Abstract
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infect and replicate primarily in human hepatocytes. Few reliable and easy accessible animal models are available for studying the immune system’s contribution to the liver disease progression during hepatitis virus infection. Humanized mouse models reconstituted with human hematopoietic stem cells (HSCs) have been developed to study human immunology, human immunodeficiency virus 1 infection, and immunopathogenesis. However, a humanized mouse model engrafted with both human immune and human liver cells is needed to study infection and immunopathogenesis of HBV/HCV infection in vivo. We have recently developed the humanized mouse model with both human immune and human liver cells (AFC8-hu HSC/Hep) to study immunopathogenesis and therapy of HCV infection in vivo. In this review, we summarize the current models of HBV/HCV infection and their limitations in immunopathogenesis. We will then present our recent findings of HCV infection and immunopathogenesis in the AFC8-hu HSC/Hep mouse, which supports HCV infection, human T-cell response and associated liver pathogenesis. Inoculation of humanized mice with primary HCV isolates resulted in long-term HCV infection. HCV infection induced elevated infiltration of human immune cells in the livers of HCV-infected humanized mice. HCV infection also induced HCV-specific T-cell immune response in lymphoid tissues of humanized mice. Additionally, HCV infection induced liver fibrosis in humanized mice. Anti-human alpha smooth muscle actin (αSMA) staining showed elevated human hepatic stellate cell activation in HCV-infected humanized mice. We discuss the limitation and future improvements of the AFC8-hu HSC/Hep mouse model and its application in evaluating novel therapeutics, as well as studying both HCV and HBV infection, human immune responses, and associated human liver fibrosis and cancer.
Keywords: humanized mice, hepatitis viruses, liver fibrosis
Approximately 500 million people are chronically infected with hepatitis B and C viruses (HBV/HCV), progressively resulting in fibrosis/cirrhosis of the liver and development of hepatocellular carcinoma (HCC) over several decades.1,2 Chronic HBV/HCV infection is associated with impaired immune responses to viral antigens and liver inflammation, leading to the liver diseases.3,4 Because of the lack of robust animal models, very little is known about how HBV/HCV evades host immunity to establish chronic infection.5-7 Furthermore, very little is known about the mechanisms of HBV/HCV-induced liver fibrosis and HCC. HBV and HCV have host species restriction, namely humans and chimpanzees. To overcome host species restriction barrier for in vivo infection and disease modeling, several small-animal models of hepatitis virus-transgenic mouse and surrogate hepatitis virus have been employed to delineate the mechanisms of chronic infection and liver disease. More recently, human-murine chimeric liver models have been developed for studying in vivo infection and evaluating therapeutics.8 This strategy was further advanced by the recent development of humanized mice with both human hepatocytes and immune cells, which enabled both hepatitis virus infection and liver immunopathogenesis.9,10 We will focus on the update of mouse models for studying HBV/HCV infection, immunopathogenesis, and liver diseases.
Transgenic mouse models of human hepatitis viruses
Transgenic mice expressing whole genome or individual genes of HBV have been widely used to investigate the mechanism of HBV replication, gene expression, and immunopathogenesis of HBV in a small-animal model.11-15 The immune system of the mice is tolerant to the viral antigen, and therefore, most of the mice do not develop liver disease. Nonetheless, adoptive transfer of HBV-specific cytotoxic T lymphocytes (CTLs) or spleen cells from syngeneic mice provides a way for immunological study.
HBV-transgenic mice with 1.3× of HBV genome can produce high level of infectious viral particles.13 The viral particles produced in the mice are morphologically indistinguishable from virus derived from human, and they are infectious when inoculated in chimpanzees.16 No liver disease developed in these mice, suggesting that HBV was not directly cytopathic.13 This transgenic mouse model was used to test the efficacy of HBV inhibitors, including nucleoside analogs reverse transcriptase inhibitors, cytokines, and small interfering RNAs.17-20 By adoptive transfer of hepatitis B surface antigen (HBsAg)-specific CTLs into HBV-transgenic mice, people found that CTLs can inhibit HBV DNA replication by noncytolytic mechanisms via release of cytokines.21 Transfer of HBsAg-specific CTLs into the mice can also lead to liver injury, and antigen-non-specific inflammatory cells recruited into the liver during the process can amplify the severity of liver damage.22 Using HBV-transgenic severe combined immunodeficient (SCID) mice, Larkin et al. report that the mice clear the HBV virus from the serum and develop chronic liver disease after adoptive transfer of syngeneic splenocytes.23 Report also shows that a subset of nonclassical natural killer T cells mediates acute hepatitis after transfer of splenocytes into the HBV-transgenic mice on the recombination activating gene (RAG)–/– or T-cell receptor (TCR)-α–/– background.24
The development of transgenic mice expressing individual genes of HBV allowed investigators to explore the role of certain viral proteins in vivo. Transgenic mice that overexpress HBsAg along with pre-S polypeptide accumulate the surface antigen in the endoplasmic reticulum (ER). These mice display low levels of hepatocellular injury and can eventually progressed to HCC.25 HCC also develop in mice that expressed high levels of the HBV X antigen (HBx) in the liver.14 Experiments with HBx transgenic mice reveal that the X protein can impair the function of p53.26
As in the study of HBV, transgenic mice expressing HCV proteins either individually or together as a polyprotein have been developed to study the effect of these proteins on liver pathology. Hepatic steatosis is a common histological feature of chronic hepatitis C. The same phenomenon is also observed in the HCV core protein transgenic mice.27 The liver of HCV core transgenic mice showed resistance to concanavalin A-induced injury, which indicated that core protein may protect HCV-infected liver cells from destruction by the immune system.28 Transgenic expression of HCV core protein in the mouse liver can lead to the development of HCCs,29 and transgenic mice harboring complete HCV polyprotein showed an increased risk of liver cancer that suggested that other HCV proteins might also play a role in the induction of HCCs.30 However, expression of HCV nonstructural proteins did not cause any spontaneous liver pathology.31,32 To overcome the immune tolerance status to HCV antigen in transgenic mice and investigate the immune response to HCV in vivo, people use the Cre-loxP recombination system to make inducible HCV protein expression transgenic mice. An anti-HCV core antibody response and an HCV-specific T-cell response were observed in the transgenic mice after induction of core transgene expression, resulting in hepatitis or liver inflammation.33,34
The HBV and HCV transgenic mouse models significantly contribute to our understanding of virus–host interaction in vivo. However, these models have important limitations. Because the mouse liver cannot be infected with HBV or HCV, we cannot study the viral entry and spread, and no covalently closed circular DNA is produced in the HBV-transgenic mice. More important, HBV or HCV proteins are expressed as self-antigens; thus, it is not possible to study host immune response in the pathogenesis process. To overcome these limitations, chimeric mice repopulated with either human hepatocytes alone or with both human hepatocytes and immune system are needed to study HBV/HCV infection and immunopathogensis.
Human-murine chimeric liver models
Currently, several types of mouse models engrafted with human hepatocytes have been established for supporting HBV/HCV infection and replication. The first reported (and also the most widely used) is the albumin (Alb)-urokinase plasminogen activator (uPA) transgenic immune-deficient mice (C.b-17/SCID/bg8 and RAG2–/– mice35) in which the uPA gene is under control of the albumin promoter. The homozygous uPA-SCID mouse overexpresses uPA in the liver, resulting in a profoundly hypofibrinogenemic state and leading to hepatocyte death. Adult human hepatocytes are intrasplenically transplanted into newborn homozygous uPA-SCID mice. The chimeric liver with high levels (up to 90%) of human hepatocytes supports efficient infection of HBV36,37 and HCV.8 Alb-uPA mice, therefore, provide an invaluable tool for studying HBV/HCV infection and for screening and evaluating antiviral therapeutics. Because of the immunodeficiency of these mice, however, neither pathogen- nor vaccine-induced immune responses can be studied, and most of the studies have focused on testing antiviral drugs. In addition, the homozygous Alb-uPA mice have a high neonatal mortality rate because of severe hemorrhage, and the survived mice also have a short lifespan after transplantation (death rate around 40% in a large cohort study38), which also limits the wide application of these mice.
The second type of chimeric human-mouse liver model uses the fumaryl acetoacetate hydrolase (Fah)/RAG2/interleukin (IL) 2-gammaC (FRG) triple mutant mice.39,40 Mutation of Fah results in the hepatic accumulation of toxic tyrosine metabolic intermediates and, thereafter, the death of mouse hepatocytes. Compared with the Alb-uPA mice, the FRG mice have a major advantage, in that the extent of liver injury can be controlled by administering and withdrawing 2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC).40 The humanized FRG mice can support robust HBV and HCV replication, and high HCV titers were detected in the blood. Third, the herpes simplex virus (HSV) thymidine kinase (TK) was used in non-obese diabetic (NOD)-SCID transgenic mice, with the HSV-TK under control of the hepatic specific Alb promoter.41 Administration of gancyclovir will lead to specific mouse hepatocyte depletion and can lead to efficient engraftment of human hepatocytes.
Another human-mouse liver model for supporting HBV/HCV infection is the ectopic transplantation of human liver tissue under the kidney capsule.42,43 However, the HBV and HCV titer is relatively low in the blood, and the duration of infection is limited because of the short-lived transplanted liver tissues.
Humanized mouse models with both human liver and immune cells
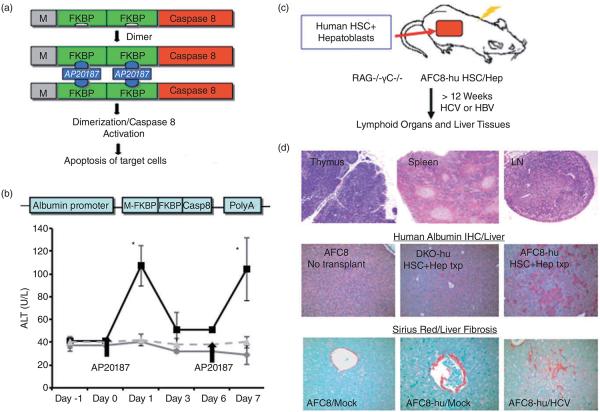
The earlier human-murine chimeric liver mouse models support robust HBV/HCV replication. However, these models lack a functional immune system; thus, it is not possible to study host immune response and hepatitis virus-induced immunopathology.8,40 Furthermore, because of the constitutively liver toxic transgene (uPA) or mutation (Fah), the poor health of uPA- or Fah-based mice humanized liver has significantly limited their general use. To overcome the problems associated with current chimeric human-murine liver mouse models, we recently developed a novel humanized mouse model (AFC8 humanized mouse model) with both human immune and liver cells.10,44 The AFC8 mouse is derived from the Balb/C-RAG2-γC-null immunodeficient mouse (double knockout [DKO]) carrying a liver-specific transgene with inducible suicidal activity. The AFC8 mouse supports development of a functional human immune system and liver cells following intrahepatic injection of human CD34+ hematopoietic stem cells (HSCs) and human liver progenitor cells (Hep) obtained from fetal liver tissue.10 AFC8-hu HSC/Hep mice supported HCV infection in the liver and generated anti-HCV human T-cell response. Additionally, HCV infection induced hepatitis and liver fibrosis, which correlated with activation of human hepatic stellate cells and expression of human fibrogenic genes (Fig. 1 and Washburn et al.10). The mechanism of human-specific fibrosis induction in the chimeric liver is not clear. The AFC8-hu mouse provides an excellent model to study how HCV or HBV infection induces human liver fibrosis in vivo.
Figure 1.
AFC8-hu Hep/hematopoietic stem cell (HSC) mice support engraftment of human immune/liver cells and hepatitis C virus (HCV) infection. (a) Inducible activation of caspase 8 through dimerization of FK506-binding protein (FKBP)-caspase 8. The chemical dimerizer AP20187 leads to activation of caspase 8 and apoptosis. (b) Mouse liver injury can be repeatedly induced in AFC8 transgenic mice. AFC8 or littermate control mice were injected with AP20187 at day 0 and day 6. ALT was measured in serum at −1, 0, 1, 3, 6, and 7 days postdrug treatment. Data represent means ± SD, P < 0.05. ( ) Control; (
) Control; ( ) APC8; (
) APC8; ( ) APC8 no drug. (c) Construction of AFC8-hu HSC/Hep mice with human CD34+ HSC and EpCAM+ hematoblasts. (d) Lymphoid organs including thymus, spleen, and lymph node (LN) are shown to demonstrate human immune cell reconstitution (top). Liver sections from control mice without transplant (left) and both control (middle) and AFC8 (right) transplanted with human HSC/Hep (AFC8-hu HSC/Hep) were stained with antihuman albumin antibody. Human albumin positive cells are shown (Middle). AFC8-hu HSC/Hep mice develop liver fibrosis after HCV infection (bottom). Liver sections from AFC8/mock, AFC8-hu/mock, and AFC8-hu/HCV mice were stained with sirius red/fast green to visualize liver fibrosis (red).
) APC8 no drug. (c) Construction of AFC8-hu HSC/Hep mice with human CD34+ HSC and EpCAM+ hematoblasts. (d) Lymphoid organs including thymus, spleen, and lymph node (LN) are shown to demonstrate human immune cell reconstitution (top). Liver sections from control mice without transplant (left) and both control (middle) and AFC8 (right) transplanted with human HSC/Hep (AFC8-hu HSC/Hep) were stained with antihuman albumin antibody. Human albumin positive cells are shown (Middle). AFC8-hu HSC/Hep mice develop liver fibrosis after HCV infection (bottom). Liver sections from AFC8/mock, AFC8-hu/mock, and AFC8-hu/HCV mice were stained with sirius red/fast green to visualize liver fibrosis (red).
Perspectives
The humanized AFC8 mouse is the first and only in vivo small animal model to recapitulate HCV infection, immune responses, and its associated liver disease, as observed in humans. It also supports infection of both HCV and human immunodeficiency virus (HIV) 1. Because HIV-1 coinfection has been reported to significantly exacerbate HCV-related liver diseases, the AFC8-hu HSC/Hep mouse allows investigation of HCV and HIV-1 coinfection in vivo.45,46 However, the human liver engraftment in the current AFC8-hu mice (~15%) is relatively low. It is difficult to support significant replication of HCV to show detectable viremia in the blood. Thus, it is important to improve it, either by more efficient depletion of mouse liver cells or by enhancing human liver cell proliferation and survival, to support efficient HCV infection. HBV infection is more efficient in other humanized mouse models with chimeric human liver. Consistently, long-term HBV infection is supported in AFC8-hu HSC/Hep mice, associated with similar immunopathogenesis and liver diseases (Bility and Su, unpubl. data, 2012). It is thus critical that the current AFC8-hu mouse model is improved that will support robust HBV/HCV infection, immunopathogenesis, and liver diseases, including fibrosis, cirrhosis, and cancer. The models will play a critical role in elucidating immune mechanisms of HBV/HCV-induced liver diseases, as well as in developing novel therapeutic strategies to treat HBV/HCV infection.
Acknowledgments
This work was supported in part by grants from University of North Carolina (UNC), University Cancer Research Fund (UCRF) innovation grant, from National Institutes of Health (NIH): UNC SPORE grants; AI076142, AA018009 (L.S.), and UNC Lineberger Comprehensive Cancer Center and UNC Infectious Disease Pathogenesis Postdoctoral Training Grants (M.T.B.).
Footnotes
Conflict of interest
The authors have no potential conflicts of interest to declare.
References
- 1.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B – United States, 1974–2008. PLoS ONE. 2011;6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir. Ther. 2011;16:1169–86. doi: 10.3851/IMP1982. [DOI] [PubMed] [Google Scholar]
- 3.Di Marco V, Lo Iacono O, Camma C, et al. The long-term course of chronic hepatitis B. Hepatology. 1999;30:257–64. doi: 10.1002/hep.510300109. [DOI] [PubMed] [Google Scholar]
- 4.Tan G, Zhao W, Liu X, Wang J, Wu Y. Immunophenotypic profile of intrahepatic and circulating lymphocytes in chronic hepatitis B patients. Hepatogastroenterology. 2011;59:1516–21. doi: 10.5754/hge11710. [DOI] [PubMed] [Google Scholar]
- 5.Gilgenkrantz H. Humanized mice for the study of hepatitis C. Med. Sci. (Paris) 2011;27:587–9. doi: 10.1051/medsci/2011276009. [DOI] [PubMed] [Google Scholar]
- 6.de Jong YP, Rice CM, Ploss A. New horizons for studying human hepatotropic infections. J. Clin. Invest. 2010;120:650–3. doi: 10.1172/JCI42338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brezillon N, Brunelle MN, Massinet H, et al. Antiviral activity of bay 41-4109 on hepatitis B Virus in humanized Alb-uPA/SCID mice. PLoS ONE. 2011;6:e25096. doi: 10.1371/journal.pone.0025096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer DF, Schiller DE, Elliott JF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001;7:927–33. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 9.Bility MT, Zhang L, Washburn ML, Curtis TA, Kovalev GI, Su L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat. Protoc. 2012;7:1608–17. doi: 10.1038/nprot.2012.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washburn ML, Bility MT, Zhang L, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–44. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chisari FV, Pinkert CA, Milich DR, et al. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985;230:1157–60. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- 12.Araki K, Miyazaki J, Hino O, et al. Expression and replication of hepatitis B virus genome in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1989;86:207–11. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J. Virol. 1995;69:6158–69. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CM, Koike K, Saito I, Miyamura T, Jay GH. Bx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–20. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 15.Chisari FV, Filippi P, McLachlan A, et al. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J. Virol. 1986;60:880–7. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–9. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 17.Julander JG, Colonno RJ, Sidwell RW, Morrey JD. Characterization of antiviral activity of entecavir in transgenic mice expressing hepatitis B virus. Antiviral Res. 2003;59:155–61. doi: 10.1016/s0166-3542(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 18.Julander JG, Sidwell RW, Morrey JD. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antiviral Res. 2002;55:27–40. doi: 10.1016/s0166-3542(01)00223-6. [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J. Virol. 2002;76:10702–7. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uprichard SL, Boyd B, Althage A, Chisari FV. Clearance of hepatitis B virus from the liver of transgenic mice by short hairpin RNAs. Proc. Natl. Acad. Sci. U. S. A. 2005;102:773–8. doi: 10.1073/pnas.0409028102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 22.Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 2004;113:1158–67. doi: 10.1172/JCI21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin J, Clayton M, Sun B, et al. Hepatitis B virus transgenic mouse model of chronic liver disease. Nat. Med. 1999;5:907–12. doi: 10.1038/11347. [DOI] [PubMed] [Google Scholar]
- 24.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–94. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 25.Chisari FV, Klopchin K, Moriyama T, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145–56. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 26.Ueda H, Ullrich SJ, Gangemi JD, et al. Functional inactivation but not structural mutation of p53 causes liver cancer. Nat Genet. 1995;9:41–7. doi: 10.1038/ng0195-41. [DOI] [PubMed] [Google Scholar]
- 27.Moriya K, Yotsuyanagi H, Shintani Y, et al. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J. Gen. Virol. 1997;78(Pt 7):1527–31. doi: 10.1099/0022-1317-78-7-1527. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura H, Govindarajan S, Aswad F, et al. HCV core expression in hepatocytes protects against autoimmune liver injury and promotes liver regeneration in mice. Hepatology. 2006;44:936–44. doi: 10.1002/hep.21360. [DOI] [PubMed] [Google Scholar]
- 29.Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat. Med. 1998;4:1065–7. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 30.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–65. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- 31.Frelin L, Brenndorfer ED, Ahlen G, et al. The hepatitis C virus and immune evasion: non-structural 3/4A transgenic mice are resistant to lethal tumour necrosis factor alpha mediated liver disease. Gut. 2006;55:1475–83. doi: 10.1136/gut.2005.085050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumder M, Steele R, Ghosh AK, et al. Expression of hepatitis C virus non-structural 5A protein in the liver of transgenic mice. FEBS Lett. 2003;555:528–32. doi: 10.1016/s0014-5793(03)01337-1. [DOI] [PubMed] [Google Scholar]
- 33.Wakita T, Taya C, Katsume A, et al. Efficient conditional transgene expression in hepatitis C virus cDNA transgenic mice mediated by the Cre/loxP system. J. Biol. Chem. 1998;273:9001–6. doi: 10.1074/jbc.273.15.9001. [DOI] [PubMed] [Google Scholar]
- 34.Wakita T, Katsume A, Kato J, et al. Possible role of cytotoxic T cells in acute liver injury in hepatitis C virus cDNA transgenic mice mediated by Cre/loxP system. J. Med. Virol. 2000;62:308–17. doi: 10.1002/1096-9071(200011)62:3<308::aid-jmv2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Dandri M, Burda MR, Torok E, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–8. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 36.Meuleman P, Libbrecht L, De Vos R, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–56. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 37.Tsuge M, Hiraga N, Takaishi H, et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology. 2005;42:1046–54. doi: 10.1002/hep.20892. [DOI] [PubMed] [Google Scholar]
- 38.Vanwolleghem T, Libbrecht L, Hansen BE, et al. Factors determining successful engraftment of hepatocytes and susceptibility to hepatitis B and C virus infection in uPA-SCID mice. J. Hepatol. 2010;53:468–76. doi: 10.1016/j.jhep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat. Biotechnol. 2007;25:903–10. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bissig KD, Wieland SF, Tran P, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J. Clin. Invest. 2010;120:924–30. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa M, Kawai K, Mitsui T, et al. The reconstituted “humanized liver” in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 2011;405:405–10. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohashi K, Marion PL, Nakai H, et al. Sustained survival of human hepatocytes in mice: a model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat. Med. 2000;6:327–31. doi: 10.1038/73187. [DOI] [PubMed] [Google Scholar]
- 43.Maeda N, Watanabe M, Okamoto S, et al. Hepatitis C virus infection in human liver tissue engrafted in mice with an infectious molecular clone. Liver Int. 2004;24:259–67. doi: 10.1111/j.1478-3231.2004.0909.x. [DOI] [PubMed] [Google Scholar]
- 44.Robinet E, Baumert TF. A first step towards a mouse model for hepatitis C virus infection containing a human immune system. J. Hepatol. 2011;55:718–20. doi: 10.1016/j.jhep.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 45.Kim AY, Chung RT. Coinfection with HIV-1 and HCV – a one-two punch. Gastroenterology. 2009;137:795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Su L. HIV-1 immunopathogenesis in humanized mouse models. Cell. Mol. Immunol. 2012;9:237–44. doi: 10.1038/cmi.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]