Abstract
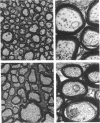
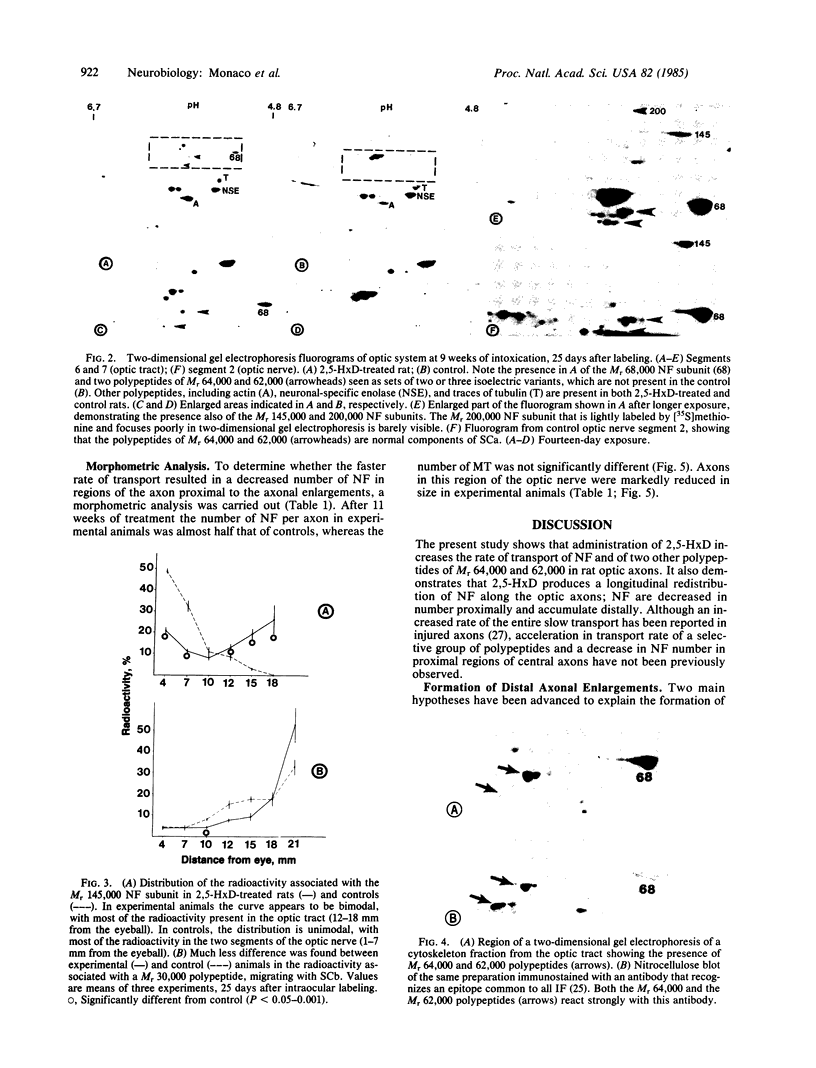
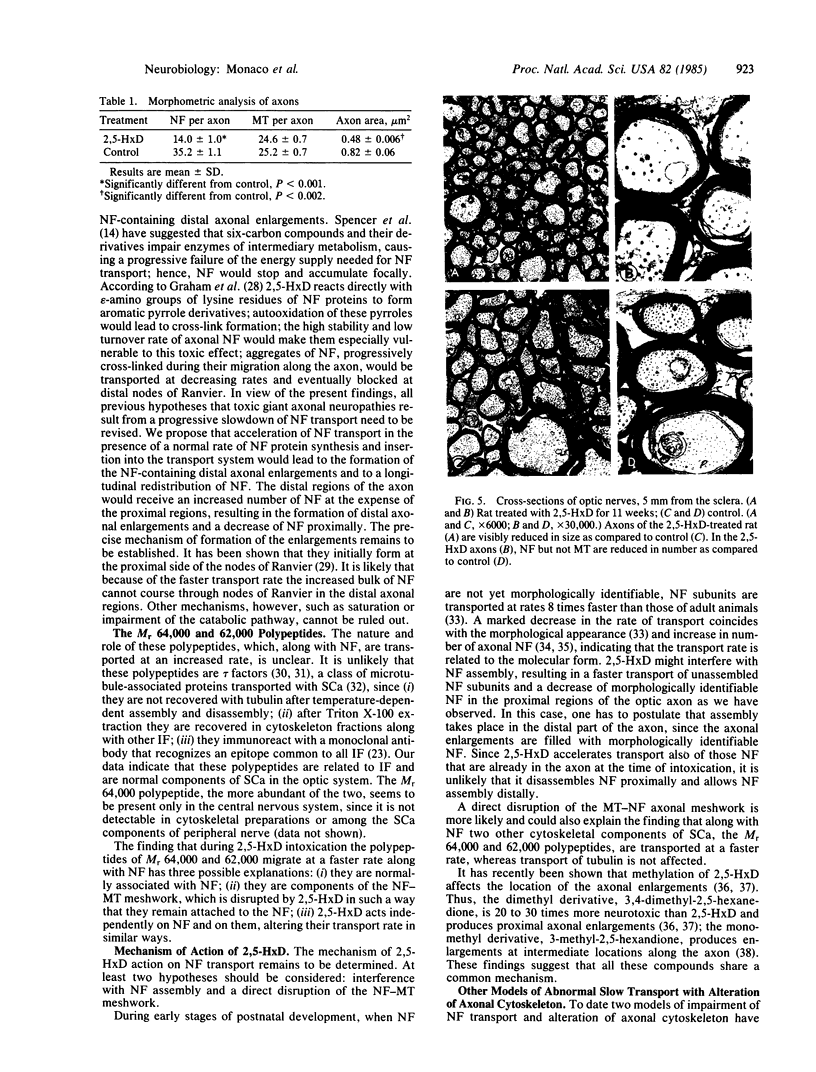
Giant axonal neuropathies are a group of acquired and inherited human diseases morphologically characterized by accumulation of neurofilaments (NF) in enlargements of preterminal regions of central and peripheral axons. Slow axonal transport was studied in the optic systems of rats treated with 2,5-hexanedione, a toxic compound that produces an experimental model of giant axonal neuropathy. The transport rate of NF and of two other polypeptides of Mr 64,000 and 62,000 were selectively increased. Other components of the slow axonal transport were not affected. Acceleration of labeled NF was also observed when 2,5-hexanedione was given after [35S]methionine administration. Morphometric analysis revealed that the number of NF and the axon size were decreased in regions of optic axons proximal to the enlargements. It is suggested that acceleration of NF transport leads to a longitudinal redistribution of NF: NF decrease proximally and increase distally, forming NF-containing enlargements. Evidence was obtained that polypeptides of Mr 64,000 and 62,000 are cytoskeletal components related to intermediate filaments, normally migrating with the component a of the slow axonal transport. The 2,5-hexanedione axon may provide insight into the pathogenesis of inherited and acquired giant axonal neuropathies and offers a model to investigate the relationship between number of NF and axonal size in central axons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N., Mendell J. R., Billmaier D. J., Fontaine R. E., O'Neill J. Toxic polyneuropathy due to methyl n-butyl ketone. An industrial outbreak. Arch Neurol. 1975 Apr;32(4):209–218. doi: 10.1001/archneur.1975.00490460025001. [DOI] [PubMed] [Google Scholar]
- Anthony D. C., Boekelheide K., Anderson C. W., Graham D. G. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. II. Dimethyl substitution accelerates pyrrole formation and protein crosslinking. Toxicol Appl Pharmacol. 1983 Dec;71(3):372–382. doi: 10.1016/0041-008x(83)90024-8. [DOI] [PubMed] [Google Scholar]
- Anthony D. C., Boekelheide K., Graham D. G. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. I. Accelerated clinical neuropathy is accompanied by more proximal axonal swellings. Toxicol Appl Pharmacol. 1983 Dec;71(3):362–371. doi: 10.1016/0041-008x(83)90023-6. [DOI] [PubMed] [Google Scholar]
- Asbury A. K., Gale M. K., Cox S. C., Baringer J. R., Berg B. O. Giant axonal neuropathy--a unique case with segmental neurofilamentous masses. Acta Neuropathol. 1972;20(3):237–247. doi: 10.1007/BF00686905. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Sipple J., Sudilovsky O., Gambetti P. Intermediate filaments of Schwann cells. J Neurochem. 1982 Mar;38(3):774–780. doi: 10.1111/j.1471-4159.1982.tb08698.x. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Velasco M. E., Sipple J., Gambetti P. Immunochemical characterization of antisera to rat neurofilament subunits. J Neurochem. 1981 Nov;37(5):1260–1265. doi: 10.1111/j.1471-4159.1981.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Bizzi A., Crane R. C., Autilio-Gambetti L., Gambetti P. Aluminum effect on slow axonal transport: a novel impairment of neurofilament transport. J Neurosci. 1984 Mar;4(3):722–731. doi: 10.1523/JNEUROSCI.04-03-00722.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M., Lasek R. J. Slow components of axonal transport: two cytoskeletal networks. J Cell Biol. 1980 Aug;86(2):616–623. doi: 10.1083/jcb.86.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J. Nerve-specific enolase and creatine phosphokinase in axonal transport: soluble proteins and the axoplasmic matrix. Cell. 1981 Feb;23(2):515–523. doi: 10.1016/0092-8674(81)90147-1. [DOI] [PubMed] [Google Scholar]
- CHOU S. M., HARTMANN H. A. AXONAL LESIONS AND WALTZING SYNDROME AFTER IDPN ADMINISTRATION IN RATS. WITH A CONCEPT--"AXOSTASIS". Acta Neuropathol. 1964 May 5;3:428–450. doi: 10.1007/BF00688453. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J Physiol. 1961 Jul;157:315–327. doi: 10.1113/jphysiol.1961.sp006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancalon P. Slow flow in axons detached from their perikarya. J Cell Biol. 1982 Dec;95(3):989–992. doi: 10.1083/jcb.95.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couri D., Abdel-Rahman M. S., Hetland L. B. Biotransformation of n-hexane and methyl n-butyl ketone in guinea pigs and mice. Am Ind Hyg Assoc J. 1978 Apr;39(4):295–300. doi: 10.1080/0002889778507761. [DOI] [PubMed] [Google Scholar]
- DiVincenzo G. D., Kaplan C. J., Dedinas J. Characterization of the metabolites of methyl n-butyl ketone, methyl iso-butyl ketone, and methyl ethyl ketone in guinea pig serum and their clearance. Toxicol Appl Pharmacol. 1976 Jun;36(3):511–522. doi: 10.1016/0041-008x(76)90230-1. [DOI] [PubMed] [Google Scholar]
- Durham H. D., Pena S. D., Carpenter S. The neurotoxins 2,5-hexanedione and acrylamide promote aggregation of intermediate filaments in cultured fibroblasts. Muscle Nerve. 1983 Nov-Dec;6(9):631–637. doi: 10.1002/mus.880060903. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970 Aug;167(4):379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- Graham D. G., Anthony D. C., Boekelheide K., Maschmann N. A., Richards R. G., Wolfram J. W., Shaw B. R. Studies of the molecular pathogenesis of hexane neuropathy. II. Evidence that pyrrole derivatization of lysyl residues leads to protein crosslinking. Toxicol Appl Pharmacol. 1982 Jul;64(3):415–422. doi: 10.1016/0041-008x(82)90237-x. [DOI] [PubMed] [Google Scholar]
- Graham D. G. Hexane neuropathy: a proposal for pathogenesis of a hazard of occupational exposure and inhalant abuse. Chem Biol Interact. 1980 Nov;32(3):339–345. doi: 10.1016/0009-2797(80)90101-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., Hoffman P. N., Clark A. W., Carroll P. T., Price D. L. Slow axonal transport of neurofilament proteins: impairment of beta,beta'-iminodipropionitrile administration. Science. 1978 Nov 10;202(4368):633–635. doi: 10.1126/science.81524. [DOI] [PubMed] [Google Scholar]
- Griffiths I. R., Kelly P. A., Carmichael S., McCulloch M., Waterston M. The relationship of glucose utilization and morphological change in the visual system in hexacarbon neuropathy. Brain Res. 1981 Oct 19;222(2):447–451. doi: 10.1016/0006-8993(81)91053-2. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Ishii N., Schaumburg H. N-hexane neuropathy. A syndrome occurring as a result of industrial exposure. N Engl J Med. 1971 Jul 8;285(2):82–85. doi: 10.1056/NEJM197107082850204. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J., Griffin J. W., Price D. L. Slowing of the axonal transport of neurofilament proteins during development. J Neurosci. 1983 Aug;3(8):1694–1700. doi: 10.1523/JNEUROSCI.03-08-01694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. B., Cavanagh J. B. The early evolution of neurofilamentous accumulations due to 2,5-hexanediol in the optic pathways of the rat. Neuropathol Appl Neurobiol. 1982 Jul-Aug;8(4):289–301. doi: 10.1111/j.1365-2990.1982.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Komiya Y. Slowing with age of the rate of slow axonal flow in bifurcating axons of rat dorsal root ganglion cells. Brain Res. 1980 Feb 10;183(2):477–480. doi: 10.1016/0006-8993(80)90484-9. [DOI] [PubMed] [Google Scholar]
- Korobkin R., Asbury A. K., Sumner A. J., Nielsen S. L. Glue-sniffing neuropathy. Arch Neurol. 1975 Mar;32(3):158–162. doi: 10.1001/archneur.1975.00490450038004. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Autilio-Gambetti L., Gambetti P. Reorganization of axoplasmic organelles following beta, beta'-iminodipropionitrile administration. J Cell Biol. 1981 Dec;91(3 Pt 1):866–871. doi: 10.1083/jcb.91.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer J., Schlote W., Bischoff A., Boltshauser E., Müller G. Generalized giant axonal neuropathy: a filament-forming disease of neuronal, endothelial, glial, and schwann cells in a patient without kinky hair. Acta Neuropathol. 1977 Nov 28;40(3):213–218. doi: 10.1007/BF00691956. [DOI] [PubMed] [Google Scholar]
- Pena S. D., Opas M., Turksen K., Kalnins V. I., Carpenter S. Immunocytochemical studies of intermediate filament aggregates and their relationship to microtubules in cultured skin fibroblasts from patients with giant axonal neuropathy. Eur J Cell Biol. 1983 Sep;31(2):227–234. [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P. S., Bischoff M. C., Schaumburg H. H. On the specific molecular configuration of neurotoxic aliphatic hexacarbon compounds causing central--peripheral distal axonopathy. Toxicol Appl Pharmacol. 1978 Apr;44(1):17–28. doi: 10.1016/0041-008x(78)90280-6. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Sabri M. I., Schaumburg H. H., Moore C. L. Does a defect of energy metabolism in the nerve fiber underlie axonal degeneration in polyneuropathies? Ann Neurol. 1979 Jun;5(6):501–507. doi: 10.1002/ana.410050602. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Schaumburg H. H. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J Neuropathol Exp Neurol. 1977 Mar-Apr;36(2):276–299. doi: 10.1097/00005072-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell M., Black M. M., Garner J. A., Lasek R. J. Axonal transport: each major rate component reflects the movement of distinct macromolecular complexes. Science. 1981 Oct 9;214(4517):179–181. doi: 10.1126/science.6169148. [DOI] [PubMed] [Google Scholar]
- Tytell M., Brady S. T., Lasek R. J. Axonal transport of a subclass of tau proteins: evidence for the regional differentiation of microtubules in neurons. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1570–1574. doi: 10.1073/pnas.81.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
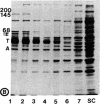
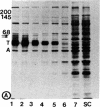
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]