Abstract
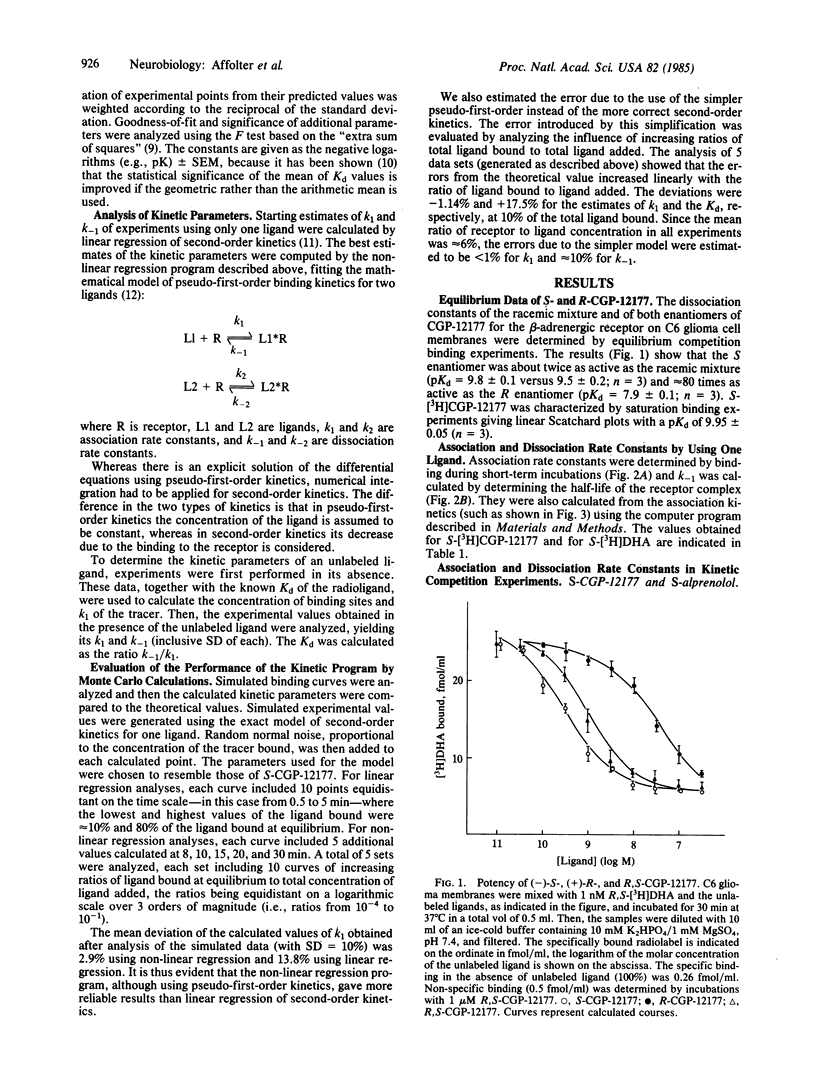
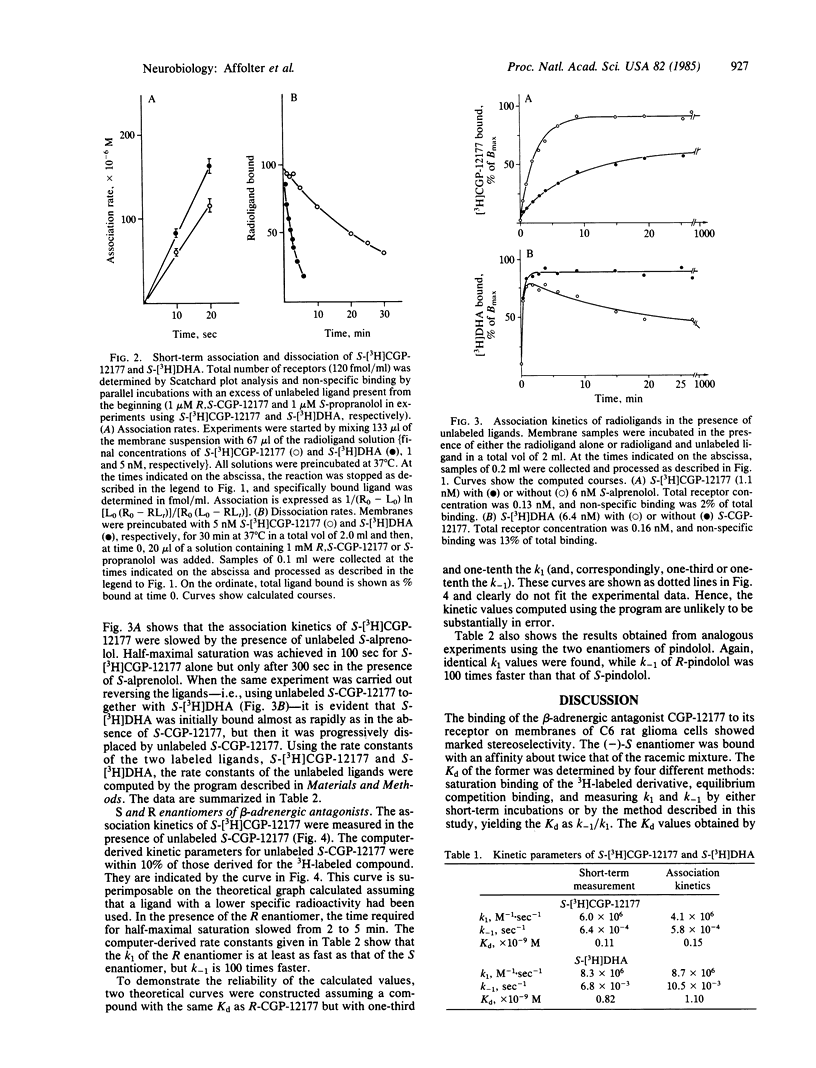
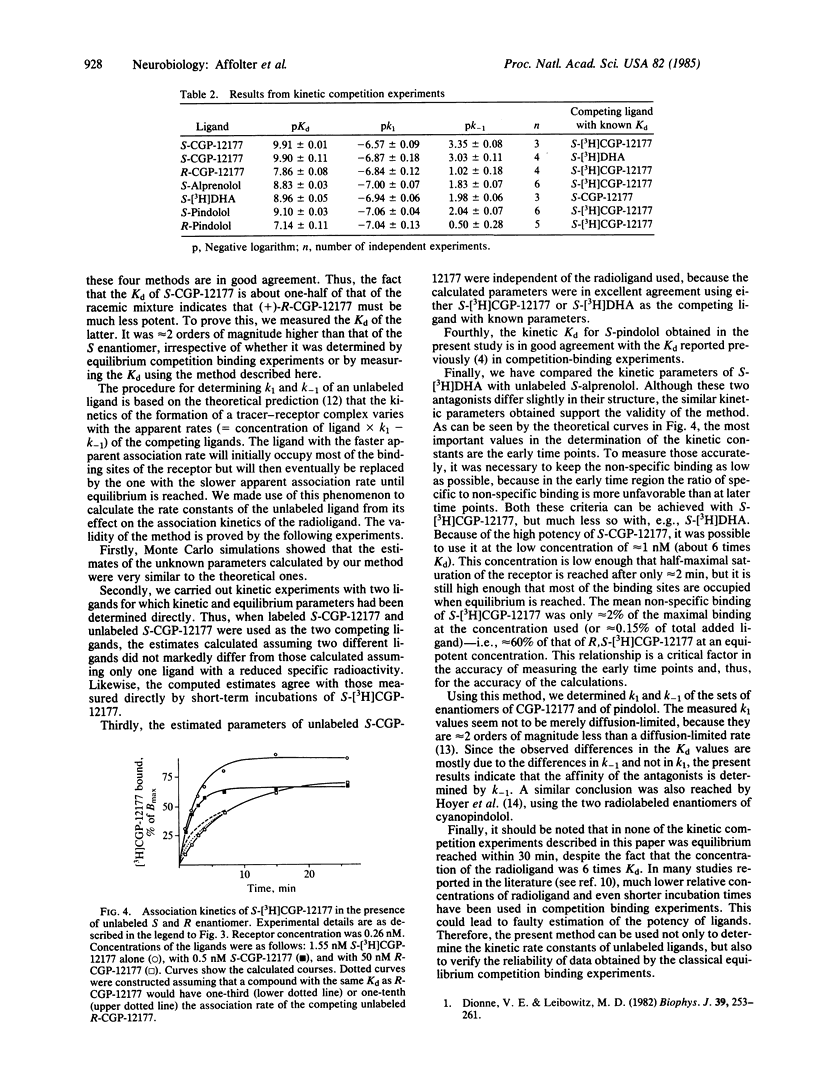
The enantiomers of the hydrophilic beta-adrenergic blocker CGP-12177 have been synthesized and the S-enantiomer radiolabeled with tritium. The dissociation constant (Kd) of the S-enantiomer for binding to the beta-adrenergic receptor is one-half of that of the racemic mixture and at least 2 orders of magnitude lower than that of the R-enantiomer. The kinetic parameters of the latter were determined by analyzing its effect on the association kinetics of (-)-S-[3H]CGP-12177. A computer program was developed that allows the association and dissociation rate constants of unlabeled ligands to be calculated. This method was validated using Monte Carlo simulations. In addition, the rate constants of unlabeled S-CGP-12177 and S-alprenolol calculated using this method were in good agreement with those of S-[3H]CGP-12177 and S-[3H]dihydroalprenolol, respectively, determined independently. The method was also used to measure the rate constants of the enantiomers of pindolol. These antagonists as well as S- and R-CGP-12177 form their receptor complexes with similar association rate constants. In contrast, the dissociation of the R-enantiomers from receptor-ligand complexes were found to be at least 100 times faster than those of the corresponding S-enantiomers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arányi P. Kinetics of the hormone-receptor interaction. Competition experiments with slowly equilibrating ligands. Biochim Biophys Acta. 1980 Mar 3;628(2):220–227. doi: 10.1016/0304-4165(80)90369-4. [DOI] [PubMed] [Google Scholar]
- Barovsky K., Brooker G. (-)-[125I]-iodopindolol, a new highly selective radioiodinated beta-adrenergic receptor antagonist: measurement of beta-receptors on intact rat astrocytoma cells. J Cyclic Nucleotide Res. 1980;6(4):297–307. [PubMed] [Google Scholar]
- De Lean A., Hancock A. A., Lefkowitz R. J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982 Jan;21(1):5–16. [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Engel G., Berthold R. Binding characteristics of (+)-, (+/-)- and (-)-[125iodo] cyanopindolol to guinea-pig left ventricle membranes. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):319–329. doi: 10.1007/BF00501172. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1977 Feb;74(2):515–519. doi: 10.1073/pnas.74.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Ross E. M., Gilman A. G. beta-Adrenergic receptor: ligand binding properties and the interaction with adenylyl cyclase. Adv Cyclic Nucleotide Res. 1977;8:1–83. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Hertel C. [3H]CGP-12177, a beta-adrenergic ligand suitable for measuring cell surface receptors. J Recept Res. 1983;3(1-2):35–43. doi: 10.3109/10799898309041921. [DOI] [PubMed] [Google Scholar]
- Staehelin M., Simons P., Jaeggi K., Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983 Mar 25;258(6):3496–3502. [PubMed] [Google Scholar]
- Weiland G. A., Molinoff P. B. Quantitative analysis of drug-receptor interactions: I. Determination of kinetic and equilibrium properties. Life Sci. 1981 Jul 27;29(4):313–330. doi: 10.1016/0024-3205(81)90324-6. [DOI] [PubMed] [Google Scholar]



