Abstract
Background
Alcohol use disorders are well recognized to be common, debilitating, and the risk of developing them is influenced by family history. The subjective response to alcohol may be determined familialy and related to the risk of developing alcoholism. The aim of this study was to evaluate differences between family history positive (FHP) and family history negative (FHN) individuals in their response to alcohol within the domains of subjective, coordination, and cognitive effects using an IV clamping method of alcohol administration.
Methods
Two groups of healthy subjects, those with a FHP (n=65) vs. those who were FHN (n=115), between the ages of 21-30, participated in three test days. Subjects were scheduled to receive placebo, low dose ethanol (target BrAC=40mg%), and high dose ethanol (target BrAC=100mg%) on three separate test days at least three days apart in a randomized order under double-blind conditions. Outcome measures included subjective effects, measures of coordination and cognitive function.
Results
Both low and high dose alcohol led to dose-related stimulant and sedative subjective effects as measured the Biphasic Alcohol Effects Scale (BAES) and subjective measures of “high” and “drowsy” measured on a visual analog scale (VAS) However, there were no effects of family history. Similar dose-related effects were observed on cognitive and coordination related outcomes, but were not moderated family history.
Conclusions
Results from this study showed that healthy individuals responded to an IV alcohol challenge in a dose-related manner; however, there were no significant differences on subjective response, or on ethanol-induced impairment of coordination or cognition, between individuals with a positive family history for alcoholism and those with a negative family history. Results suggest that FH may not be a specific enough marker of risk, particularly in individuals who are beyond the age where alcohol use disorders often develop.
Introduction
Alcohol use disorders are well recognized to be common, debilitating, and genetically influenced. A family history of alcoholism has been reliably identified as a risk factor for the development of alcohol use disorders in numerous studies (Goodwin et al. 1973; Cotton 1979; Cloninger et al. 1981; Dawson et al. 1992). The subjective response to alcohol, including the balance of positive and negative effects experienced by individuals (or “reward valance” of alcohol), may be familial determined and related to the risk of developing alcoholism (Morean and Corbin 2010).
In a series of elegant studies first conducted by Schuckit and colleagues, it was demonstrated that sons of alcoholic fathers exhibited a low level of response (LR) to oral alcohol administration in a laboratory setting, compared to young men without a family history of alcoholism (Schuckit 1980; Schuckit et al. 1984); the low response was specific to some of the negative effects of alcohol. LR individuals also demonstrated an increased risk for the subsequent development of alcohol use disorders (Schuckit 1994; Schuckit and Smith 1996; Schuckit et al. 2000; Schuckit et al. 2001). Numerous studies have supported the intergenerational transmission of the subjective response to alcohol as a pathophysiologic mechanism for the genetic risk of alcoholism (Schuckit et al. 2005). It has been hypothesized that individuals with a family history positive (FHP) for alcohol use disorders who are at higher risk may be less sensitive to the effects of alcohol, and at increased likelihood to consume more alcohol, because they lack the feedback signal to stop drinking experienced by family history negative (FHN) individuals.
Several groups have replicated the initial studies and some have taken a step further, suggesting that family history effects include differences in the biphasic response to alcohol, that is both the stimulant effects that occur while blood alcohol levels are rising and the sedative effects more common while blood alcohol levels are falling. A “differentiator model” (DM) hypothesis has been proposed and posits that individuals with a positive family history for alcoholism demonstrate both increased and decreased sensitivity to alcohol (Newlin and Thomson 1990). As conceptualized by this model, FHP individuals show increased sensitivity to the rewarding, excitatory effects of alcohol on the ascending limb of the blood alcohol curve (BAC) and decreased sensitivity to the negative effects common to the descending limb (e.g. sedation, depression, anxiety). The results of some studies have offered at least partial support for this hypothesis; for example college students with a family history had higher levels of stimulation, but lower levels of sedation during alcohol consumption than students without a family history (Erblich et al. 2003). In partial support, FHP subjects reported greater feelings of intoxication, including stimulation but also sedation, during initial alcohol IV administration than FHN subjects, that adapted after time (Morzorati et al. 2002). Similarly (King et al. 2002; Morzorati et al. 2002; Erblich et al. 2003). It should be noted, however, that several studies have failed to find clear differences between FHP and FHN subjects, and do not clearly support either the LR or DM model (de Wit and McCracken 1990; Evans and Levin 2003). Further, several groups have identified potential confounding factors, such as drinking history (Ramchandani et al. 2002; King et al. 2011).
While most studies have been conducted using oral alcohol paradigms, oral alcohol administration has several limitations including, for example, variable absorption and blood alcohol levels (BALs), even when controlling for factors such as gender and weight. The administration of a fixed intravenous (IV) dose presents similar issues, as equivalent doses still lead to different BALs (Davidson et al. 1997). Another way to administer alcohol is to establish a steady state blood alcohol level using an IV alcohol infusion that is titrated to a breathalyzer reading and clamped at a steady state (Ramchandani et al. 1999; Subramanian et al. 2002). This breath alcohol clamping (BrAC) procedure allows for direct comparisons of effects at a specific blood alcohol level. It has been used safely in healthy individuals with targeted BALs ranging from 50-150 mg/dl (Ramchandani et al. 1999; Morzorati et al. 2002; Subramanian et al. 2002) and has been used in studies evaluating the pharmacokinetics of alcohol, the metabolism of alcohol, genetic variations in alcohol metabolism enzymes (reviewed in (Ramchandani and O'Connor 2006). More recently its use has been extended to neuroimaging studies, eg. using functional magnetic resonance imaging (fMRI) to understand the effects of alcohol on risk taking behavior (Gilman et al. 2011) and using proton magnetic resonance to document effect of alcohol on levels of neurotransmitters such as glutamate (Gomez et al. 2012). Using the IV clamp method, previous studies have shown subjective differences between FHP and FHN individuals on measures of high, intoxication, and stimulation during the initial phases of the clamp, greater acute tolerance in FHP individuals on measures of intoxication, and family history-related differences on saccadic eye movement, where FHP individuals had significantly greater latency in saccades compared to FHN in response to alcohol measures at BrAC levels of 60mg% (Blekher et al. 2002; Morzorati et al. 2002).
The aim of this study was to further evaluate differences between FHP and FHN individuals in their response to alcohol within the domains of subjective, coordination, and cognitive effects using the BrAC method, targeting two different doses of ethanol: 40mg% and 100mg%. We hypothesized that there would be a blunting of the negative effects of ethanol administration (e.g. anxiety, dysphoria, perceptual disturbances) in individuals at high-risk to develop alcoholism relative to healthy subjects who were not at high risk based on family history. We also sought to determine if dose-response effects seen at high and low alcohol levels differ significantly between FHN and FHP individuals.
Methods
Subjects
Healthy subjects (n = 180) were recruited by advertisement and compensated for their participation. Inclusion criteria included: 1) males and females, 2) between the ages of 21 and 30 years, and 3) medically and neurologically healthy on the basis of history, physical examination, electrocardiogram and screening laboratories. Exclusion criteria included: 1) DSM-IV psychiatric or substance use disorder (excluding alcohol abuse) by history on psychiatric evaluation, including structured diagnostic interview (SCID), 2) meeting criteria for alcohol abuse and expressing an interest in stopping alcohol use/seeking treatment or current enrollment in treatment for alcoholism or having sought treatment in the last 6 months, 3) individuals with a history of counseling or psychotherapy, except family therapy centered around another family member, 4) unwillingness to remain alcohol-free for 48 hours prior to each test day, 5) positive urine toxicology on test days for drugs, including marijuana, cocaine, benzodiazepines, amphetamines and opioids, 6) for women, positive pregnancy test at screening or intention to engage in unprotected sex during the study, 7) alcohol naïve, and 8) adoptees with no contact with family members. Family history positive (FHP) subjects were required to have a biological father and another first- or second-degree biological relative with histories of alcoholism by the Family History Assessment Module (FHAM) developed by COGA. Biological mothers had to be without a history of alcoholism, in order to ensure that any effects were not the result of direct toxicity of alcohol during pregnancy. Family history negative (FHN) subjects were required to have no family history of alcoholism in any first- or second-degree relatives. Subjects had to reliably report on first and second degree relatives to be included in the study Institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine approved this study.
Procedures
After signing informed consent, subjects underwent baseline screening, including structured interview, physical examination, and laboratory testing, including urine toxicology. Prior to participation in the study, subjects were advised regarding the potential risks of ethanol administration and were informed that they would receive ethanol on 2 out of 3 test days. Subjects were scheduled to receive placebo, low dose ethanol (target BrAC=40mg%), and high dose ethanol (target BrAC=100mg%) on three separate test days at least three days apart in a randomized order under double-blind conditions.
Prior to each test session, participants were instructed not to consume alcohol or caffeine for 48 hours and were asked to fast overnight. They presented to the Biological Studies Unit of the VA Connecticut Healthcare System, West Haven campus, at approximately 9:00 AM. All subjects were given a standardized breakfast at the beginning of each test day and crackers during the test day, as requested. Prior to testing, subjects underwent urine drug screening for toxicology and breathalyzer screening. Provided that these tests were negative, an intravenous line was placed. Subjects received infusion of placebo or one of two ethanol doses (target BrAC=40mg% or target BrAC=100mg%) intravenously for approximately 20 minutes, until the target BrAC was achieved. Once the BrAC was achieved, (40mg% or 100mg%), it was maintained using the clamp procedure for 60 minutes. Test days lasted approximately 8 hours in total.
Subjective intoxication ratings were obtained at baseline, 10 minutes after starting the infusion, immediately upon reaching the target alcohol level, and at 30, 80, 110, 140, and 200 minutes after reaching the target alcohol level using the Number of Drinks Scale (NDS), and Visual Analogue Scale (VAS) for ‘high,’ ‘drowsy,’ and ‘tired.’ The Biphasic Alcohol Effects Scale (BAES) (Martin et al. 1993) was also used and the sum of the respective items for each subscale was reported (0-70). Subjects also reported on similarity to alcohol as measured by the Visual Analogue Scales of Similarity to Drugs of Abuse (VASSDA). All subjects had used alcohol in the past and were asked to assess alcohol-like effects. The VASSDA consists of VAS's (0 = not at all, 7 = extremely) measuring the perceived similarity of the administered agent to ethanol and other drugs, and had been used in a previous challenge study conducted by this group (Perrino et al. 2008; Dickerson et al. 2010). Subjects were asked to report on the number of drinks they felt like they had consumed using the NDS, which has also been used in several previous challenge studies conducted by this group (Perrino et al. 2008; Dickerson et al. 2010). The BAES measures both the stimulating and sedating effects associated with ethanol intoxication. The stimulating effects are associated with the ascending limb of ethanol intoxication and include feeling energized, excited, stimulated, talkative, ‘up,’ and vigorous. The sedating effects are associated with the descending limb of ethanol intoxication and include feeling ‘down,’ heavy headed, inactive, sedated, sluggish, having slow thoughts, and difficulty in concentrating. Perceived intensities of feeling ‘high,’ ‘drowsy,’ and ‘tired’ were assessed using a VAS (0 = not at all, 7 = extremely).
Changes in hand-to-eye coordination were assessed using the Grooved Pegboard Test (Lafayette Instrument Company). This is a manipulative dexterity test, consisting of a board with randomly positioned slots into which the subject inserts pegs under timed conditions (Ruff and Parker 1993).
Assessment for changes in cognitive function included use of the Hopkins Verbal Learning Test (HVLT). The HVLT is word list learning test of verbal memory and has the advantage of six different versions that permit multiple episodes of testing (Brandt 1991). The procedures associated with the test allow some degree of distinction between immediate recall, delayed recall, and recognition. Subjects could not leave the testing facility until a BrAC of 20 mg% was achieved. Subjects were cleared for discharge by a study physician and the Mini Mental State Examination (MMSE) (Folstein et al. 1975) was used as a safety measure, with the stipulation that any subject with a post-infusion score of <27 would stay overnight, prior to consideration for discharge.
Data Analysis
Data were checked for normality prior to analysis using Kolmogorov-Smirnov test statistics and normal probability plots. The outcomes for the peg board task and total recall on the HVLT were sufficiently normal. Linear mixed models with alcohol (placebo, low-, high-dose) and trial (1-3) included as within-subjects factors and group (FHP vs. FHN) as a between-subject factor was used to analyze total recall from the HVLT. The pegboard task was analyzed using the same model described above with time (baseline, +30min) replacing trial as a within-subjects factor. All other outcome measures were skewed, particularly during the placebo condition – i.e., infrequent response – across all time points, and unsuccessfully transformed. Thus, these outcomes were analyzed using the nonparametric approach for repeated measures data by Brunner (Brunner et al. 2002) where the data were first ranked, and then fitted using a mixed effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS). These models included both alcohol (placebo, low-, high-dose) and time (study time points) as within-subjects explanatory factors and family history status (FHP vs. FHN) as a between-subjects factor. A secondary analysis was conducted adding gender as a factor. These models allowed for testing of all main and interactive effects of group, drug and time. All reported p-values are Bonferroni adjusted, applied within but not across domains. Data were analyzed using SAS, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Subject Characteristics
As shown in Table 1, 180 individuals participated in this study, of which 165 (92%) completed all 3 test days. Of the 15 subjects who partially completed the protocol, eight received placebo only, one 40% mg only, three 100% mg only, one both placebo and 100% mg, and two both 40% mg and 100% mg. Reported reasons for dropout varied: 8 subjects withdrew from the study due to scheduling conflicts, 5 either never returned calls or never showed up for a test day, 1 subject moved out of state, and 1 subject experienced vomiting on the second test day (100% mg) and was withdrawn from the study. All subjects were included in the analysis (n = 180). The sample included 65 FHP and 115 FHN individuals.
Table 1. Study Participants.
| Variable | Totals (n=180) | FHN (n= 115) | FHP (n = 65) |
|---|---|---|---|
|
| |||
| Gender* | n, % | n, % | n, % |
| Female | 88 (48.9) | 56 (48.7) | 32 (49.2) |
| Male | 92 (51.1) | 59 (51.3) | 33 (50.8) |
|
| |||
| Ethnicity* | n, % | n, % | n, % |
| Caucasian | 139 (77.2) | 88 (76.5) | 51 (78.5) |
| African American | 24 (13.3) | 14 (12.2) | 10 (15.4) |
| Other | 17 (9.5) | 13 (11.3) | 4 (6.1) |
|
| |||
| Mean (SE) | Mean (SE) | Mean (SE) | |
|
| |||
| Age* | 24.1 (0.2) | 24.1 (0.2) | 24.1 (0.4) |
|
| |||
| Years of education* | 16.1 (0.2) | 16.24 (0.2) | 15.9 (0.3) |
|
| |||
| # of Drinking Days past 30 days* | 5.87 (0.3) | 6.1 (0.5) | 5.5 (0.5) |
|
| |||
| # of Drinks during past 30 days* | 17.61(1.6) | 16.21 (1.4) | 20.1 (3.5) |
|
| |||
| Drinks per drinking day | 2.82 (1.8) | 2.62 (1.3) | 3.19 (2.4) |
|
| |||
| Age began to drink regularly* | 17.4 (0.2) | 17.6 (0.2) | 17.1 (0.3) |
No significant differences between groups
Overall, the sample included slightly more males (n= 92, 51%) than females, had a mean age of 24.1 ± 2.6 years (range, 21-30), and was predominantly Caucasian (n=139, 77%). All subjects had at least a high school education (12 years) with an average of 16.1 ± 2.0 years of education. The average age at which subjects began drinking was 17.4 ± 2.0 years. The average number of drinking days within the past 30 days before infusion was 5.9 ± 4.5 (range, 0-28), and the average number of standard drinks within the past 30 days before infusion was 17.6 ± 21.0 (range, 0-185). There were no differences in baseline variables between groups.
Alcohol Dose (BrAC)
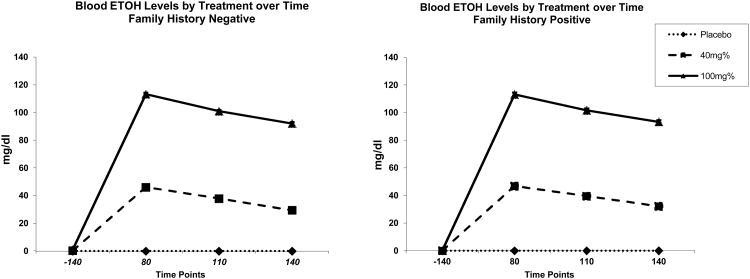
Target BrAC levels were reached during the infusion for both the low dose (average BrAC level =0.04, sd= 0.003) and the high dose (average BrAC= 0.1; sd=0.004) test days. Mean blood alcohol levels over time for each of the challenge days, by FH status, is presented in Figure 1.
Figure 1. Blood Alcohol Levels (mg/dl) over Time by Family History.
Subjective Intoxication
Number of Drinks Scale
Subjects rated the low dose as equivalent to 2.0 standard drinks (SD = 1.5) and the high dose as equivalent to 3.8 standard drinks (SD = 1.8). No differences were observed between family history negative and family history positive subjects [history by treatment by time effect: num df = 6.8, ATS = 0.82, p = 0.56]. (Due to zero variance at baseline for placebo and low dose, and only 3 non-zero values among high dose, this analysis was based on change from baseline.)
Perceived similarity to alcohol
Both low and high dose ethanol showed perceived similarities to alcohol in a dose-related manner [treatment by time effect: num df = 8.2, ATS = 74.7, p < 0.0001]. Post-hoc analyses showed that both low and high dose ethanol significantly resembled alcohol compared with placebo 10 minutes into the infusion, immediately upon reaching the target dose, and at 30, 80 (data says 60, not 80), 110, and 140 minutes after reaching the target (all p < 0.001). No differences were observed between family history negative and family history positive subjects [family history by treatment by time effect: num df = 8.2, ATS = 1.1, p = 0.35].
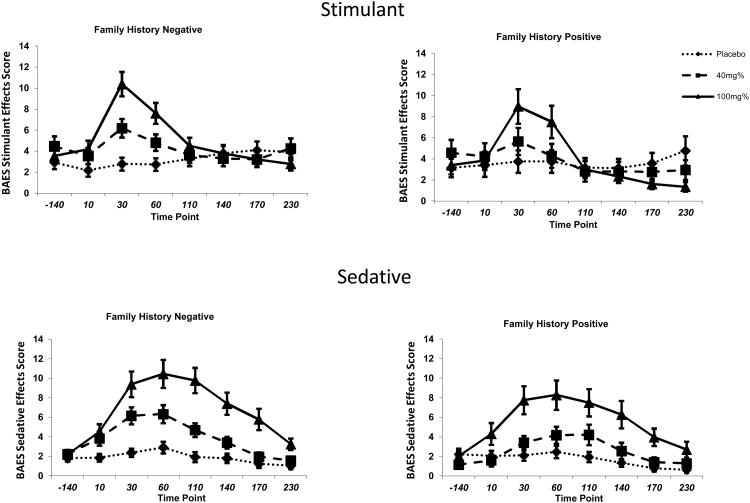
Biphasic Alcohol Effects Scale
As shown in Figure 2, both low and high dose ethanol produced significant effects in a dose-related manner associated with the ascending limb (stimulant subscale of the BAES) of alcohol intoxication [treatment by time effect: num df = 9.5, ATS = 22.2, p <0.0001] and the descending limb (sedative subscale of the BAES) of alcohol intoxication [treatment by time effect, num df = 9.8, ATS = 13.0, p < 0.0001]. The range of the BAES subscales is similar to the range described in other studies by our group (Krystal et al. 1997) and other groups (Martin et al. 1993) using oral alcohol administration paradigms. Low and high dose ethanol produced significantly greater stimulatory and sedative effects compared with placebo. High dose ethanol produced significantly greater stimulatory and sedative effects compared to low dose. No differences were observed between family history negative and family history positive subjects in the stimulant effects of alcohol intoxication [history by treatment by time effect: num df = 9.5, ATS = 0.52, p = 0.87] or the sedative effects of alcohol intoxication [history by treatment by time effect: num df = 9.8, ATS = 0.85, p = 0.58].
Figure 2. Biphasic Alcohol Effects Scale (BAES) subscales over Time by Family History.
Self-reported high and drowsiness
On the ‘high’ VAS, both low and high dose ethanol showed significant dose-related effects [treatment by time effect: num df = 8.6, ATS = 22.7, p < 0.0001]. These increases were the most pronounced in the high dose ethanol condition where ‘high’ levels were significantly greater compared with both placebo and low dose ethanol immediately upon reaching the target dose, and at 30, 60, 110, 140, 170, and 230 minutes after reaching the target (all p < 0.013). Low dose ethanol produced significant increases compared with placebo 10 minutes into the infusion, immediately upon reaching the target dose, and at 30, 60, 110, and 140 minutes after reaching the target (all p < 0.01). No differences were observed between family history negative and family history positive subjects [history by treatment by time effect: num df = 8.6, ATS = 0.58, p = 0.81].
On the ‘drowsy’ VAS, both low and high dose ethanol showed significant dose-related effects [treatment by time effect: num df = 10.5, ATS = 7.8, p < 0.0001]. Both low and high dose ethanol condition increased ‘drowsy’ levels compared with both placebo at 30 minutes after reaching the target and beyond (all p < 0.05). High dose ethanol produced more significant increases compared with low dose at 110, 140, 170, and 230 minutes after reaching the target (all p < 0.004). No differences were observed between family history negative and family history positive subjects [history by treatment by time effect: num df = 10.5, ATS = 0.93, p = 0.51].
Coordination
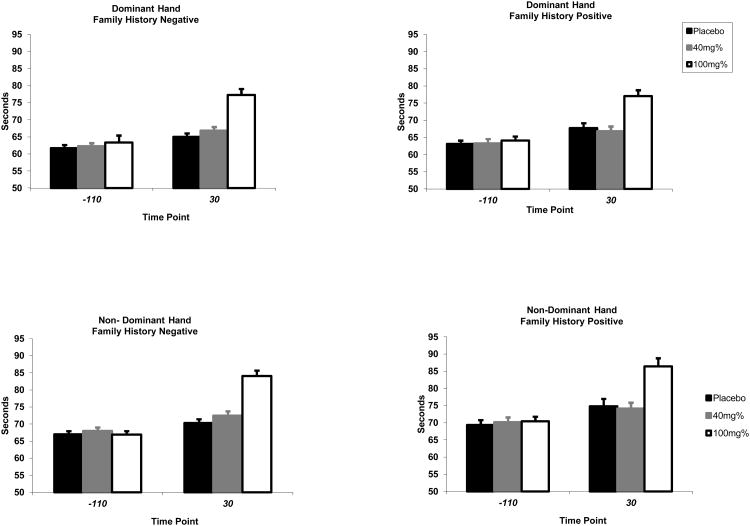
Dominant hand reaction times during the Peg Board task were significantly increased post-infusion due to ethanol administration [treatment by time effect: F(2, 831) = 35.3, p < 0.0001]. Post-infusion, observed times were greater during high dose administration compared to both placebo and low dose ethanol (all p < 0.0001). However, low dose was indistinguishable from placebo (p = 0.38). No differences were observed between family history negative and family history positive subjects [history by treatment by time effect: F(2,831) = 0.78, p = 0.46] (see Figure 3).
Figure 3. Time (in seconds) to Complete a Measurement of Dexterity Using the Grooved Peg Board at Baseline and 30 Minutes into Infusion by Family History (Dominant and Non-Dominant Hand).
Cognition
Both low and high dose ethanol adversely impaired memory in a dose-related fashion, as measured by immediate recall across 3 trials [treatment by trial effect: F(4, 1335) = 3.5, p < 0.008] and delayed recall [treatment effect: num df = 1.98, ATS = 171.5, p < 0.0001] from the Hopkins Verbal Learning Test. Adjustment for levels of drowsiness did not affect these results. No differences in cognition were observed between family history negative and family history positive subjects (all p>0.35).
Additional Analyses
Co-varying the previous analyses for total drinks in the past 30 days did not change the study results. Similar results were also found using alternate methods of analysis, including those examining peak change from baseline. A secondary analysis, using gender as a factor, was conducted on all outcome variables. There was a significant effect of gender on the VAS “high” [history by treatment by time by gender effect: num df = 8.66, ATS = 2.67, p = 0.005]. Post hoc analysis showed that among females only, there was a FH effect where FHP had a more robust response to alcohol than FHN. There was also a gender effect on similarity to alcohol [history by treatment by time by gender effect: num df = 8.22, ATS = 2.15, p = 0.03]; post hoc analysis suggest that there was a FH effect on males only where FHN males had a more robust effect than FHP males. There were no other gender effects on any of the other variables.
Discussion
Results from this study showed, as expected, that healthy individuals responded to an IV alcohol challenge in a time-dependent, dose-related manner on measures of subjective intoxication, and showed impairment in coordination and cognitive measures. High dose ethanol generally produced greater effects than low dose ethanol, which in turn were greater than placebo. In contrast to a priori expectations, however, there were no significant differences on subjective response, or on ethanol-induced impairment of coordination or cognition, between individuals with a family history positive for alcoholism and those with a negative family history.
The results from this study conflict with the results of most, but not all, studies comparing subjective and other alcohol-induced effects in FHP vs. FHN subjects. Family history differences have not been universally observed, including the recent findings of a large, placebo-controlled laboratory alcohol challenge study that showed no family history effects for acute subjective or objective alcohol responses (King et al. 2011). Those authors noted that in contrast to prior studies which focused largely on white male participants, their results were observed in a diverse sample of both men and women, with various racial backgrounds. These considerations may be similarly relevant to our present study findings, as our sample included both male (53%) and female (47%) participants, as well as individuals from different racial and ethnic backgrounds. Secondary analyses do show differential FH response between males and females in some of the subjective response to alcohol. Interestingly, there was a FH response noted among females only for subjective intoxication, where FHP females reported a more robust response. However, there was a FH effect among males only for the perceived similarity to alcohol where FHN males had a more robust effect than FHP males.
Other confounding variables influencing response in previous studies include differences in patterns of alcohol consumption between FHP and FHN individuals. For example, in King et al.'s (King et al. 2011) study, alcohol produced greater stimulant/rewarding effects and lower sedative and cortisol responses in heavy versus light drinkers and it was noted that it was not possible to determine whether these responses were constitutional or acquired with repeated exposure to alcohol. In the present study, there were no differences between groups in measures of drinking suggesting that different patterns of alcohol consumption did not influence the results.
This leaves the question why there were no differences between FHP and FHN in the current study. Even after testing for acute (within-session) tolerance as outlined by Ramchandani (Ramchandani et al. 1999) et al , no family history effects were observed in either the index of initial response to alcohol or the index of acute adaptation to alcohol. Previous studies utilizing the IV clamp method have found differences between FHP and FHN groups at BrAC levels of 0.06 (Blekher et al. 2002; Morzorati et al. 2002). Therefore, it may not be surprising that our study did not find family history-associated differences at the lower ethanol dose, though differences at the higher dose would be expected. While previous studies also used within-subjects, placebo-controlled designs, that work differed from the present study in several ways, including less stringent FHP criteria (at least two first- or second-degree relatives with alcohol dependence) and a larger age range (21-39 years). This study is the largest IV study to date that controlled for differences in baseline alcohol consumption, used stringent FH criteria. The lack of effect suggests that FH may be not be a specific enough marker for risk, particularly in individuals past the age where alcohol use disorders can develop. Studying younger subjects would be interesting, but unfortunately is limited by ethical and legal constraints. Other more specific markers, such as genotypes may be more relevant in identifying risk. This work is ongoing and being conducted by our group and others (Petrakis et al. 2011; Roh et al. 2011; Uhart et al. 2013).
Limitations of the current study include the fact that participation was limited to subjects in the age range of 21-30, the lower limit of which was necessitated by legal requirements. Furthermore, subjects were healthy young adults, with relatively limited drinking histories. Research was conducted in a laboratory setting and utilized intravenous administration of ethanol, a paradigm that bears little resemblance to the real-world conditions in which alcohol is typically consumed. Nonetheless, effects of dosing and time on subjective intoxication, coordination, and cognitive measures were significant and consistent with a priori expectations, with the exception of lack of family history effects.
In conclusion, this large, placebo-controlled study found that healthy individuals responded to intravenous BrAC alcohol challenge in a time-dependent, dose-related manner on measures of subjective intoxication, and showed impairment in coordination and cognitive measures. In contrast to expectations, however, no significant differences were seen in responses between family history positive and family history negative individuals. It is likely that a combination of factors, including not only family history but also complex biological, psychological, and environmental influences, determine response to alcohol in ways that have yet to be completely understood.
Acknowledgments
Funding: The authors acknowledge the important contributions of Angelina Genovese, R.N.C., M.B.A., Elizabeth O'Donnell, R.N., and Michelle Lynn SanPedro, R.N., Willie Ford of the Neurobiological Studies Unit of the VA Connecticut Healthcare System, West Haven Campus, West Haven, CT. In addition, the authors acknowledge support for this work from the Department of Veterans Affairs (Alcohol Research Center), and National Institute on Alcohol Abuse and Alcoholism (CTNA - 2P50-AA012870-07).
References
- Blekher T, Ramchandani VA, Flury L, Foroud T, Kareken D, Yee RD, Li TK, O'Connor S. Saccadic Eye Movements Are Associated With a Family History of Alcoholism at Baseline and After Exposure to Alcohol. Alc Clin Exp Res. 2002;26:1568–1573. doi: 10.1097/01.ALC.0000033121.05006.EF. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5(2):125–142. [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38(8):861–8. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. Journal of Studies on Alcohol. 1979;40(1):89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Davidson D, Camara P, Swift R. Behavioral effects and pharmacokinetics of low-dose intravenous alcohol in humans. Alcoholism, Clinical & Experimental Research. 1997;21(7):1294–9. [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcoholism: Clinical & Experimental Research. 1992;16(3):572–5. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcoholism, Clinical & Experimental Research. 1990;14(1):63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, Edgecombe J, Acampora G, Krystal JH, Petrakis I. Ethanol-like effects of thiopental and ketamine in healthy humans. J Psychopharmacol. 2010;24(2):203–11. doi: 10.1177/0269881108098612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M, Erblich B, Bovbjerg DH. Biphasic stimulant and sedative effects of ethanol: are children of alcoholics really different? Addictive Behaviors. 2003;28(6):1129–39. doi: 10.1016/s0306-4603(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in females with a paternal history of alcoholism. Psychopharmacology. 2003;169(1):10–20. doi: 10.1007/s00213-003-1474-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addict Biol. 2011;17(2):465–78. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71(3):239–46. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Archives of General Psychiatry. 1973;28(2):238–43. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- King A, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek M. Hypothalamic-pituitary-adrenocortical (HPA) Axis Response and Biotransformation of Oral Naltrexone: Preliminary Examination of Relationship to Family History of Alcoholism. Neuropsychopharmacology. 2002;26(6):778–88. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry. 2011;68(4):389–99. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, DSouza DC, Trevisan L, Krasnicki S, Charney DS. Interactive effects of high dose intravenous glycine and oral D-cycloserine in healthy human subjects. Biological Psychiatry. 1997;41:74–74. [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical & Experimental Research. 1993;93:8–15. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcoholism: Clinical & Experimental Research. 2010;34(3):385–95. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O'Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcoholism: Clinical & Experimental Research. 2002;26(8):1299–306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychological Bulletin. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Perrino AC, Jr, Ralevski E, Acampora G, Edgecombe J, Limoncelli D, Petrakis IL. Ethanol and pain sensitivity: effects in healthy subjects using an acute pain paradigm. Alcoholism: Clinical & Experimental Research. 2008;32(6):952–8. doi: 10.1111/j.1530-0277.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Yang BZ, Krystal JH, Edens E, Koretski J, Limoncelli D, Gelernter J. Role of Gaba-Related Genes in Mediating Subjective Response to Iv Ethanol in Healthy Subjects. Alcoholism-Clinical and Experimental Research. 2011;35(6):131a–131a. [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li TK, O'Connor S. Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg% clamp. Journal of Studies on Alcohol. 2002;63(6):734–44. doi: 10.15288/jsa.2002.63.734. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S. Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health. 2006;29(4):286–90. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger JJ, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcoholism: Clinical & Experimental Research. 1999;23(8):1320–30. [PubMed] [Google Scholar]
- Roh S, Sachio M, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, Higuchi S. Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alc Clin Exp Res. 2011;35(3):400–407. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993;76(3 Pt 2):1219–30. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41(3):242–9. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151(2):184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcoholism: Clinical & Experimental Research. 2001;25(3):323–9. [PubMed] [Google Scholar]
- Schuckit MA, Morrison CR, Gold EO. A pragmatic alcoholism treatment outcome scale. American Journal of Drug & Alcohol Abuse. 1984;10(1):125–31. doi: 10.3109/00952998409002660. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53(3):202–10. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Danko GP. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcohol Clin Exp Res. 2005;29(11):1921–7. doi: 10.1097/01.alc.0000187154.94681.65. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol & Alcoholism. 2000;35(3):242–8. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Subramanian M, Heil S, Kruger M, Collins K, Buck P, Zawacki T, Abbey A, Sokol R, Diamond M. A three-stage alcohol clamp procedure in human subjects. Alcoholism: Clinical and Experimental Research. 2002;26(10):1479–83. doi: 10.1097/01.ALC.0000034038.41972.36. [DOI] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, McCaul ME, Guo X, Yan X, Kranzler HR, Li N, Wand GS. GABRA2 markers moderate the subjective effects of alcohol. Addict Biol. 2013;18(2):357–369. doi: 10.1111/j.1369-1600.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]