Abstract
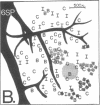
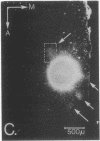
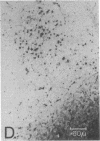
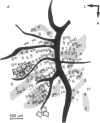
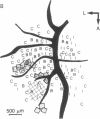
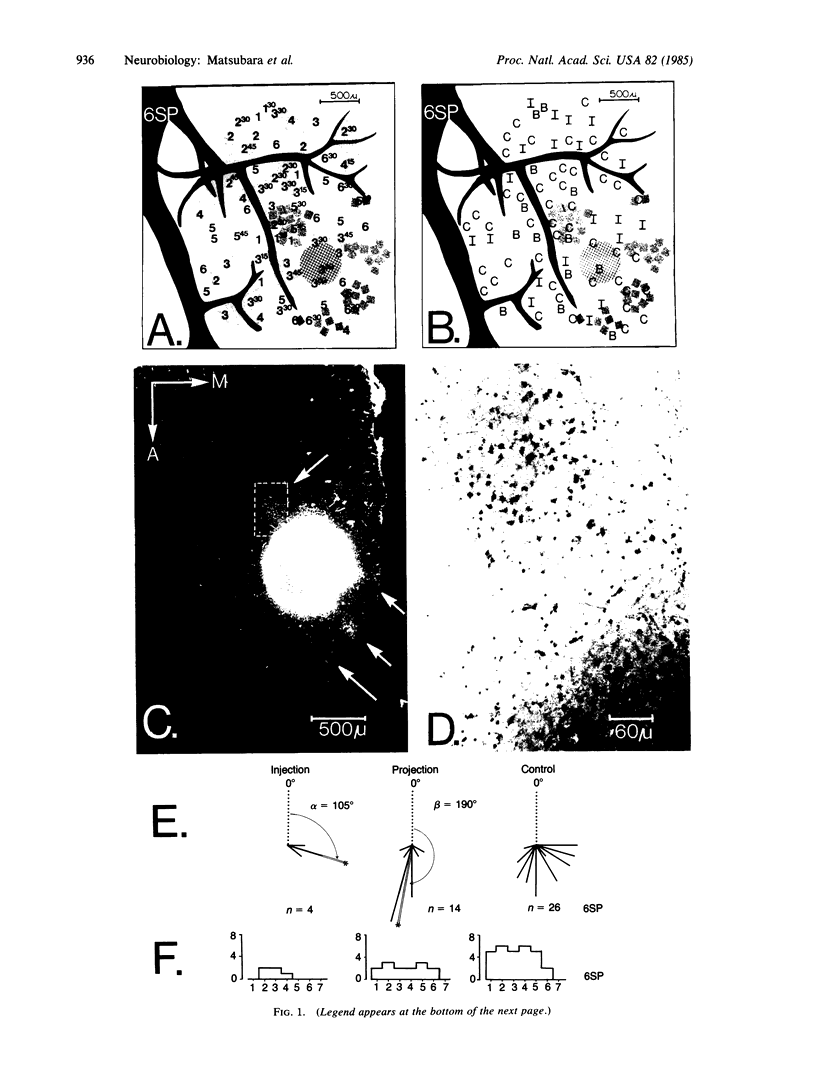
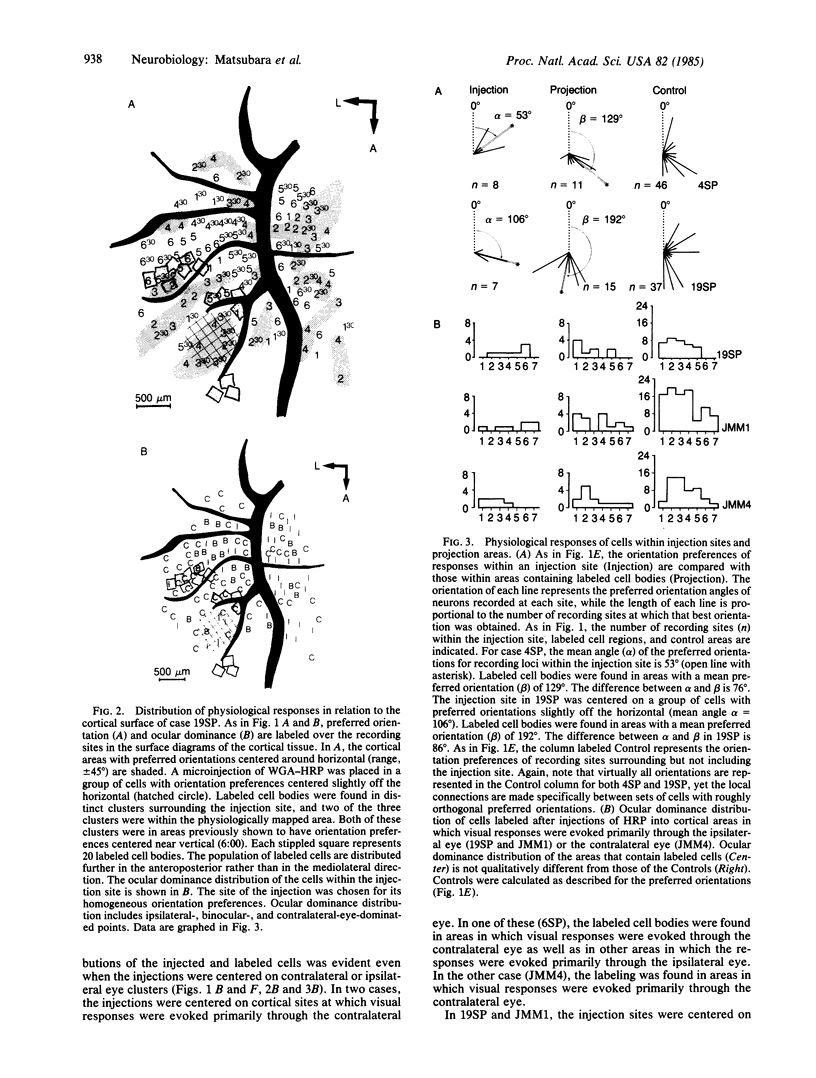
The functional organization of intrinsic connections within area 18 of cat visual cortex was studied using combined electrophysiological and anatomical techniques. Physiological recordings were first used to map the distribution of orientation preference, ocular dominance, and receptive-field location relative to the cortical surface. Next, localized injections of lectin-conjugated horseradish peroxidase were made into physiologically identified regions within area 18. We found that (i) the local cortical interconnections are made preferentially between cell populations with orthogonal preferred orientations and are independent of the ocular dominance of the cortical cells, (ii) the map of visual space in the cortex is anisotropic with the magnification factor for vertical at least twice that for horizontal visual space, and (iii) the pattern of cortical projections compensates for the functional asymmetry so that a population of interconnected cells represents a roughly circular region of visual space.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. 14C-deoxyglucose mapping of orientation subunits in the cats visual cortical areas. Exp Brain Res. 1979;37(3):609–613. doi: 10.1007/BF00236828. [DOI] [PubMed] [Google Scholar]
- COLONNIER M. THE TANGENTIAL ORGANIZATION OF THE VISUAL CORTEX. J Anat. 1964 Jul;98:327–344. [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976 May 28;25(2):139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Schwartz M. L. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982 May 14;216(4547):755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Goldman P. S., Nauta W. J. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977 Feb 25;122(3):393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., Stryker M. P. Anatomical demonstration of orientation columns in macaque monkey. J Comp Neurol. 1978 Feb 1;177(3):361–380. doi: 10.1002/cne.901770302. [DOI] [PubMed] [Google Scholar]
- Imig T. J., Brugge J. F. Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J Comp Neurol. 1978 Dec 15;182(4):637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- Imig T. J., Reale R. A. Patterns of cortico-cortical connections related to tonotopic maps in cat auditory cortex. J Comp Neurol. 1980 Jul 15;192(2):293–332. doi: 10.1002/cne.901920208. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Burton H., Porter R. Commissural and cortico-cortical "columns" in the somatic sensory cortex of primates. Science. 1975 Nov 7;190(4214):572–574. doi: 10.1126/science.810887. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Specificity of cortico-cortical connections in monkey visual system. Nature. 1983 Aug 11;304(5926):531–534. doi: 10.1038/304531a0. [DOI] [PubMed] [Google Scholar]
- Mitchison G., Crick F. Long axons within the striate cortex: their distribution, orientation, and patterns of connection. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3661–3665. doi: 10.1073/pnas.79.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone M. C., Burr D. C., Maffei L. Functional implications of cross-orientation inhibition of cortical visual cells. I. Neurophysiological evidence. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):335–354. doi: 10.1098/rspb.1982.0078. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S., Humphrey A. L. Anatomical binding of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J Comp Neurol. 1982 Jul 20;209(1):41–58. doi: 10.1002/cne.902090105. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Intrinsic laminar lattice connections in primate visual cortex. J Comp Neurol. 1983 May 20;216(3):303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Widespread periodic intrinsic connections in the tree shrew visual cortex. Science. 1982 Mar 19;215(4539):1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Topographic organization of orientation columns in the cat visual cortex. A deoxyglucose study. Exp Brain Res. 1981;44(4):431–436. doi: 10.1007/BF00238836. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Rosenquist A. C., Palmer L. A. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979 Jun 15;185(4):657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Macnichol E. F., Jr, Wagner H. G. Glass Insulated Platinum Microelectrode. Science. 1960 Nov 4;132(3436):1309–1310. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]