Abstract
Autophagy selectively removes abnormal or damaged organelles such as dysfunctional mitochondria. The mitochondrial permeability transition (MPT) is a marker of impaired mitochondrial function that is evident in hepatic ischemia/reperfusion (I/R) injury. However, the relationship between mitochondrial dysfunction and autophagy in I/R injury is unknown. Cultured rat hepatocytes and mouse livers were exposed to anoxia/reoxygenation (A/R) and I/R, respectively. Expression of autophagyrelatedprotein7(Atg7),Beclin-1,andAtg12,autophagyregulatoryproteins,wasanalyzedbywestern blots. Some hepatocytes were incubated with calpain 2 inhibitors or infected with adenoviruses encoding green fluorescent protein (control), Atg7, and Beclin-1 to augment autophagy. To induce nutrient depletion, a condition stimulating autophagy, hepatocytes were incubated in an amino acid–free and serum-free medium for 3 hours prior to onset of anoxia. For confocal imaging, hepatocytes were coloaded with calcein and tetramethylrhodamine methyl ester to visualize onset of the MPT and mitochondrial depolarization, respectively. To further examine autophagy, hepatocytes were infected with an adenovirus expressing green fluorescent protein–microtubule-associated protein light chain 3 (GFP-LC3) and subjected to A/R. Calpain activity was fluorometrically determined with succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin. A/R markedly decreased Atg7 and Beclin-1 concomitantly with a progressive increase in calpain activity. I/R of livers also decreased both proteins. However, inhibition of calpain isoform 2, adenoviral overexpression, and nutrient depletion all substantially suppressed A/R-induced loss of autophagy proteins, prevented onset of the MPT, and decreased cell death after reoxygenation. Confocal imaging of GFP-LC3 confirmed A/R-induced depletion of autophagosomes, which was reversed by nutrient depletion and adenoviral overexpression.
Conclusion
Calpain 2–mediated degradation of Atg7 and Beclin-1 impairs mitochondrial autophagy, and this subsequently leads to MPT-dependent hepatocyte death after A/R.
Hepatocyte ischemia during liver transplantation, resection, and shock causes anoxia, depletion of glycolytic substrates, loss of adenosine triphosphate (ATP), and acidosis.1 When blood flow returns to hepatocytes, the normal oxygen concentration and acid-base balance are restored; however, paradoxically, hepatocyte injury is prominent upon reperfusion.1 The mechanisms underlying ischemia/reperfusion (I/R) injury are multifactorial, including increased Ca2+, formation of reactive oxygen species (ROS), activation of injurious catabolic enzymes, and mitochondrial dysfunction.2 Reperfusion causes onset of the mitochondrial permeability transition (MPT), leading to uncoupling of oxidative phosphorylation and necrotic cell death.1,3-5 Onset of the MPT also induces large-amplitude swelling of mitochondria and apoptosis by releasing proapoptotic factors that are normally sequestered in the mitochondrial intermembrane space.6,7 Thus, MPT onset is the common mechanism that culminates in either necrosis or apoptosis after I/R of liver.3
Autophagy degrades both long-lived cytoplasmic proteins and surplus or dysfunctional organelles by lysosome-dependent mechanisms.8 Because nearly all liver proteins are long-lived, macroautophagy (referred to as autophagy hereafter) is a primary catabolic process of hepatic proteins.9 Recent evidence supports organelle-specific, selective autophagy, especially for clearance of peroxisomes (pexophagy) and mitochondria (mitophagy).10,11 Autophagy, much like apoptosis, is regulated by a number of proteins including autophagy-related protein 7 (Atg7).12 This ubiquitin E1-like protein activates both the Atg5-Atg12-Atg16 complex and the conjugation of phosphatidylethanolamine to microtubule-associated protein light chain 3 (LC3; Atg8).12 In addition, Beclin-1 is a mammalian orthologue of yeast Atg6 that promotes the formation of autophagosomes13 and may act as a tumor suppressor.13 The exact role of Atg7 and Beclin-1 in ischemic liver is, however, unknown.
Because mitophagy is the endogenous quality control machinery of mitochondria, an important question has been posed: why do ischemic livers undergo mitochondrial dysfunction even in the presence of mitophagy? Accordingly, the aim of the present study was to investigate the relation between anoxia/reoxygenation (A/R)–induced hepatocyte mitochondrial injury and autophagy. Our results indicate that A/R injury results in increased calpain activity, decreased induction of autophagic regulatory proteins, and limited autophagosome formation with consequent onset of the MPT and hepatocyte death.
Materials and Methods
Hepatocyte Isolation and Culture
Animals received humane care according to protocols approved by the Institutional Care and Use Committee of the University of Florida. Hepatocytes were isolated from 1-day-fasted male Sprague-Dawley rats (250-300 g) by the collagenase perfusion method and cultured overnight in Waymouth's medium, as previously described.3
A/R
To simulate tissue ischemia, hepatocytes were incubated at 37°C in Krebs–Ringer–hydroxyethylpiperazine-N-2 ethanesulfonic acid (KRH) buffer at pH 6.2 in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI) for 4 hours.3,4 To simulate reoxygenation and return to physiological pH during reperfusion, anaerobic KRH at pH 6.2 was replaced with aerobic KRH at pH 7.4. In some experiments, nutrient depletion was induced by incubation of hepatocytes in amino acid–depleted and serum-depleted aerobic KRH at pH 7.4 for 3 hours prior to beginning A/R. Some hepatocytes were treated with 50 μM acetyl-Leu-Leu-methioninal (ALLM; Calbiochem, San Diego, CA) or z-Leu-Leu-Tyr-fluoromethylketone (zLLY-fmk), calpain 2 inhibitors,14,15 or 5-50 μM lacta-cystin or MG-132 (Calbiochem), proteasome inhibitors,16 during A/R.
In Vivo I/R
Total hepatic ischemia in male C57BL/6 mice (20-25 g) was induced by occlusion of the portal triad for 45 minutes, as previously described.17 Reperfusion was initiated by removal of a microvascular clamp. Liver biopsies from the left lateral lobe were collected during I/R and immediately frozen in liquid nitrogen for the analysis of autophagy proteins. Control animals were sham-operated.
Necrosis and Apoptosis Assay
Necrosis and apoptosis were assessed by propidium iodide (PI) fluorometry and caspase 3 western blotting, respectively, as previously described.3
Western Blot Analysis
Hepatocyte lysates were prepared, and expression of Atg7 and Atg12 was detected with primary polyclonal antibodies (a gift from Dr. W.A. Dunn, Jr). Beclin-1 was detected with polyclonal Beclin-1 antibody (Cell Signaling Technology, Danvers, MA). Expression of calpains 1 and 2 and caspase 3 was analyzed by polyclonal antibodies (Cell Signaling Technology). Changes in protein expression were determined with IMAGE J software (National Institutes of Health, Bethesda, MD).
Adenovirus Construction and Transfection
The adenoviruses expressing Atg7 (AdAtg7) and Beclin-1 (Ad-Beclin-1) were constructed with Atg7 complementary DNA (a gift from Dr. W.A. Dunn, Jr) and Beclin-1 complementary DNA (a gift from Dr. B. Levine) with the ViraPower Adenoviral Gateway Expression kit (Invitrogen). Hepatocytes were infected with AdAtg7, AdBeclin-1, and an adenovirus encoding green fluorescent protein–microtubule-associated protein light chain 3 (AdGFP-LC3; a gift from Dr. X.-M. Yin) at the concentration of 10 plaque-forming units/cell in hormonally defined medium.18 For the control infection, hepatocytes were incubated with an adenovirus encoding green fluorescent protein (AdGFP). Infection efficiency was evaluated through the counting of green fluorescent protein–positive hepatocytes from coverslips. More than 250 cells were viewed under an inverted fluorescence microscope (Olympus, Center Valley, PA), and the infection percentage was determined.
Confocal Microscopy
Hepatocytes cultured on glass coverslips were coloaded with 100 nM tetramethylrhodamine methyl ester (TMRM), 1 μM calcein-AM, and 3 μM PI in KRH to monitor the mitochondrial membrane potential, onset of the MPT, and cell death, respectively, as previously described.4 For visualization of lysosomes, hepatocytes were incubated overnight in Waymouth's medium containing 1 mg/mL rhodamine-dextran (70 kDa). Confocal images were collected with a gas-tight Perfusion Open Closed-Reduced (POC-R) chamber (Zeiss, Jena, Germany) using an inverted Zeiss 510 META laser scanning confocal microscope equipped with a 63× oil-immersion planapochromat lens (numerical aperture, 1.4).
For visualization of autophagosomes during anoxia, hepatocytes were infected with AdGFP-LC3 and incubated in anoxic KRH at pH 6.2 in the presence of exogenous oxygen-consuming respiratory complexes (3.3% Oxyrase, Oxyrase, Mansfield, OH) to prevent back-diffusion of oxygen into the gas-tight chamber.5
Measurement of Calpain Activity
The activity of calpains was fluorometrically assessed with a membrane-permeable calpain substrate, 20 μM succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (SLLVY-AMC; Sigma, St. Louise, MO), in the presence and absence of ALLM.19
ATP Measurement
Cellular levels of ATP in cultured rat hepatocytes were luminometrically determined by a luciferin/luciferase ATP assay kit (Promega, Madison WI).3
Data Analysis
Differences between means were compared with analysis of variance with a level of significance of P < 0.05. Data were expressed as means ± standard error. All experiments are representative of at least three different cell isolations.
Results
Loss of Atg7 and Beclin-1 After A/R of Cultured Rat Hepatocytes
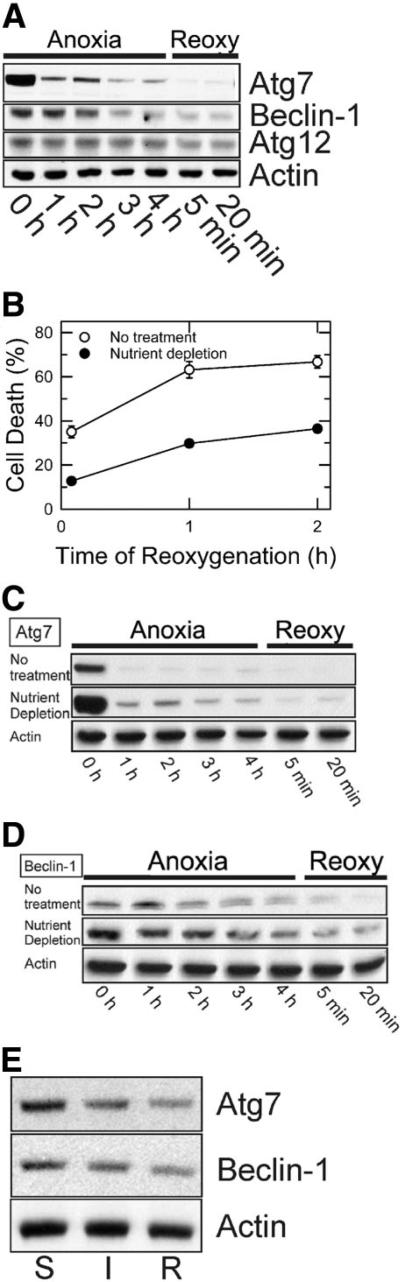
As a first step toward establishing a mechanistic correlation between autophagy and A/R-induced cell death, we determined changes in expression of autophagy proteins, including Atg7, Atg12, and Beclin-1. Western blot analysis from hepatocyte lysates showed a marked decrease in Atg7 after a short period of anoxia. Densitometric analysis revealed that after 1 hour of anoxia, Atg7 decreased to 15.1% ± 2.5% (n = 5) of normal values, whereas Beclin-1 remained unchanged (Fig. 1A). Prolonged anoxia caused a substantial loss of both Atg7 and Beclin-1. After 4 hours of anoxia, Atg7 and Beclin-1 decreased to 8.5% ± 0.7% and 31.9% ± 9.3% of normal values, respectively. Reoxygenation did not recover expression of Atg7 and Beclin-1. After 20 minutes, Atg7 and Beclin-1 further decreased to 0.7% ± 0.1% and 11.3% ± 2.3%, respectively. In contrast, A/R caused a minor change in Atg12.
Fig. 1.
Suppression of Atg7 and Beclin-1 loss by nutrient depletion. Hepatocytes were incubated in anaerobic KRH buffer at pH 6.2 for up to 4 hours to simulate ischemia and then reoxygenated in aerobic KRH at pH 7.4 to simulate reperfusion. Nutrient depletion was simulated by incubation of cells in aerobic KRH at pH 7.4 for 3 hours prior to anoxia. (A) Atg7, Beclin-1, and Atg12 expression during A/R was analyzed by western blotting. (B) After 4 hours of anoxia in the presence and absence of nutrient depletion, hepatocytes were reoxygenated for 2 hours. Necrosis was measured by PI fluorometry. Values are means ± standard error. Cell death was significantly reduced by nutrient depletion (P < 0.0001 versus no treatment). Changes in (C) Atg7 and (D) Beclin-1 expression in the presence and absence of nutrient depletion were determined by western blotting. (E) Mouse livers were exposed to 45 minutes of total ischemia (I) and subsequently 30 minutes of reperfusion (R). Changes in Atg7 and Beclin-1 were analyzed by western blotting and compared with a sham control (S).
Protection by Nutrient Depletion Against A/R Injury
After 4 hours of anoxia, hepatocytes were reoxygenated for 2 hours, and necrosis was fluorometrically determined. Necrosis increased to more than 66% within 2 hours (Fig. 1B). To determine if enhanced autophagy could abrogate cell death, hepatocytes were exposed to nutrient depletion, a condition stimulating autophagy.8,20 Cell death was markedly diminished by nutrient depletion (Fig. 1B). Autophagic stimulation after nutrient depletion was confirmed by western blotting (Fig. 1C,D). Although nutrient depletion substantially raised initial expression of Atg7 and Beclin-1, both proteins were decreased during A/R, and this suggests that the cellular events responsible for autophagic protein loss are not affected by nutrient depletion. Expression of other proteins such as total and phosphorylated Akt and extracellular signal-regulated kinase 1/2, however, did not change after nutrient depletion (data not shown). Cumulatively, these results suggest that cytoprotection by nutrient depletion is associated with mechanisms other than classic survival pathways. Instead, enhanced autophagy is likely to protect hepatocytes against A/R injury.
Loss of Autophagy Proteins After In Vivo I/R
To investigate whether loss of autophagy proteins also occurs after in vivo I/R, expression of Atg7 and Beclin-1 was assessed after I/R (Fig. 1E). After 45 minutes of ischemia, Atg7 and Beclin-1 decreased to 58% and 66%, respectively, of a sham control. Reperfusion for 30 minutes further decreased Atg7 and Beclin-1 to 38.1% and 40.3%, respectively.
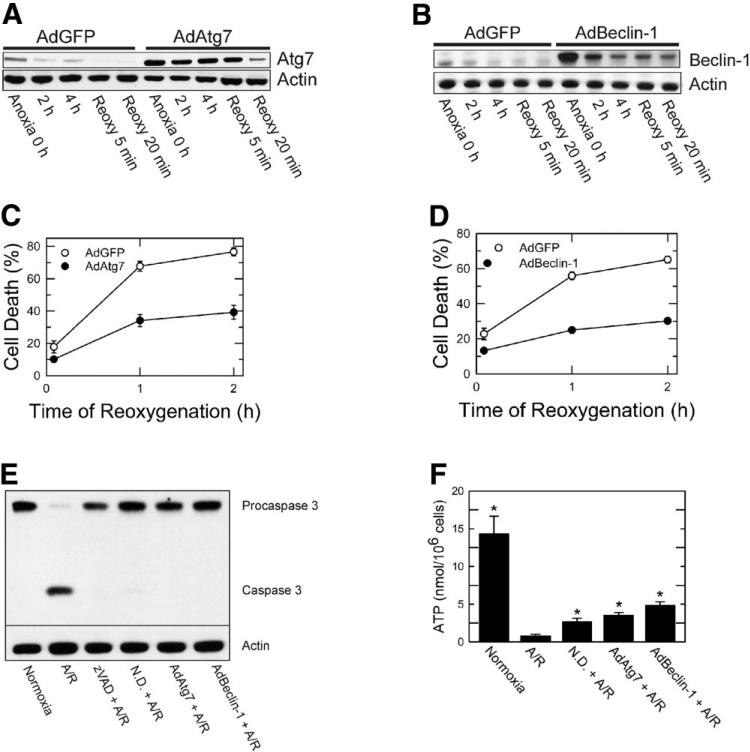
Protection by Adenoviral Overexpression of Atg7 or Beclin-1 Against A/R Injury
To investigate specific roles of Atg7 and Beclin-1 in reoxygenation-induced cell death, hepatocytes were infected with AdAtg7 or AdBeclin-1. AdGFP was used for viral control. Infection efficiency was over 95% (data not shown). AdAtg7 infection increased Atg7 expression by 280% (0 hour of anoxia) compared to AdGFP (Fig. 2A). Similarly, Beclin-1 expression increased by 300% after AdBeclin-1 treatment (Fig. 2B).
Fig. 2.
Cytoprotection by AdAtg7 and AdBeclin-1 against A/R. Hepatocytes were infected with AdAtg7 or AdBeclin-1 (10 plaque performing units/cell) and then subjected to A/R. AdGFP was used for viral control. Western blot analysis of (A) Atg7 and (B) Beclin-1 was performed during A/R. Infection of hepatocytes with (C) AdAtg7 or (D) AdBeclin-1 prevented reoxygenation-induced necrotic death (P < 0.0001 versus AdGFP). Values are means ± standard error. (E) Hepatocytes were subjected to 4 hours of anoxia and subsequently reoxygenated for 9 hours. Western blot analysis of procaspase 3 and caspase 3 was performed under the conditions of normoxia, A/R, zVAD (50 μM), nutrient depletion (N.D.), and adenoviral overexpression. (F). Hepatocellular ATP concentration was determined after 20 minutes of reoxygenation. *P < 0.05 versus A/R.
To examine whether overexpression of autophagy proteins confers cytoprotection, hepatocytes infected with AdAtg7 or AdBeclin-1 were subjected to A/R, and necrosis was determined. Overexpression of either Atg7 or Beclin-1 significantly reduced necrosis (Fig. 2C,D; P < 0.0001). Therefore, overexpression of either Atg7 or Beclin-1 was cytoprotective against lethal reoxygenation injury to hepatocytes.
Inhibition of A/R-Mediated Apoptosis by Autophagy
Because A/R also causes caspase 3–dependent hepatocellular apoptosis,3 we determined whether autophagy augmentation inhibits caspase 3 activation (Fig. 2E). Reoxygenation of anoxic hepatocytes for 9 hours caused a cleavage of inactive procaspase 3 (35 kDa) to active caspase 3 (17 kDa). Caspase 3–dependent apoptosis was, however, blocked by N-benzyloxycarbonyl-valyl-alanylaspartyl-fluoromethylketone (zVAD), a pan caspase inhibitor, nutrient depletion, and overexpression of Atg7 or Beclin-1. Thus, these data strongly suggest that autophagy prevents caspase 3–dependent apoptosis after A/R.
Effect of Autophagy Stimulation on ATP
We also examined how autophagy stimulation affects ATP after A/R (Fig. 2F). After 4 hours of anoxia and 20 minutes of reoxygenation, hepatocellular ATP decreased from 14. 3 ± 2.37 to 0.77 ± 0.26 nmol/106 cells. However, when hepatocytes were exposed to nutrient depletion and either AdAtg7 or AdBeclin-1 prior to A/R, 18%-34% of basal ATP was restored after reoxygenation. Thus, all three manipulations that stimulate autophagy substantially increased ATP content.
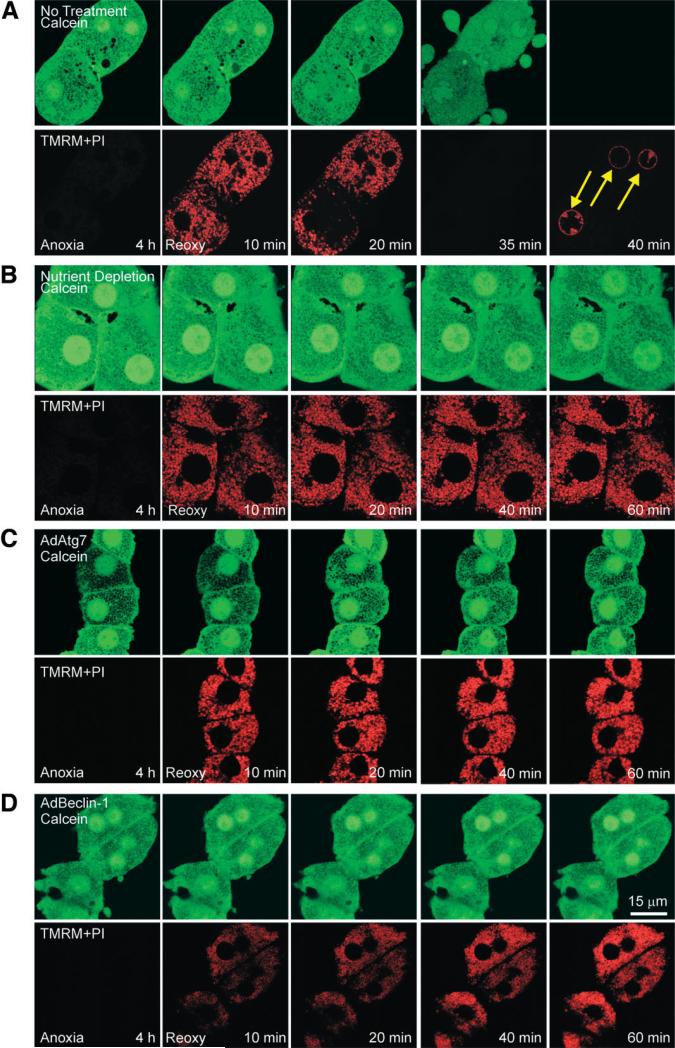
Blockade of the MPT by Enhanced Autophagy
Onset of the MPT is the causative mechanism of hepatocyte death after A/R.3 Because nutrient depletion and adenoviral overexpression both prevented hepatocyte death, we examined whether cytoprotection is associated with blockade of the MPT. The MPT, membrane potential, and cell death were monitored with laser scanning confocal microscopy of calcein, TMRM, and PI, respectively.4 Calcein is a green-fluorescing dye and loads into the cytosol and nucleus but is excluded by normal mitochondria that have closed permeability transition pores.4 TMRM electrophoretically accumulates into mitochondria in response to the negative mitochondrial membrane potential.4 PI labels nuclei only when the integrity of plasma membranes is lost.4 Because polarized mitochondria take up TMRM while simultaneously excluding green-fluorescing calcein, mitochondria appear as small, dark, and round voids, where each void represents a single, polarized mitochondrion.
After 4 hours of anoxia, TMRM fluorescence was barely detectable (Fig. 3A). However, at the same time, calcein was excluded by mitochondria, and this indicated that permeability transition pores were remaining closed during anoxia. When anoxic hepatocytes were reoxygenated, these mitochondria began to take up TMRM within 10 minutes. However, after 20 minutes, mitochondria of one hepatocyte began to lose TMRM fluorescence; this event was indicative of mitochondrial depolarization (Fig. 3A). Subsequently, all mitochondria lost TMRM at 35 minutes. As mitochondria lost membrane potential, calcein redistributed from cytosol to mitochondria, and this indicated the MPT. After 35 minutes, dark mitochondrial voids were completely filled with calcein, showing widespread MPT. Numerous blebs were observed at this time. A few minutes later, calcein fluorescence disappeared, and PI labeled nuclei (arrows) because of the failure of the plasma membrane barrier and cell death.
Fig. 3.
MPT inhibition by enhanced autophagy. After 4 hours of anoxia, hepatocytes were reoxygenated, and confocal images of calcein (green) and TMRM and PI (red) were simultaneously collected at 10, 20, 40, and 60 minutes. (A) Hepatocytes were reoxygenated with no other treatment. At the end of anoxia, calcein was excluded by mitochondria, and this was indicative of permeability pore closure. After 10 minutes of reoxygenation, mitochondria became repolarized without calcein redistribution into mitochondria. After 20 minutes, calcein redistributed into mitochondria, indicating onset of the MPT, and TMRM began to disappear, indicating mitochondrial depolarization. Within minutes, cytosolic calcein was lost, and PI labeled nuclei (arrows), indicating cell death. (B) Under the condition of nutrient depletion, mitochondrial voids in the calcein fluorescence were persistently present, and TMRM was continuously evident throughout reoxygenation. (C) AdAtg7 or (D) AdBeclin-1 infection prevented the MPT and cell death.
Reoxygenation of nutrient-depleted hepatocytes demonstrated persistent repolarization of mitochondria (Fig. 3B). Moreover, mitochondria continued to exclude calcein, and hepatocytes were viable. These results demonstrated that nutrient depletion inhibited onset of the MPT and necrotic death after A/R. Similarly, adenoviral overexpression of both Atg7 and Beclin-1 promoted sustained mitochondrial repolarization and blocked the MPT onset after reoxygenation (Fig. 3C,D). Collectively, all three manipulations stimulating autophagy prevented MPT-dependent necrosis after A/R.
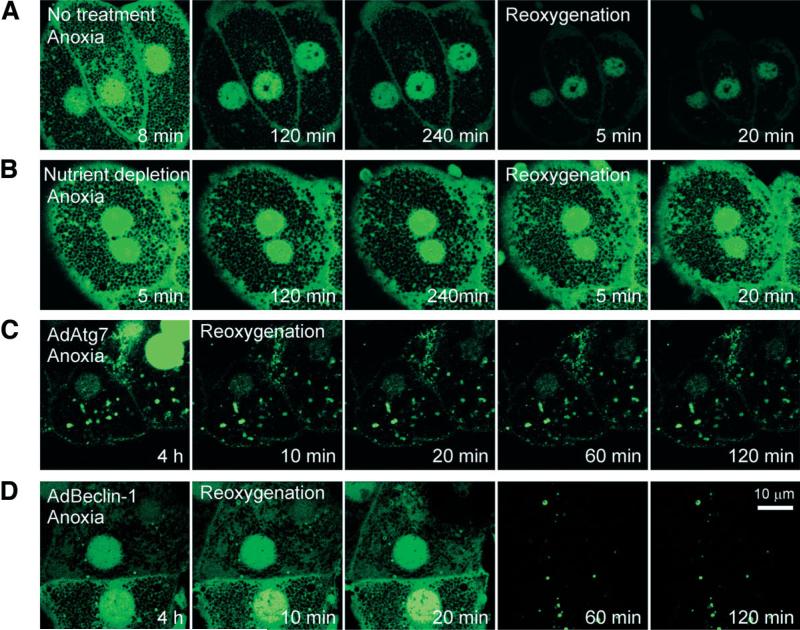
Changes in Autophagosome Formation After A/R
Stimulation of autophagy converts LC3 I to phosphatidylethanolamine-conjugated LC3 II by Atg7.21 LC3 II has widely been used as a biomarker of autophagosome formation.21 To confirm defective autophagy after A/R, hepatocytes were infected with AdGFP-LC3. After 8-12 hours of culture, a robust expression of green fluorescent protein–microtubule-associated protein light chain 3 (GFP-LC3) was evident. Some autophagosomes, as characterized by punctate, green-fluorescing structures,21 were detected after 8 minutes of anoxia (Fig. 4A). Diffuse green fluorescence was also observed both in nuclei and in the area near plasma membranes; this result was similar to that of a previous study.22 Prolonged anoxia, however, markedly decreased the number of autophagosomes, which was not recovered by reoxygenation (Fig. 4A). Contrarily, nutrient depletion substantially increased the initial number of autophagosomes (Fig. 4B). After 2 hours of anoxia, fluorescence in both cytosolic and nuclear compartments decreased, but punctate structures remained evident during anoxia and even after reoxygenation. Thus, these results not only confirmed autophagic stimulation by nutrient depletion but support our conclusion that autophagy is impaired during A/R.
Fig. 4.
Autophagosome formation after A/R. Hepatocytes infected with AdGFP-LC3 were exposed to A/R, and changes in autophagosome formation were monitored by confocal microscopy. (A) After 8 minutes of anoxia, autophagosomes (green-fluorescing, punctate structures) were evident. After prolonged anoxia, most autophagosomes disappeared. Note a further decrease in autophagosomes after reoxygenation. (B) Nutrient depletion delayed loss of autophagosome. Autophagosomes were persistently present in hepatocytes coinfected with (C) AdAtg7 or (D) AdBeclin-1.
Hepatocytes coinfected with AdGFP-LC3 and AdAtg7 showed numerous autophagosomes after 4 hours of anoxia (Fig. 4C). Although nuclear fluorescence was lost after reoxygenation, many autophagosomes were persistently present. In hepatocytes coinfected with AdGFP-LC3 and AdBeclin-1, a robust green fluorescence in cytosol and nuclei persisted during the early phase of reoxygenation but disappeared at later time points (Fig. 4D). Taken together, these results showed that overexpression of Atg7 or Beclin-1 considerably suppressed A/R-dependent depletion of autophagosomes.
Autophagy and the MPT
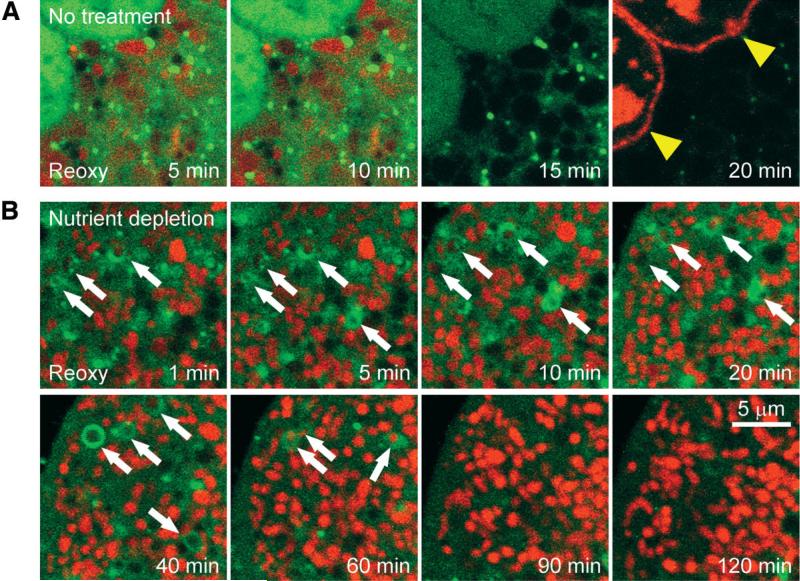
Because the MPT has been proposed as a molecular signal initiating mitophagy,20 we investigated the cause-and-effect role of the MPT in mitophagy. Hepatocytes infected with AdGFP-LC3 were subjected to A/R with TMRM and PI. Some hepatocytes were exposed to nutrient depletion. In the control group (no treatment), mitochondria depolarized after a brief repolarization (Fig. 5A). The cell then lost viability (arrowheads). Although green-fluorescing, dotlike structures were detected, those were substantially smaller in size than the individual mitochondria and furthermore did not colocalize with mitochondria, suggesting overall failure of mitophagy. In the nutrient depletion group, some mitochondria were enveloped by autophagosomes, green-fluorescing, ringlike structures (Fig. 5B, arrows). Surprisingly, the lumen of autophagosomes contained red-fluorescing (polarized) mitochondria. These mitochondria remained polarized until 40 to 60 minutes of reoxygenation. After 90 minutes, autophagosomes disappeared, but the cell remained viable. Because mitochondrial depolarization signifies the MPT, sequestration of polarized mitochondria in autophagosomes strongly suggests that autophagosome formation precedes onset of the MPT and, importantly, signaling pathways other than the MPT likely trigger mitophagy. Additionally, these results demonstrated that mitophagy protected hepatocytes against lethal A/R injury and strongly support our conclusion that defects in autophagy cause the MPT-dependent necrosis after A/R.
Fig. 5.
Sequestration of polarized mitochondria in autophagosomes. Confocal images of AdGFP-LC3, TMRM, and PI were collected after reoxygenation. (A) In the control group, green-fluorescing structures surrounding repolarized mitochondria were markedly smaller than the neighboring mitochondria, and this indicated mitophagic failure. Mitochondria released TMRM, and the cell lost viability thereafter (arrowheads). (B) After nutrient depletion, some mitochondria after reoxygenation were enveloped by autophagosomes but remained polarized until 40 to 60 minutes (arrows).
A Minor Role of Lysosomes in Impaired Autophagy After A/R
To examine whether impaired autophagy results from defective lysosomes, hepatocytes were loaded with rhodamine-dextran and subjected to A/R. Rhodamine-dextran (70 kDa) is taken up by endocytosis to become concentrated in lysosomes.23 Confocal images showed that at 6 minutes of anoxia, many punctate, redfluorescing lysosomes were distributed throughout cytoplasm with a preferential accumulation in the perinuclear region (Fig. 6). The number and location of lysosomes remained unchanged during 4 hours of anoxia and in the early phase of reoxygenation. A substantial loss occurred after 30 minutes of reoxygenation, and cell death occurred thereafter (Fig. 6, arrows). Because lysosomal rupture is preceded by the MPT in hypoxic hepatocytes,24 persistent appearance of lysosomes suggests a minor role of lysosomes in dysfunctional autophagy after A/R. Instead, defective autophagosome formation as a consequence of Atg7 and Beclin-1 depletion is likely to cause failure of autophagy.
Fig. 6.
Lysosomes after A/R. Hepatocytes were labeled with rhoda-mine dextran and PI and subsequently subjected to A/R. The number and location of the lysosomes were assessed by confocal microscopy. At 35 minutes of reoxygenation, nuclei were labeled with PI (arrows).
Degradation of Atg7 and Beclin-1 by Calpains
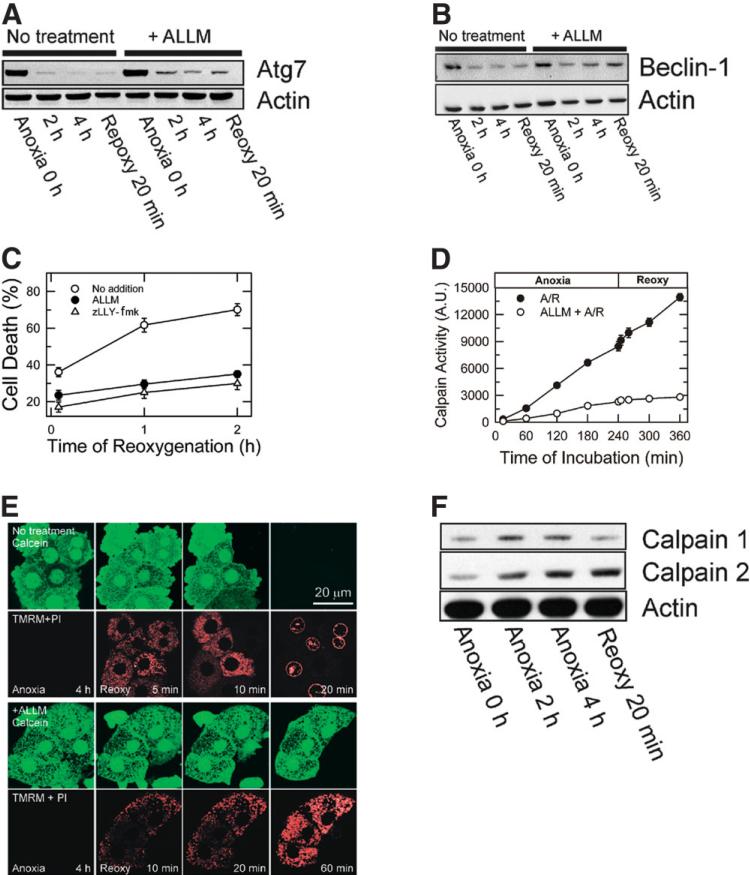
To investigate the role of calpains in Atg7 and Beclin-1 depletion, hepatocytes were incubated with 50 μM ALLM or zLLY-fmk, membrane-permeable calpain isoform 2 inhibitors.14,15 After subjection to A/R, expression of Atg7 and Beclin-1 was analyzed. Calpain 2 inhibition substantially suppressed loss of Atg7 and Beclin-1(Fig. 7A,B) and prevented cell death (Fig. 7C). However, ALLN and PD151746, specific inhibitors of calpain isoform1, did not prevent loss of autophagy proteins or necrosis (data not shown). In additional experiments, we examined the effects of lactacystin and MG-132, proteasome inhibitors, on Atg7 and Beclin-1 expression and cell death. However, these proteasome inhibitors failed to suppress loss of autophagy proteins and hepatocyte death (data not shown), and this suggested that proteasomes did not contribute to autophagy protein loss.
Fig. 7.
Suppression of Atg7 and Beclin-1 loss by calpain 2 inhibition. Hepatocytes were subjected to A/R in the presence and absence of 50 μM ALLM or zLLY-fmk, specific calpain 2 inhibitors. Changes in (A) Atg7 and (B) Beclin-1 were determined by western blotting. (C) Inhibition of calpain 2 prevented cell death after reoxygenation (P < 0.0001 versus no treatment). (D) Calpain activity was determined by SLLVY-AMC fluorometry. (E) Confocal images of calcein and TMRM in control (upper panels) and ALLM-treated cells (lower panels). (F) Changes in calpain 1 and 2 expression were analyzed by western blotting after A/R.
To further investigate whether A/R alters calpains, changes in calpain activity were fluorometrically determined with SLLVY-AMC.19,25 Cleavage of the amide bond by calpains liberates a highly fluorescent AMC molecule. Calpain activity increased by 25-fold and 41-fold after 4 hours of anoxia and after 2 hours of reoxygenation, respectively, compared to the activity at 15 minutes of anoxia (Fig. 7D). Incubation of hepatocytes with ALLM blocked A/R-dependent calpain stimulation to a level comparable to that of normoxic hepatocytes (data not shown). The cytoprotective effect of ALLM was directly associated with its inhibition of the MPT because ALLM blocked the MPT onset, mitochondrial depolarization, and cell death after reoxygenation (Fig. 7E). The importance of calpain isoform 2 was further evaluated with western blotting (Fig. 7F). Calpain 2 expression increased to 344.6% and 417.8% after 4 hours of anoxia and after 20 minutes of reoxygenation, respectively. Although calpain 1 expression was elevated during anoxia, reoxygenation decreased expression of calpain 1 to basal levels. Thus, these results showed an integral role of calpain 2 in reoxygenation injury to hepatocytes.
Discussion
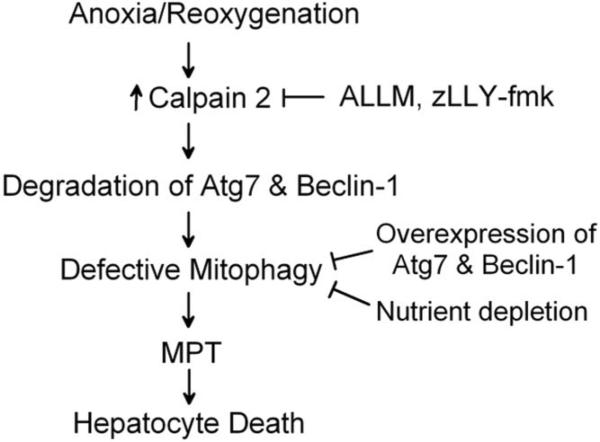
I/R injury has a profound impact on the burden of liver diseases, but efforts to improve liver function after I/R have not been successful, mostly because of an incomplete understanding of the pathogenesis of I/R injury. Although the liver constitutively expresses a robust mitochondrial quality control system, namely mitophagy, reoxygenation of anoxic hepatocytes causes the MPT and mitochondrial dysfunction. Our findings reveal that cal-pain 2 activation during simulated I/R degrades key autophagy proteins, Atg7 and Beclin-1, leading to impaired autophagy. Furthermore, I/R of in vivo livers substantially decreases both proteins. Consequently, defective autophagy culminates in onset of the MPT and hepatocyte death after reoxygenation (Fig. 8).
Fig. 8.
Scheme of A/R-induced impairment of mitophagy. Stimulation of calpain 2 after A/R hydrolyzes Atg 7 and Beclin-1, causing defective mitophagy. Because impaired mitophagy fails to remove dysfunctional mitochondria, the mitochondria laden with ROS and calcium undergo the MPT, which in turn leads to uncoupling of oxidative phosphorylation, energetic failure, ATP depletion, and ultimately cell death. Because the MPT causes cell death after A/R, blockade of the MPT onset by calpain 2 inhibition prevents cell death. Likewise, enhanced autophagy by nutrient depletion and Atg7 or Beclin-1 overexpression increases auto-phagy expression, which facilitates the formation of autophagosomes and mitophagy, leading to cell survival.
Atg7 is a ubiquitin E1-like protein that activates a glycine residue of carboxy terminal in Atg12 and generates an Atg5-Atg12-Atg16 complex, a structure essential for autophago-some formation.12 Atg7 also plays a major role in activation of LC3. Therefore, loss of Atg7 would impair autophagosome formation, and this was confirmed by confocal imaging of virally infected hepatocytes expressing GFP-LC3 (Figs. 4 and 5). The importance of Atg7 has recently been reported in Atg7-deficient transgenic animals that develop a neurodegenerative disorder and accumulate abnormal mitochondria in the liver,26 and this is consistent with our results. Similar to Atg7, the depletion of Beclin-1 caused A/R injury to hepatocytes (Figs. 1 and 2). The cytoprotective role of Beclin-1 has been shown in A/R injury to the HL-1 cardiomyocyte cell line.27
Our results imply that depletion of Atg7 and Beclin-1 is caused by calpains, especially isoform 2 (Fig. 7). Although lysosomal defects could be attributed to impaired autophagy, confocal imaging suggests a minor role of lysosomes in dysfunctional mitophagy (Fig. 6). Instead, defective autophagosome formation as a consequence of Atg7 and Beclin-1 depletion causes failure of mitophagy. In I/R of liver, inhibition of calpains improves tissue viability and hepatic function.28,29 Moreover, calpains have been recently suggested to play an integral role in regulation of autophagy.30 Because calcium is overloaded after I/R, Ca2+-activated calpains may hydrolyze autophagy proteins. However, calpain inhibition alone did not fully recover Atg7 and Beclin-1 expression (Fig. 7A,B). Thus, calpain 2 is, at least in part, involved in loss of autophagy proteins in A/R, but other factors are also likely contributing. Identification of these factors warrants future investigation.
In hepatocytes, Atg7 was diminished by over 80% during 1 hour of anoxia, whereas a substantial decrease in Beclin-1 became evident after prolonged anoxia (Fig. 1). It remains to be elucidated why Atg7 is more vulnerable to anoxia than Beclin-1. Western blotting of normoxic hepatocytes in the presence of cycloheximide, an inhibitor of protein synthesis,11 showed that a half-life time of Atg7 and Beclin-1 synthesis is on the order of minutes and hours, respectively, suggesting a rapid protein turnover of Atg7 (data not shown). Because ATP is required for autophagosome formation, lack of ATP during anoxia, together with a fast turnover of Atg7, is likely to inhibit new protein synthesis, leading to a net loss of Atg7. However, stable expression of Beclin-1 with a slower turnover may permit tolerance against anoxic stress. Alternatively, calpains may preferentially cleave Atg7 over other proteins. In neutrophils, calpains rapidly cleave Atg5 to liberate a 24-kDa fragment, whereas Beclin-1 is resistant to calpain-dependent proteolysis;31 this suggests a varying sensitivity of autophagy proteins to calpains. One interesting finding is that overexpression of either Atg7 or Beclin-1 alone was sufficient to prevent MPT-dependent necrosis and apoptosis, although infection of Atg7 did not affect expression of Beclin-1 or vice versa, suggesting that Atg7 and Beclin-1 form autophagosomes in a separate pathway, but the signaling pathway afterwards may converge at an event regulating mitochondrial functional integrity.
Signaling molecules that initiate mitophagy remain elusive. In this study, we used an adenovirus to express GFPLC3 in rat hepatocytes, a model ideal for imaging the autophagy process.22 Time-lapse confocal microscopy showed that individual mitochondria sequestered in the lumen of autophagosomes maintained mitochondrial membrane potential for more than 40 minutes (Fig. 5B). Although the MPT has been proposed as a cellular signal triggering mitophagy in normoxic conditions,20 our data suggest that signals other than the MPT contribute to triggering mitophagy after A/R. Although future study is needed to characterize the molecular signal, a recent study suggested that MPT onset increases mitophagy only under the conditions of low-intensity stresses such as starvation and normoxia.32 Additionally, in GFP-LC3 hepatocytes, formation of autophagosomes after photodamage is not inhibited by cyclosporin A, an MPT blocker.32 Thus, under the conditions of severe stresses such as A/R, events upstream of the MPT may initiate mitophagy. ROS, adenosine monophosphate–activated protein kinase, and calcium have been recently proposed to regulate autophagy.33-35
Whether autophagy protects or promotes tissue injury in different pathological conditions is the subject of ongoing controversy. Overexpression of Atg7 or Beclin-1 prevented both necrosis and caspase 3–mediated apoptosis (Fig. 2) and stimulated ATP recovery after A/R (Fig. 2F). Moreover, overexpression of either Atg7 or Beclin-1 did not induce apoptosis (Fig. 2E). Cumulatively, our results support the protective role of autophagy against cell death. Timely removal of abnormal mitochondria by mitophagy is indispensable to hepatocellular function. Accelerated generation of ROS from defective mitochondria has great potential to harm neighboring normal mitochondria and ultimately causes cell death. ROS formation promotes the MPT.5 Because mitochondrial DNA is prone to ROS-mediated stress due to a weak DNA repair system, defective mitophagy leads to accumulation of ROS, DNA mutation, and cell injury. On the other hand, excessive autophagy may be cytotoxic because uncontrolled clearance of cellular constituents and organelles would deplete proteins and metabolites essential for normal cellular function. Additionally, unregulated release of lysosomal enzymes such as cathepsins and acid pro-teases may injure tissues.
In conclusion, A/R of hepatocytes causes loss of both Atg7 and Beclin-1 by calpain 2 activation, which in turn limits mitophagy. Defective mitophagy subsequently culminates in onset of the MPT and ultimately cell death after reoxygenation. Stimulation of autophagy through nutrient depletion and overexpression of Atg7 or Beclin-1 inhibits MPT-dependent hepatocyte necrosis and apoptosis and enhances ATP recovery after reoxygenation. Thus, strategies enhancing mitophagy may be a novel approach to improve hepatocyte viability and function after I/R injury.
Acknowledgment
We thank Dr. Beth Levine and Dr. Xiao-Ming Yin for Beclin-1 complementary DNA and adenovirus expressing GFP-LC3, respectively. We are also grateful to Dr. John J. Lemasters and Dr. Insil Kim for technical help.
Supported by the Research Career Development Award of the University of Florida.
Abbreviations
- AdAtg7
adenovirus encoding autophagy-related protein 7
- AdBeclin-1
adenovirus encoding Beclin-1
- AdGFP
adenovirus encoding green fluorescent protein
- AdGFP-LC3
adenovirus encoding green fluorescent protein–microtubule-associated protein light chain 3
- ALLM
acetyl-Leu-Leu-methioninal
- A/R
anoxia/reoxygenation
- Atg
autophagy-related protein
- ATP
adenosine triphosphate
- GFP-LC3
green fluorescent protein–microtubule-associated protein light chain 3
- I/R
ischemia/reperfusion
- KRH
Krebs–Ringer–hydroxyethylpiperazine-N-2 ethanesulfonic acid
- LC3
microtubule-associated protein light chain 3
- MPT
mitochondrial permeability transition
- PI
propidium iodide
- ROS
reactive oxygen species
- SLLVY-AMC
succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin
- TMRM
tetramethylrhodamine methyl ester
- zLLY-fmk
z-Leu-Leu-Tyr-fluoromethylketone
- zVAD
N-benzyloxycarbonyl-valyl-alanyl-aspartyl-fluoromethylketone
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3:527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim J-S, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 4.Kim J-S, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. HEPATOLOGY. 2004;39:1533–1543. doi: 10.1002/hep.20197. [DOI] [PubMed] [Google Scholar]
- 5.Kim J-S, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia/reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 6.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 7.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 8.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortimore GE, Poso AR, Lardeux BR. Mechanism and regulation of protein degradation in liver. Diabetes Metab Rev. 1989;5:49–70. doi: 10.1002/dmr.5610050105. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle DL, Lewin AS, Dunn WA., Jr Selective autophagy of peroxisomes in methylotrophic yeasts. Eur J Cell Biol. 1993;60:283–290. [PubMed] [Google Scholar]
- 11.Kissova I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–39074. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 12.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 13.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 14.Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun. 2002;291:321–331. doi: 10.1006/bbrc.2002.6453. [DOI] [PubMed] [Google Scholar]
- 15.Dutt P, Arthur JS, Croall DE, Elce JS. m-Calpain subunits remain associated in the presence of calcium. FEBS Lett. 1998;436:367–371. doi: 10.1016/s0014-5793(98)01167-3. [DOI] [PubMed] [Google Scholar]
- 16.Mezey E, Rennie-Tankersley L, Potter JJ. Liver alcohol dehydrogenase is degraded by the ubiquitin-proteasome pathway. Biochem Biophys Res Commun. 2001;285:644–648. doi: 10.1006/bbrc.2001.5226. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Kubota S, Nagaya M, Ozaki S, Nagafuchi H, Akashi K, et al. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124:59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Hatano E, Bradham CA, Stark A, Iimuro Y, Lemasters JJ, Brenner DA. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J Biol Chem. 2000;275:11814–11823. doi: 10.1074/jbc.275.16.11814. [DOI] [PubMed] [Google Scholar]
- 19.Bronk SF, Gores GJ. pH-dependent nonlysosomal proteolysis contributes to lethal anoxic injury of rat hepatocytes. Am J Physiol. 1993;264(pt 1):G744–G751. doi: 10.1152/ajpgi.1993.264.4.G744. [DOI] [PubMed] [Google Scholar]
- 20.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic. 2006;7:129–145. doi: 10.1111/j.1600-0854.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 23.Gores GJ, Nieminen AL, Wray BE, Herman B, Lemasters JJ. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989;83:386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahrebelski G, Nieminen AL, al Ghoul K, Qian T, Herman B, Lemasters JJ. Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study. HEPATOLOGY. 1995;21:1361–1372. [PubMed] [Google Scholar]
- 25.Sasaki T, Kikuchi T, Yumoto N, Yoshimura N, Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984;259:12489–12494. [PubMed] [Google Scholar]
- 26.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 28.Kohli V, Madden JF, Bentley RC, Clavien PA. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999;116:168–178. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Sakon M, Umeshita K, Okuyama M, Shiozaki K, Nagano H, et al. Prednisolone suppresses ischemia-reperfusion injury of the rat liver by reducing cytokine production and calpain mu activation. J Hepatol. 2001;34:278–283. doi: 10.1016/s0168-8278(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 30.Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, et al. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 32.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 35.Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K(+)/H(+) exchange activity, osmotic swelling and mitophagy. Cell Death Differ. 2007;14:1647–1656. doi: 10.1038/sj.cdd.4402167. [DOI] [PubMed] [Google Scholar]