNA is consistent with the significant reduction of MyHC protein expression in smhyc1-MO injected zebrafish embryos (Cordina et al., 2010).
Fig. 3. The nuclear localization of smyhc1 pre-mRNA in smyhc1 ATG-MO injected embryos.
A–D: in situ hybridization shows smyhc1 mRNA localization in the control (A, C) or smyhc1-MO (B, D) injected embryos at 24 hpf. Nuclear localization was observed in the smyhc1-MO injected embryos (B, D). E and F: nucleus localization in slow muscles revealed by immunostaining with anti-Prox1 antibody (E), together with F59 antibody (F) for slow muscles in wild type embryo at 24 hpf. G and H: in situ hybridization shows the cytosolic localization of smyhc1 mRNA in smyd1 knockdown (G) or Hsp90α1(slo) mutant (H) embryos at 24 hpf.
To rule out the possibility that the nuclear localization was due to disruption of thick filament organization in slow muscles of zebrafish embryos, we analyzed smyhc1 mRNA distribution in smyd1b knockdown and Hsp90α1mutant embryos that also showed disorganized myosin thick filaments (Tan et al., 2006; Du et al., 2008). The data showed that unlike the smyhc1-MO, disruption of thick filament organization by smyd1b knockdown or hsp90α1 mutation had no effect on the nuclear to cytoplasmic exportation of smyhc1 pre-mRNA (Fig. 3G and H). Together, these data indicate that the nuclear localization was not caused by defective thick filament organization, confirming the gene-specific effect of defective splicing in nuclear localization of pre-mRNA.
2. Knockdown of smyhc1 expression resulted in defective M-line organization in slow muscles
Previous studies have demonstrated that knockdown of smyhc1 does not affect the specification of slow muscle precursors and early formation of slow muscles in zebrafish embryos (Cordina et al., 2010). However, knockdown of smyhc1 expression resulted in defective organization of thick and thin filaments as well Z-line structures (Cordina et al., 2010). The effect on M-line structure in slow muscles could not be analyzed due to the lack of slow muscle-specific M-line markers available in zebrafish. A recent identification of a myomesin-3-rfp insertion line [myom3(mnGt0067)] from gene trapping provided a unique model to visualize M-line organization in slow muscles of zebrafish embryos (Clark et al., 2011). Consistent with the slow muscle specific expression of myomesin-3, the myomesin-3-RFP fusion protein is specifically expressed in slow muscles of zebrafish embryos and showed the M-line localization (Clark et al., 2011; Li et al., 2011).
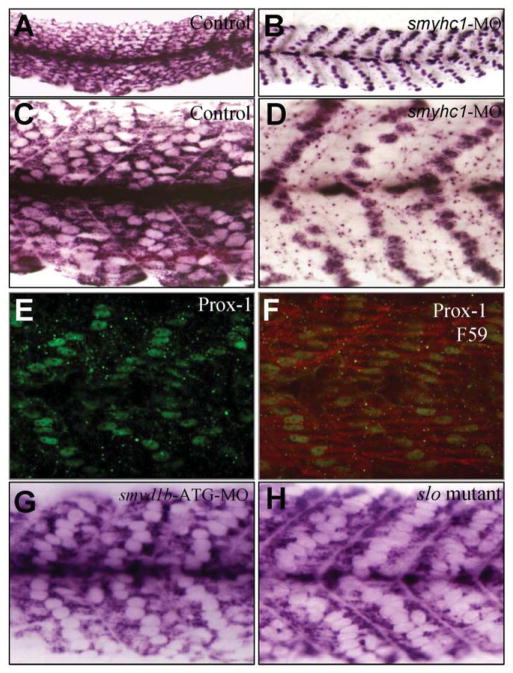
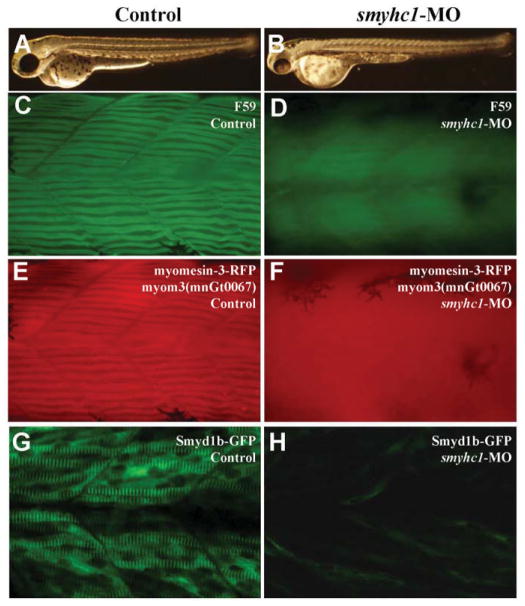
To test whether myosin thick filaments are required for the sarcomeric localization of myomesin, we knocked down the expression of smyhc1 in myom3(mnGt0067) zebrafish embryos using the smyhc1-MO (Fig. 4A–D). The sarcomeric localization of myomesin-3-RFP was examined in the control and smyhc1-MO injected embryos (Fig. 4E and F). The data showed that knockdown of smyhc1 completely diminished the M-line localization of myomesin-3-RFP (Fig. 4F). To assess whether this also affected the M-line localization of other proteins, we compared the M-line localization of Smyd1-GFP in skeletal muscles of the control and smyhc1 knockdown zebrafish embryos. The data showed that knockdown of smyhc1 also blocked the M-line localization of Smyd1-GFP fusion protein in slow muscles of zebrafish embryos (Fig. 4H). Together, these data demonstrated that disruption of myosin thick filaments abolished the M-line localization of myomesin-3 and Smyd1-GFP, suggesting that the thick filaments may be required for M-line organization in slow muscles of zebrafish embryos.
Fig. 4. Effects of smyhc1 knockdown on the M-line localization of myomesin-3-RFP and smyd1b-GFP in zebrafish embryos.
A and B: morphological comparison of the control (A) and smyhc1-MO injected (B) embryos at 48 hpf. C and D: immunostaining with antibody (F59) shows the Smyhc1 expression and thick filament organization in the control (C) and smyhc1-MO injected (D) embryos at 24 hpf. E and F: sarcomeric localization of myomesin-3-RFP in the control (E) or smyhc1-MO injected (F) zebrafish embryos at 24 hpf. G and H: sarcomeric localization of Smyd1b-GFP in the control (G) or smyhc1-MO injected (H) zebrafish embryos at 24 hpf
3. Hsp90α1 and Unc45b are required for myomesin-3-RFP M-line organization in slow muscles
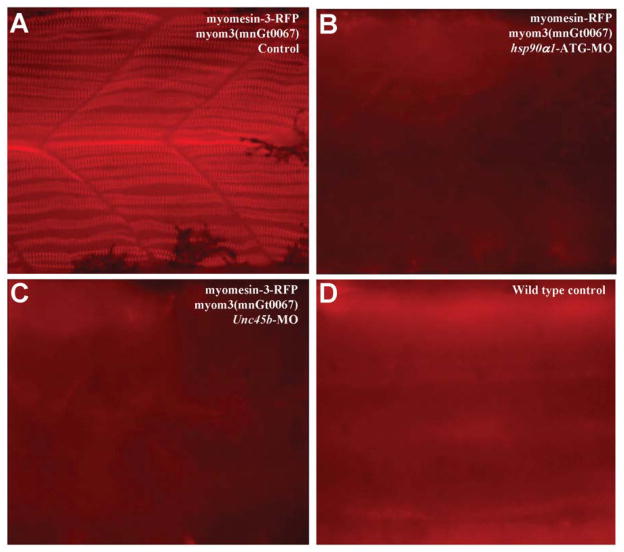
It has been shown that Hsp90α1 and Unc45b are myosin chaperones that play a pivotal role in myosin folding and sarcomere assembly (Etard et al., 2007; Wohlgemuth et al., 2007; Du et al., 2008; Hawkins et al., 2008; Bernick et al., 2010). Knockdown or mutation of hsp90α1 or Unc45b leads to complete disruption of sarcomere organization in thick and thin filaments of both slow and fast muscles (Etard et al., 2007; Wohlgemuth et al., 2007; Du et al., 2008; Hawkins et al., 2008; Bernick et al., 2010). However, their effects on M-line organization have not been analyzed in slow muscles. To characterize the function of Hsp90α1 and Unc45b on M-line organization, we analyzed myomesin-3-RFP localization in hsp90α1 or Unc45b knockdown embryos. The results showed that knockdown of hsp90α1 or Unc45b completely abolished the sarcomeric localization of myomesin-3-RFP in slow muscles (Fig. 5B, C). Together, these data indicate that Hsp90α1 and Unc45b may play a direct role in M-line organization. Alternatively, because Hsp90α1 and Unc45b are required for myosin thick filament assembly, the disruption of myomesin-3-RFP M-line localization could result indirectly from the defective thick filament organization from hsp90α1 and Unc45b knockdown.
Fig. 5. Effects of hsp90α1 and Unc45b knockdown on the sarcomeric localization of myomesin-3-RFP in slow muscles of zebrafish embryos.
Sarcomeric localization of myomesin-3-RFP in slow muscles of myom3(mnGt0067) fish embryos (A), hsp90α1 (B) or Unc45b (C) knockdown myom3(mnGt0067) embryos, or wild type control embryos (D) at 48 hpf.
4. Disruption of myomesin-3 locus by RFP trapping had no effect on sarcomere formation in slow muscles
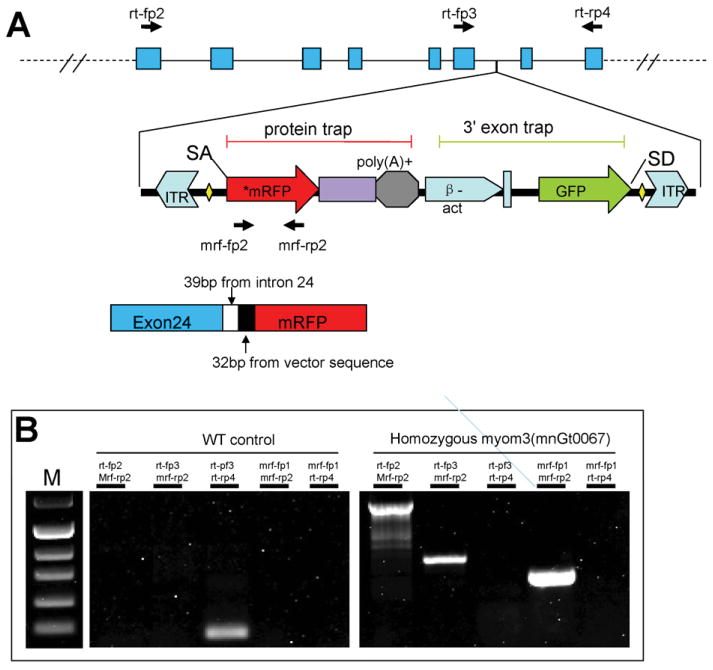
The gene trap integration site in myom3(mnGt0067) fish has been recently mapped to an intron site of myomesin-3 gene (Clark et al., 2011). It has been reported that RFP gene trap integration resulted in over 99% reduction of the normal myomesin-3 gene expression (Clark et al., 2011). To better characterize the integration site and the effect of RFP insertion on myomesin-3 gene expression, we analyze the expression of myomesin-3-RFP chimeric mRNA transcripts by RT-PCR and its sequence. Sequence analysis revealed that the RFP integration site was located within the intron 24 of myomesin-3 (Fig. 6A). The RFP integration resulted in the production of a myomesin-3-RFP fusion protein that includes the N-terminal 957 aa residues of myomesin-3 followed by 13 aa residues coded by part of intron 24 plus a 11 aa residues coded by sequence upstream of the RFP coding sequence in the vector, and finally the RFP sequence (Fig. 6A). The myomesin-3-RFP fusion protein lacks the C-terminal sequence of 219 aa residues.
Fig. 6. Diagram shows the gene trap integration in myomesin-3 gene and PCR strategy for analyzing the expression of myomesin-3 and myomesin-3-RFP mRNA transcripts.
A:. The RFP gene trap is integrated at the intron 24 of myomesin-3. A cDNA fragment covering the junction site of myomesin-3 and RFP fusion was amplified by RT-PCR. Additional sequence of 39 bp from intron 24 and 32 bp from the vector sequence upstream of RFP was found in the myomesin-3-RFP fusion transcript. myomesin-3 GenBank accession No. XM_001921030. B: RT-PCR results show the expression of myomesin-3 mRNA transcripts or myomesin-3-RFP fusion products in wild type or homozygous myom3(mnGt0067) fish embryos.
To determine the effect of RFP integration on the myomesin-3 mRNAs expression, we carried out RT-PCR to amplify myomesin-3 transcripts and myomesin-3-rfp fusion mRNA transcripts from total RNAs of the control or myomesin-3-RFP homozygous fish embryos as shown in Fig. 6A. The data showed a strong expression of myomesin-3-rfp fusion transcripts in myom3(mnGt0067) homozygous zebrafish embryos. However, no myomesin-3 transcripts could be detected in the homozygous zebrafish embryos. This is consistent with the report that integration of the RFP trap significantly reduced the expression of the normal myomesin-3 mRNA transcripts to less than 1% of the normal levels (Clark et al., 2011).
To determine whether disruption of myomesin-3 expression by the RFP insertion could affect the M-line localization of myomesin-3, we analyzed the myomesin-3-RFP localization in myom3(mnGt0067) homozygous fish embryos and showed a normal M-line localization of myomesin-3-RFP in slow muscles (Fig. 7C), suggesting that the myomesin-3 N-terminal region contains the sequence required for the myomesin-3 M-line localization. To determine whether disruption of myomesin-3 expression by the RFP insertion could affect sarcomere organization in slow muscles, we characterized the organization of thick and thin filaments and Z-lines in myom3(mnGt0067) homozygous fish embryos (Fig. 8). The results showed that blocking myomesin-3 expression by RFP insertion had little or no effect on the sarcomere organization. Both thick and thin filaments as well as Z-lines appeared normal in slow muscles of myom3(mnGt0067) homozygous fish embryos (Fig. 8 B, E and H). Together, these data indicate that the sarcomere organization was not affected by the RFP insertion in the myomesin-3 gene locus. This is unexpected considering that the RFP insertion in myomesin-3 resulted in the production of a truncated myomesin-3, and a 99% reduction in the expression of wild type myomesin-3 mRNA transcripts (Clark et al., 2011).
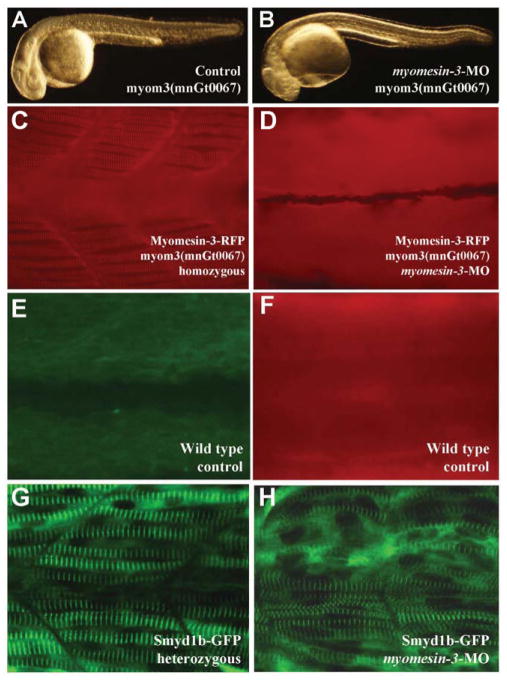
Fig. 7. The effect of RFP gene trap insertion and myomesin-3 knockdown on M-line organization in slow muscles of zebrafish embryos.
A and B: morphological comparison of the control (A) and myomein-3 ATG-MO injected (B) embryos at 24 hpf. C and D: the M-line localization of myomesin-3-RFP in myom3(mnGt0067) homozygous control (C) or myomesin-3 knockdown (D) embryos at 48 hpf. E and F: negative controls of wild type embryos for GFP (E) and RFP (F) at 28 hpf and 48 hpf, respectively. G and H: sarcomeric localization of Smyd1b-GFP at the M-lines in Smyd1b-GFP heterozygous control (G) or myomesin-3 knockdown (H) zebrafish embryos at 28 hpf.
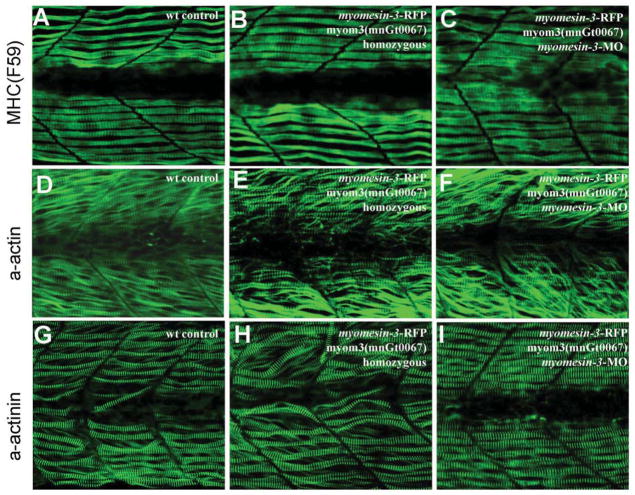
Fig. 8. The sarcomere organization in slow muscles of homozygous myomesin-3-RFP and myomesin-3 knockdown zebrafish embryos.
A–C: anti-MyHC antibody (F59) staining shows the thick filament organization in slow muscles of the control (A), homozygous myom3(mnGt0067) (B), or myomesin-3 knockdown (C) embryos at 28 hpf. D–F: α-actin immunostaining shows the organization of thin filaments in slow muscles of the control (D), homozygous myom3(mnGt0067) (E), or myomesin-3 knockdown (F) embryos at 28 hpf. G–I: α-actinin immunostaining shows the organization of Z-lines in slow muscles of the control (G), homozygous myomesin-3-RFP (H), or myomesin-3 knockdown (I) embryos at 28 hpf.
5. Knockdown of myomesin-3 had no effect on sarcomere organization in slow muscles of zebrafish embryos
To rule out the possibility that the myomesin-3-RFP might retain some myomesin-3 function involved in M-line organization, we performed a knockdown experiment in zebrafish embryos to directly inhibit myomesin-3 protein expression using a myomesin-3 specific translation blocker (myomesin-3 ATG-MO). The myomesin-3 ATG-MO was injected into myom3(mnGt0067) embryos. The knockdown embryos appeared morphologically normal (Fig. 7B). The knockdown efficiency was monitored by examining myomesin3-RFP expression in the injected embryos at 48 hpf. The data showed that the myomesin-3 ATG-MO was highly effective in knocking down the expression of myomesin3-RFP (Fig. 7D). To assess the effect of myomesin-3 knockdown on M-line organization, we examined the M-line localization of Smyd1-GFP fusion protein (Xu and Du, unpublished). The data showed that M-line localization of Smyd1b-GFP was normal in myomesin-3 knockdown embryos (Fig. 7H), suggesting that the M-line structure was not disrupted by myomesin-3 knockdown. To determine the effect of myomesin-3 knockdown on the organization of other sarcomere structures, the myomesin-3 ATG-MO injected embryos were analyzed by immunostaining with antibodies against myosin thick filament, α-actin thin filament and α-actinin on the Z-lines. The data showed that knockdown of myomesin-3 had no effect on thick and thin filament formation and Z-line organization (Fig. 8C, F and I). Collectively, these data indicate that myomesin-3 is dispensable for sarcomere organization in slow muscles of zebrafish embryos.
DISCUSSION
In this study, we analyzed the role of myosin heavy chain 1 and myomesin-3 in sarcomere organization in slow muscles of zebrafish embryos. We demonstrated that knockdown of smyhc1 expression completely disrupted the sarcomeric localization of myomesin-3-RFP and Smyd1b-GFP on M-lines in skeletal muscles of zebrafish embryos. In contrast, disruption of myomesin-3 expression by RFP insertion or knockdown had little effect on sarcomere organization in slow muscles. Together, these studies indicate that myosin thick filaments are required for M-line organization; however, myomesin-3 is dispensable for sarcomere organization and M-line formation in slow muscles.
Myosin thick filaments are required for M-line localization of myomesin-3 in slow muscles
By using the gene-specific knockdown approach, we demonstrated that myosin thick filaments are required for M-line localization of myomesin-3 and possibly M-line organization in zebrafish slow muscles. Knockdown of smyhc1 expression completely abolished the M-line localization of myomesin-3-RFP and Smyd1b-GFP. The muscle phenotype was slow muscle specific, no disruptive effect was observed in fast muscles (Data not shown), consistent with previous findings with other sarcomere structures (Cordina et al., 2010). The smyhc1 knockdown phenotype indicates a critical role for myosin thick filaments in the M-line organization.
Genetic and biochemical analyses have shown that chaperone-mediated myosin folding and assembly is an integral part of myofibrillogenesis during muscle development. Mutations of Uun-45, a myosin chaperone in Caenorhabditis elegans, result in paralyzed animals with severe myofibril defects in body wall muscles (Epstein et al., 1992, 1974; Barral et al., 1998; Landsverk et al., 2005; Moerman et al., 2006). Recent studies showed that Hsp90α1, which binds to UNC-45 and forms a complex with newly synthesized myosin proteins, also plays a vital role in myosin folding and myofibril assembly (Barral et al., 1998; Srikakulam et al., 2004; Etard et al., 2007; Du et al., 2008; Hawkins et al., 2008). Knockdown or mutation of hsp90α1 or Unc45b resulted in defective myofibril organization in skeletal muscles (Etard et al., 2007; Du et al., 2008; Hawkins et al., 2008). Consistent with their function in thick filament assembly and sarcomere organization, we demonstrated in this study that knockdown of Unc45b or hsp90α1 completely abolished the sarcomeric localization of myomesin-3-RFP. The M-line defect in Unc45b and hsp90α1 knockdown embryos could be explained by their direct function on M-line organization. Alternatively, the effect could result indirectly from the disruption of myosin thick filament organization in Unc45b and hsp90α1 knockdown embryos.
Loss of myomesin-3 had little effects on myofibril organization
It has been suggested that myomesin plays a key role in myofibrillogenesis. Knockdown of myomesin by siRNA interference disrupted M-band structure leads to sarcomere disorganization in cultured neonatal rat cardiomyocytes (Fukuzawa et al., 2008). However, direct evidence from knockout or mutant models has yet to be obtained although cellular and biochemical studies have provided some indirect evidence supporting this idea. It has been shown that myomesin molecules bind with their N-terminal domains to the myosin rod, and myomesin can form antiparallel dimmers via their C termini, which might cross-link the neighboring thick filaments (Obermmann et al., 1997; Auerbach et al., 1999; Langer et al., 2005). In addition, myomesin can bind tightly to the titin C-terminal end extended into the M-line. The close interactions of myomesin with myosin and titin are critical for the sarcomere stabilization. Consistent with this idea, targeted homozygous C-terminal deletion of M-band titin prevents sarcomere formation in cardimyocytes (Musa et al., 2006). Moreover, M-line titin homozygous C-terminal truncations cause early-onset myopathy with fatal cardiomyopathy (Carmignac et al., 2007).
Surprisingly, data from our studies demonstrated that knockdown of myomesin-3 expression had little or no effect on myofibril organization in slow muscles of zebrafish embryos. The M-line localization of myomesin-3-RFP and smyd1b-GFP appeared normal in homozygous myom3(mnGt0067) insertion mutant or myomesin-3 knockdown embryos, respectively. Other sarcomeric structures, including thick and thin filaments as well as Z-lines, also appeared normal. This is in contrast to the myosin knockdown embryos that had little or no organized thin filaments, M- and Z-lines. Collectively, these data indicate that myomesin-3 is either not required for M-line organization in slow muscles or other myomesin genes expressed in slow muscles may have a redundant function that prevent the characterization of myomesin-3 knockdown effects. Consistent with this idea, our recent studies showed that zebrafish genome contains at least five myomesin genes (Xu and Du, unpublished).
Interestingly, we demonstrated that myomesin-3-RFP fusion protein showed a clear M-line localization in slow muscles of zebrafish embryos. It suggests that the N-terminal 957 aa residues retained in myomesin-3-RFP are sufficient to target myomesin-3-RFP onto M-lines. This is consistent with previous report that the unique myomesin N-terminal domain of approximately 138 residues is sufficient for binding with myosin in the central region of light meromyosin (Obermann et al., 1997). In addition, the three fibronectin type III repeats (My4-6) at the N-terminal region are sufficient to confer myomesin binding to a single titin immunoglobulin domain (Obermann et al., 1997). The M-line localization of myomesin-3-RFP fusion protein could be explained by its ability to bind with myosin and titin filaments.
Nuclear localization of defectively spliced smyhc1 RNA transcripts
We demonstrated in this study that smyhc1-MO targeted to the ATG site and the splicing acceptor sequence (…AG) of intron 1 could block the intron 1 splicing and resulted in the nuclear retention of defectively spliced smyhc1 pre-mRNA transcripts. This is consistent with previous reports that blocking pre-mRNA splicing by MO could lead to production of defectively spliced RNA transcripts with intron sequences that retain the RNA transcripts within the nucleus (Yan et al., 2002; Hans et al., 2004). It has been suggested that blocking the splicing donor sequence of intron 1 could most likely result in the production of RNA transcripts with an intron insertion (Morcos, 2007). Our studies showed that targeting to the splicing acceptor of the first intron could also block intron 1 splicing and generate defectively spliced transcripts. In fact, such defective splicing is not limited to intron 1. It has been shown that MOs targeted to internal intron sequences could also produce defectively spliced mRNA transcripts that were unable to transport to the cytoplasm (Yan et al., 2002; Hans et al., 2004). Blocking the nuclear exportation of mRNA transcripts is thus a very effective approach to knock down gene expression for functional studies. In addition, blocking pre-mRNA splicing is also a powerful method to investigate the molecular regulation of pre-mRNA splicing in general, and to knock down the expression of specific isoform from alternative splicing.
Acknowledgments
This study was supported by a research grant MB-8716-08 from United States–Israel Binational Agriculture Research and Development Fund to SJD and a NIH grant DA14546 to SCE. Liangyi Xue was supported by a Pao Yu-Kong and Pao Zhao-Long Scholarship for Chinese Scholars Studying Abroad from Ningbo University, China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Agarkova I, ehler Elisabeth, lange Stephan, schoenauer Roman, perriard Jean-claude. M-band: a safeguard for sarcomere stability? J Muscle Res Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- Agarkova I, Schoenauer R, Ehler E, Carlsson L, Carlsson E, Thornell LE, Perriard JC. The molecular composition of the sarcomeric M-band correlates with muscle fiber type. Eur J Cell Biol. 2004;83:193–204. doi: 10.1078/0171-9335-00383. [DOI] [PubMed] [Google Scholar]
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Auerbach D, Bantle S, Keller S, Hinderling V, Leu M, Ehler E, Perriard JC. Different domains of the M-band protein myomesin are involved in myosin binding and M-band targeting. Mol Biol Cell. 1999;10:1297–1308. doi: 10.1091/mbc.10.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral JM, Bauer CC, Ortiz I, Epstein HF. Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J Cell Biol. 1998;143:1215–1225. doi: 10.1083/jcb.143.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson-Richardson RJ, Daggett DF, Cortes F, Neyt C, Keenan DG, Currie PD. Myosin heavy chain expression in zebrafish and slow muscle composition. Dev Dyn. 2005;233:1018–1022. doi: 10.1002/dvdy.20380. [DOI] [PubMed] [Google Scholar]
- Bernick EP, Zhang PJ, Du S. Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos. BMC Cell Biol. 2010;11:70. doi: 10.1186/1471-2121-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignac V, Salih MAM, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- Clark KJ, Balciunas D, Pogoda HM, Ding Y, Westcot SE, Bedell VM, Greenwood TM, Urban MD, Skuster KJ, Petzold AM, Ni J, Nielsen AL, Patowary A, Scaria V, Sivasubbu S, Xu X, Hammerschmidt M, Ekker SC. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat Methods. 2011;8:506–515. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina M, Li J, Gutiérrez J, Kao JP, Du SJ. Loss of Smyhc1 or Hsp90a function results in different effects on myofibril organization insSkeletal muscles of zebrafish embryos. PloS ONE. 2010;5:e8416. doi: 10.1371/journal.pone.0008416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps RM, Suggs JA, Bernstein SI. Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J. 1999;18:1793–1804. doi: 10.1093/emboj/18.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci USA. 2008;105:554–559. doi: 10.1073/pnas.0707330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elworthy S, Hargrave M, Knight R, Mebus K, Ingham PW. Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity. Development. 2008;135:2115–2126. doi: 10.1242/dev.015719. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Bernstein SI. Genetic approaches to understanding muscle development. Dev Biol. 1992;154:231–244. doi: 10.1016/0012-1606(92)90064-n. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–580. doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strähle U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol. 2007;308:133–143. doi: 10.1016/j.ydbio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel MJ. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: Implications for hereditary myopathies. Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Hammer RE, Hübner N, Monti J, Witt CC, McNabb M, Richardson JA, Granzier H, Labeit S, Herz J. Conditional expression of mutant M-line titins results in cardiomyopathy with altered sarcomere structure. J Biol Chem. 2003;278:6059–6065. doi: 10.1074/jbc.M211723200. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Hawkins TA, Haramis AP, Etard C, Prodromou C, Vaughan CK, Ashworth R, Ray S, Behra M, Holder N, Talbot WS, Pearl LH, Strähle U, Wilson SW. The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development. 2008;135:1147–1156. doi: 10.1242/dev.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Lange S, Himmel M, Auerbach D, Agarkova I, Hayess K, Fürst DO, Perriard JC, Ehler E. Dimerisation of myomesin: implications for the structure of the sarcomeric M-band. J Mol Biol. 2005;345:289–298. doi: 10.1016/j.jmb.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Landsverk ML, Epstein HF. Genetic analysis of myosin II assembly and organization in model organisms. Cell Mol Life Sci. 2005;62:2270–2282. doi: 10.1007/s00018-005-5176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu J, Bian YH, Rotllant P, Shen T, Chu W, Zhang J, Schneider M, Du SJ. Smyd1b_tv1, a key regulator of sarcomere assembly, is localized on the M-line of skeletal muscle fibers. PLoS ONE. 2011;6:e28524. doi: 10.1371/journal.pone.0028524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman DG, Williams BD. Sarcomere assembly in C. elegans muscle. WormBook. 2006:1–16. doi: 10.1895/wormbook.1.81.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H, Meek S, Gautel M, Peddie D, Smith AJ, Peckham M. Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation. J Cell Sci. 2006;119:4322–4331. doi: 10.1242/jcs.03198. [DOI] [PubMed] [Google Scholar]
- Morcos PA. Achieving targeted and quantifiable alteration of mRNA splicing with Morpholino oligos. Biochem Biophys Res Commun. 2007;358:521–527. doi: 10.1016/j.bbrc.2007.04.172. [DOI] [PubMed] [Google Scholar]
- Obermann WM, Gautel M, Weber K, Fürst DO. Molecular structure of the sarcomeric M Band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenauer R, Lange S, Hirschy A, Ehler E, Perriard JC, Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. J Mol Biol. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004;117:641–652. doi: 10.1242/jcs.00899. [DOI] [PubMed] [Google Scholar]
- Siegert R, Perrot A, Keller S, Behlke J, Michalewska-Wludarczyk A, Wycisk A, Tendera M, Morano I, Ozcelik C. A myomesin mutation associated with hypertrophic cardiomyopathy deteriorates dimerisation properties. Biochem Biophys Res Commun. 2011;405:473–479. doi: 10.1016/j.bbrc.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Tan X, Rotllant J, Li H, De Deyne P, Du SJ. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc Natl Acad Sci USA. 2006;103:2713–2718. doi: 10.1073/pnas.0509503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth SL, Crawford BD, Pilgrim DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol. 2007;303:483–492. doi: 10.1016/j.ydbio.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, Chung BC, Westerfield M, Haffter P, Hopkins N, Kimmel C, Postlethwait JH. A zebrafish sox9 gene required for cartilage morphogenesis. Development. 2002;129:5065–5079. doi: 10.1242/dev.129.21.5065. [DOI] [PubMed] [Google Scholar]