Abstract
Rationale
Adiponectin enhances insulin action and induces nitric oxide–dependent vasodilatation. Insulin delivery to muscle microcirculation and transendothelial transport are 2 discrete steps that limit insulin's action. We have shown that expansion of muscle microvascular surface area increases muscle insulin delivery and action.
Objective
To examine whether adiponectin modulates muscle microvascular recruitment thus insulin delivery and action in vivo.
Methods and Results
Overnight fasted adult male rats were studied. We determined the effects of adiponectin on muscle microvascular recruitment, using contrast-enhanced ultrasound, on insulin-mediated microvascular recruitment and whole-body glucose disposal, using contrast-enhanced ultrasound and insulin clamp, and on muscle insulin clearance and uptake with 125I-insulin. Globular adiponectin potently increased muscle microvascular blood volume without altering microvascular blood flow velocity, leading to a significantly increased microvascular blood flow. This was paralleled by a ≈30% to 40% increase in muscle insulin uptake and clearance, and ≈30% increase in insulin-stimulated whole-body glucose disposal. Inhibition of endothelial nitric oxide synthase abolished globular adiponectin-mediated muscle microvascular recruitment and insulin uptake. In cultured endothelial cells, globular adiponectin dose-dependently increased endothelial nitric oxide synthase phosphorylation but had no effect on endothelial cell internalization of insulin.
Conclusions
Globular adiponectin increases muscle insulin uptake by recruiting muscle microvasculature, which contributes to its insulin-sensitizing action.
Keywords: adiponectin, endothelium, insulin delivery, microcirculation, muscle
Adiponectin circulates as both a full-length form (full-length adiponectin [fAd]) and a C-terminal globular domain (globular adiponectin [gAd]) that bind to adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) to exert many distinct effects.1–3 Ample evidence has confirmed that adiponectin has insulin-sensitizing properties and hypoadiponectinemia is linked to the development of insulin resistance and type 2 diabetes mellitus in humans.3–5 Indeed, plasma levels of adiopnectin are decreased in the insulin-resistant states and replenishment of adiponectin ameliorates insulin resistance in liver, adipose tissue, and skeletal muscle.6–9 In Wistar rats, adenovirus-mediated overexpression of adiponectin augments skeletal muscle insulin sensitivity.10 The mechanisms underlying the insulin-sensitizing effect of adiponectin have been extensively studied but remain to be defined. Adiponectin activates AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α, and increases fatty acid oxidation and glucose uptake, each of which can contribute to increased insulin sensitivity.1–3 In addition, adiponectin has been shown to increase muscle insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of insulin receptor substrate 1.11
Adiponectin also is a potent vasodilator.12,13 This effect is mediated through a nitric oxide (NO)–dependent pathway because inhibition of NO synthase blocks adiponectin's vasodilatory effect, and in cultured endothelial cells adiponectin directly increases NO production.14–16 Conversely, hypoadiponectinemia is associated with impaired vasoreactivity in humans,17 which could contribute to the development of cardiovascular complications of diabetes mellitus.
We and others have shown that factors that increase endothelial production of NO, such as systemic administration of insulin,18,19 glucagon-like peptide 1,20 or an angiotensin II type 1 receptor blocker21,22 all potently vasodilate the muscle precapillary arterioles to increase microvascular perfusion, and this action is associated with increased insulin action in muscle. As muscle microvasculature provides the principal endothelial surface area for substrate exchange between plasma and muscle interstitium, and resting blood flow in muscle is relatively low, even a small increase in the muscle microvascular blood volume (MBV) can significantly increase substrate and hormonal exchanges, including that of insulin, resulting in increased insulin delivery and action in muscle. Indeed, insulin's microvascular action is closely coupled with its metabolic action, and inhibition of insulin-mediated microvascular recruitment with NO synthase inhibitor decreases insulin-stimulated glucose disposal by ≈40%.18,19
In the current study, we hypothesized that adiponectin enhances muscle insulin action by regulating muscle microvascular recruitment and muscle insulin uptake in vivo. Our results indicate that gAd potently recruits muscle microvasculature via an NO-dependent pathway by expanding muscle MBV, which leads to increased muscle insulin delivery and insulin-stimulated whole-body glucose disposal.
Methods
Animal Preparations and Experimental Protocols
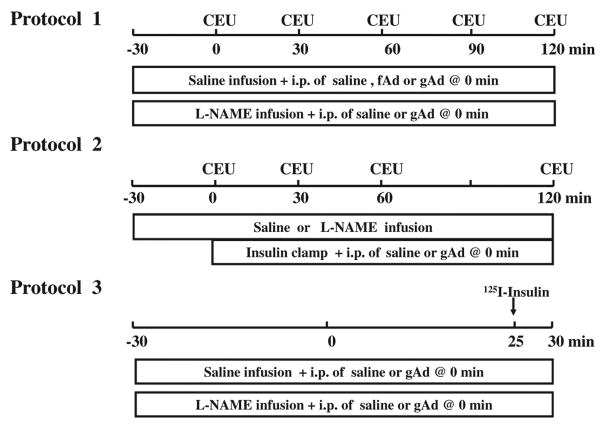
Overnight fasted, adult, male Sprague-Dawley rats (250–360 g; Charles River Laboratories, Wilmington, MA) were studied. Rats were housed at 22±2°C on a 12-hour light-dark cycle and fed standard laboratory chow and water ad libitum before the study. After being anesthetized with pentobarbital sodium (50 mg/kg intraperitoneal [IP]; Abbott Laboratories, North Chicago, IL), rats were placed in a supine position on a heating pad to ensure euthermia and intubated to maintain a patent airway. The carotid artery and the jugular vein were cannulated with PE50 polyethylene tubing (Fisher Scientific, Newark, DE) for arterial blood pressure monitoring, arterial blood sampling, and various infusions. After a 30- to 45-minute baseline period to assure hemodynamic stability and stable anesthesia, rats were studied under the following 3 protocols (Figure 1).
Figure 1. Experimental protocols.
CEU indicates contrast-enhanced ultrasound; fAd, full-length adiponectin; gAd, globular adiponectin; and L-NAME, NG-nitro-l-arginine methyl ester.
In protocol 1 (Figure 1, top panel), rats received an IP injection of either fAd (0.2 μg/g body weight) or gAd (0.2 or 0.4 μg/g body weight), or saline 30 minutes after initiation of systemic infusion of either NG-nitro-l-arginine methyl ester (L-NAME; 50 μg/kg per minute; Sigma-Aldrich, St. Louis, MO) or saline. Both fAd and gAd were obtained from Biovision Inc (Mountain view, CA). Hindlimb skeletal muscle MBV and microvascular blood flow velocity (MFV) were measured before and then every 30 minutes after IP injection using contrast-enhanced ultrasound, as previously described.18,20,22–24 Microvascular blood flow was calculated as the product of MBV and MFV. Plasma NO concentrations were determined at time 0, 30, 60, 90, and 120 minutes, as described below.
In protocol 2 (Figure 1, middle panel), rats received a 2-hour infusion of insulin (3 mU/kg per minute) after IP injection of either saline or gAd (0.4 μg/g body weight) in the presence or absence of L-NAME infusion (50 μg/kg per minute). Hindlimb skeletal muscle MBV and MFV were measured before and then every 30 minutes after IP injection using contrast-enhanced ultrasound. Arterial blood glucose was determined every 10 minutes using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN), and 30% dextrose (30% wt/vol) was infused at a variable rate to maintain blood glucose within 10% of basal.25,26 Steady-state whole-body glucose disposal rates were calculated. Skeletal muscle MBV, MFV, and microvascular blood flow were determined at time 0, 30, 60, and 120 minutes. Rats were then euthanized, gastrocnemius muscle freeze-clamped for later measurement of Akt and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation using Western blotting, as described previously.25,26
In protocol 3 (Figure 1, lower panel), rats received a systemic infusion of either saline or L-NAME for 60 minutes (from −30 to 30 minutes). At time 0, gAd (0.4 μg/g body weight) or saline was injected intraperitoneally. Each rat then received a bolus injection of 125I-insulin administered intravenously (1.5 μCi; PerkinElmer Inc, Boston, MA) 25 minutes later and was euthanized at 30 minutes. Blood and gastrocnemius were obtained for determination of muscle clearance of insulin and muscle 125I-insulin uptake.
Throughout the study, mean arterial blood pressure was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA; and AD Instruments, Inc, Colorado Springs, CO). Pentobarbital sodium was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study. Adiponectin was given as a single-dose intraperitoneal injection because of its long plasma half-life of ≈3 to 6 hours.27,28 The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85–23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia.
Determination of Hindleg Glucose Uptake
Carotid arterial and femoral venous blood glucose concentrations were determined using an Accu-Chek Advantage blood glucometer (Roche), as previously described,20,22 and hindleg glucose uptake (mg/dL) was calculated as the arterial-venous glucose differences.
Measurement of Plasma NO, Insulin, and Adiponectin Levels
Plasma NO levels were measured using 280i Nitric Oxide Analyzer (GE Analytical Instruments), according to the manufacturer's instructions. In brief, ice-cold ethanol was mixed with plasma samples at a ratio of 2:1, kept at 0°C for 30 minutes, and then centrifuged at ≈14 000 RPM for 5 minutes. The supernatant was then used for NO analysis, based on a gas-phase chemiluminescent reaction between NO and ozone. Plasma insulin concentrations were determined using a rat insulin ELISA assay kit (Mercodia AB, Uppsala, Sweden). Plasma adiponectin was measured using an ab108784 Adiponectin Rat ELISA kit (Abcam, San Francisco, CA), which recognizes both full-length and golublar adiponectin.
Determination of Muscle Uptake of 125I-Insulin
125I-insulin was used as tracer to track the muscle clearance and uptake of native insulin, as described previously.20,21,24 Five minutes after a bolus injection of 1.5 μCi 125I-insulin administered intravenously, blood sample was collected and each rat was then flushed with 120 mL ice-cold saline (10 mL/min) via the carotid artery catheter. Gastrocnemius muscle was then dissected from the right hindlimb. Protein-bound 125iodine in blood and muscle samples was precipitated with 30% trichloroacetic acid and radioactivity was measured using a γ-counter. Muscle clearance of 125I-insulin (muscle 125I-insulin [disintegrations per minute/g dry weight]/blood 125I-insulin [disintegrations per minute/mL]/5 minutes) and muscle 125I-insulin uptake (muscle 125I-insulin [disintegrations per minute/g dry weight]/5 minutes) were calculated.
Culture of Endothelial Cells and Determination of Endothelial Insulin Uptake
Endothelial cell insulin uptake was assessed using 125I-insulin, as previously reported.29-32 In brief, bovine aortic endothelial cells in primary culture were purchased from Lonza (Walkersville, MD). Cells between passages 3 to 6 were cultured in 6-well plates until 80% confluence, serum starved for 18 to 22 hours, and then incubated with prewarmed N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-binding buffer (0.1 mol/L HEPES, 0.12 mol/L NaCl, 5 mmol/L KCl, 1.2 mmol/L MgSO4, 8 mmol/L glucose, and 1% bovine serum albumin; pH 7.8) containing 200 pmol/L 125I-insulin in the presence or absence of unlabelled regular insulin (2 μmol/L) or various concentrations of gAd (0.1, 1, 2, 3 μg/mL) at 37°C for 15 minutes. The reaction was stopped by transferring the culture plates onto ice. Cells were washed once with ice-cold HEPES-binding buffer, then twice with ice-cold acid solution (0.5 mol/L NaCl, 0.2 mol/L acetic acid, pH 3.0) to remove surface-bound 125I-insulin, and then lysed with 0.5 mL 1 mol/L NaOH on ice for 1 hour. Aliquots of cell lysate were used for protein content determination and radioactivity quantification using a gamma counter. After subtracting the nonspecific binding, endothelial insulin uptake was calculated and expressed as cpm/μg protein.
Determination of Protein Phosphorylation in Muscle and Endothelial Cells
Phosphorylation of endothelial nitric oxide synthase (eNOS), Akt, ERK1/2, and AMPK in muscle and cultured endothelial cells were determined by Western blotting, as described previously.21,33,34 Primary antibodies against phospho-eNOS (Ser1177), total eNOS, phospho-Akt (Ser473), total Akt, phospho-ERK (Thr202/Tyr204), total ERK1/2, phospho-AMPK (Thr172), and total AMPK were obtained from Cell Signaling Technology (Beverly, MA). All blots were developed using enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). Autoradiographic films were scanned densitometrically and quantified using ImageQuant 3.3 software. Both the total and phosphospecific densities were quantified and the ratios of phosphospecific to total density calculated.
Statistical Analysis
All data are presented as mean±SEM. Statistical analyses were performed with SigmaStat 3.1.1 software (Systat Software, Inc), using student t test or ANOVA with post hoc analysis as appropriate. P<0.05 was considered statistically significant.
Results
Adiponectin Increases Skeletal Muscle Microvascular Perfusion via a NO-Dependent Pathway
We first tested whether adiponectin exerts vasodilatory action in the muscle microvasculature and, if so, whether this effect was NO-dependent. We injected rats intraperitoneally with fAd (0.2 μg/g body weight) or gAd (0.2 or 0.4 μg/g body weight) and then measured muscle microvascular parameters every 30 minutes for 2 hours. As shown in Figure 2, both fAd and gAd recruited muscle microvasculature by increasing MBV without altering MFV. This resulted in an ≈2-fold increase in muscle microvascular blood flow (Figure 2C). At the dose of 0.2 μg/g body weight, gAd seemed to be more effective than fAd in inducing muscle microvascular recruitment, and this effect lasted for 90 minutes. At a higher dose (0.4 μ/g body weight), gAd-induced microvascular recruitment lasted for the entire 2 hours. As such, we used gAd 0.4 μg/g body weight for all subsequent studies. The increase in muscle microvascular recruitment was paralleled by a significant increase in the plasma NO levels (Figure 2D). Neither fAd nor gAd at the doses selected affected blood glucose, blood pressure, or plasma insulin concentrations throughout the study (Table). Simultaneous infusion of L-NAME increased mean arterial blood pressure by ≈20 to 30 mm Hg without affecting blood glucose levels. However, it completely abolished the high-dose gAd-induced microvascular recruitment.
Figure 2. Adiponectin increases skeletal muscle microvascular perfusion and plasma NO levels.
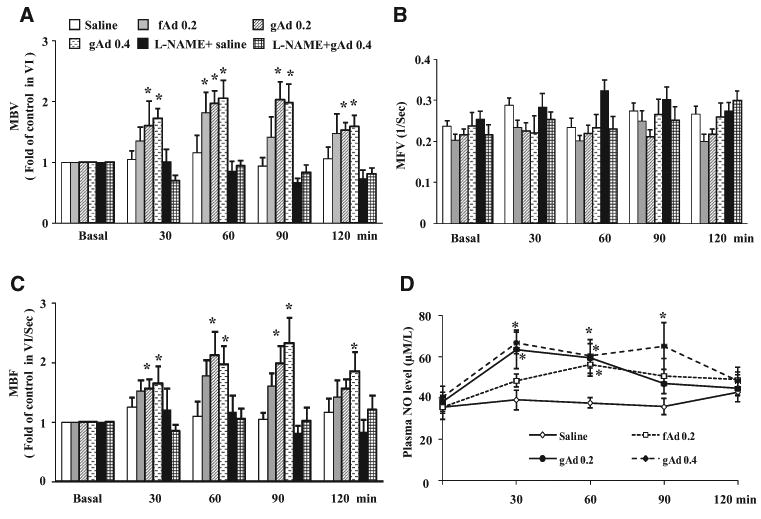
Each rat was given an intraperitoneal injection of either full-length adiponectin (fAd; 0.2 μg/g body weight), globular adiponectin (gAd; 0.2 or 0.4 μg/g body weight), or saline in the absence or presence of NG-nitro-l-arginine methyl ester (L-NAME) infusion. Contrast-enhanced ultrasound (CEU) measurements were done before and then every 30 minutes after IP injection. A, Microvascular blood volume (MBV). B, Microvascular blood flow velocity (MFV). C, Microvascular blood flow (MBF). D, Plasma NO levels. n=5 to 6. Compared with saline control *P<0.05 (ANOVA).
Table. Changes in Blood Pressure, Blood Glucose Levels, Plasma Insulin, and Adiponectin Concentrations.
| 0 min | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| MAP, mm Hg | |||||
| Saline | 102.2±2.3 | 101.5±1.5 | 101.7±2.6 | 98.3±3.3 | 99.0±2.6 |
| fAd 0.2 | 107.0±1.9 | 100.3±1.7 | 103.3±0.6 | 104.3±3.1 | 103.2±2.8 |
| gAd 0.2 | 102.2±2.9 | 100.3±3.2 | 98.8±4.0 | 97.0±3.8 | 96.2±2.5 |
| gAd 0.4 | 113.5±1.6 | 102.7±1.8 | 101.7±1.2 | 100.0±2.8 | 101.8±2.9 |
| L-NAME+Saline | 127.4±2.5* | 131.4±2.1* | 129.0±2.4* | 130.6±1.7* | 131.8±1.7* |
| L-NAME+gAd 0.4 | 131.0±0.9* | 129.4±2.2* | 130.8±1.4* | 128.6±3.3* | 131.4±1.2* |
| Blood glucose concentrations, mg/dL | |||||
| Saline | 92.4±5.3 | 94.3±2.8 | 93.3±3.3 | 98.5±4.0 | 96.6±5.5 |
| fAd 0.2 | 93.7±8.4 | 96.3±9.8 | 97.8±10.1 | 99.0±6.8 | 98.5±7.2 |
| gAd 0.2 | 96.0±5.7 | 97.2±2.7 | 95.5±4.1 | 97.3±5.43 | 93.8±3.3 |
| gAd 0.4 | 86.6±6.6 | 87.0±10.5 | 86.3±11.0 | 92.3±11.0 | 88.3±10.5 |
| L-NAME+Saline | 89.0±5.0 | 93.2±4.7 | 97.5±5.9 | 94.6±6.1 | 95.0±4.6 |
| L-NAME+gAd 0.4 | 93.0±1.0 | 97.7±4.0 | 95.6±5.5 | 92.6±6.7 | 93.0±4.6 |
| Plasma insulin concentrations, pmol/L | |||||
| Saline | 104.4+9.4 | 87.8+7.1 | 93.3±7.1 | 99.9±6.7 | 98.9±7.4 |
| fAd 0.2 | 104.2±7.1 | 111.5±11.1 | 88.2±7.2 | 83.9±6.6 | 104.3±6.6 |
| gAd 0.2 | 111.1±11.6 | 122.6±12.5 | 91.1±12.0 | 103.0±7.8 | 106.1±6.5 |
| gAd 0.4 | 108.1±10.8 | 114.3±2.2 | 103.8±9.5 | 103.0±7.6 | 102.7±9.3 |
| L-NAME+Saline | 127.9±23.0 | 118.2+4.8 | 99.3±14.5 | 117.2±9.1 | 118.6±13.4 |
| L-NAME+gAd 0.4 | 109.0±16.8 | 113.2±12.9 | 113.7±11.6 | 133.3±17.0 | 112.1±12.3 |
| Plasma adiponectin concentrations, μg/mL | |||||
| Saline | 5.7±0.12 | 6.06+0.14 | 5.21±0.41 | 5.55±0.23 | 5.45±0.47 |
| fAd 0.2 | 5.81±0.74 | 6.97±0.50 | 7.69±0.25 | 8.49±0.77* | 8.61±0.71* |
| gAd 0.2 | 5.66±0.68 | 7.74±0.46 | 7.59±0.80 | 7.91±0.79 | 7.81±0.45 |
| gAd 0.4 | 5.76±0.50 | 9.65±1.04* | 9.47±0.66* | 9.65±0.32* | 9.81±0.83* |
Values are mean±SEM. n=5-6. fAd 0.2 indicates full-length adiponectin 0.2 μg/g body weight; gAd 0.2, globular adiponectin 0.2 μg/g body weight; gAd 0.4, globular adiponectin 0.4 μg/g body weight; L-NAME, NG-nitro-l-arginine methyl ester; and MAP, mean arterial blood pressure.
Compared with saline group,
P<0.05.
Adiponectin Enhances Muscle Metabolic Responses to Insulin
As muscle microvascular recruitment is associated with increased insulin action in muscle, we next examined whether adiponectin could modulate insulin-mediated glucose disposal during insulin infusion. Insulin infusion raised plasma insulin concentrations from 113.1±8.6 to 621.1±42.9 pmol/L in the saline group and from 118.4±11.7 to 612.2±43.7 pmol/L in the gAd group (n=5; NS between groups). Administration of gAd before the initiation of insulin significantly increased insulin-mediated whole-body glucose disposal by ≈30% over the entire clamp period (P<0.001, ANOVA) and at steady state (90–120 minutes; P<0.05; Figure 3A and 3B). Coinfusion of L-NAME completely abolished gAd-induced increases in insulin-stimulated glucose disposal. Infusion of gAd alone (0.4 μg/g body weight) did not significantly alter hindleg [A]–[V] glucose differences (3.5±0.4, 4.9±0.5, 4.2±0.4, 4.6±0.3, and 4.8±0.4 mg/dL for baseline, 30 minutes, 60 minutes, 90 minutes, and 120 minutes, respectively; n=6; P=0.107, ANOVA). Neither blood glucose concentrations nor femoral blood flows changed during gAd-alone infusion (P>0.05).
Figure 3. Globular adiponectin (gAd) augments insulin-stimulated whole-body glucose disposal.
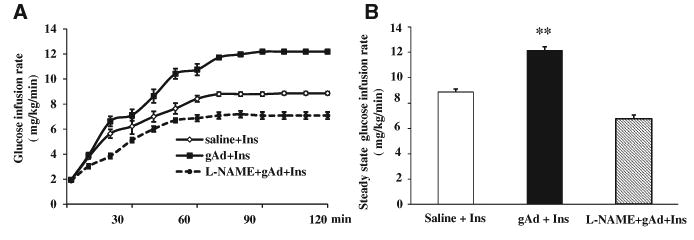
Each rat received IP injection of saline or gAd (0.4 μg/g body weight) followed by a 2-hour euglycemic hyperinsulinemic clamp (3 mU/kg per minute) in the presence or absence of NG-nitro-l-arginine methyl ester (L-NAME) infusion. A, Time course of glucose infusion rate (GIR). Compared with saline group, P<0.001 (ANOVA). B, Steady-state GIR. Compared with saline control and L-NAME groups, **P<0.01. n=5 to 6.
To further confirm that adiponectin indeed modulated insulin signaling in muscle, we examined the phosphorylation status of Akt and ERK1/2, two intermediate signaling molecules in the insulin-signaling pathway, in biopsied gastrocnemius samples at the conclusion of insulin infusion (Figure 4). Administration of gAd at either dose did not significantly increase muscle Akt phosphorylation. Insulin infusion alone increased muscle Akt phosphorylation by >2-fold (P<0.05). In the presence of gAd, insulin-mediated Akt phosphorylation further increased to 3-fold of the control level (P<0.001). In contrast, gAd at 0.4 μg/g body weight enhanced muscle ERK1/2 phosphorylation to the extent seen with insulin alone (P<0.05, ANOVA), and the combination of gAd and insulin did not further increase the extent of ERK1/2 phosphorylation.
Figure 4. Effects of globular adiponectin (gAd) on muscle Akt and ERK1/2 phosphorylation.
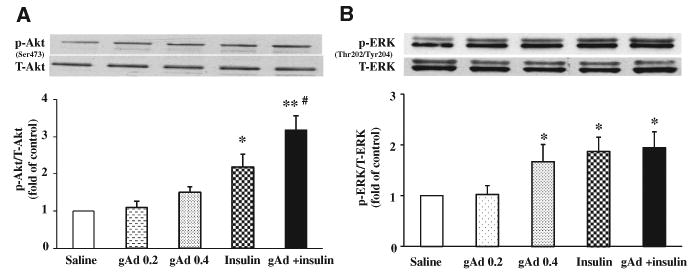
A, Changes in muscle Akt phosphorylation. B, Changes in muscle ERK phosphorylation. Compared with saline control, *P<0.05, **P<0.01. Compared with insulin #P<0.05. n=3 to 4. gAd 0.2 indicates gAd 0.2 μg/g body weight; gAd 0.4, gAd 0.4 μg/g body weight; insulin, 3 mU/kg per minute; gAd+insulin, gAd 0.4 μg/g body weight + insulin 3 mU/kg per minute.
Adiponectin Increases Muscle 125I-Insulin Uptake via an NO-Dependent Pathway
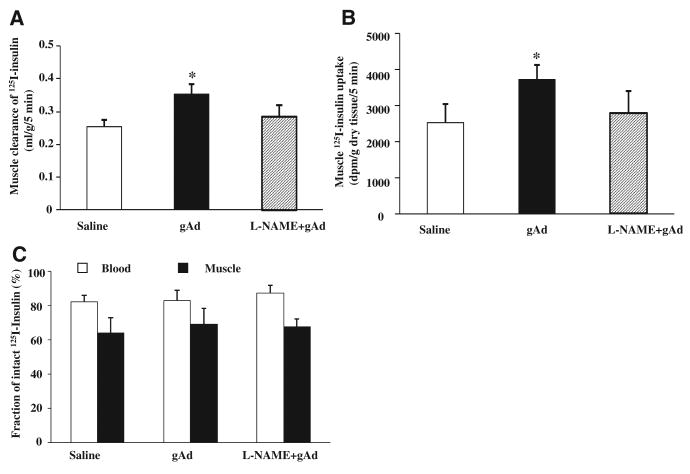
As insulin action in muscle is closely correlated with interstitium muscle insulin concentrations,35 we next examined whether the increased metabolic responses to insulin after gAd administration was secondary to increased muscle uptake of insulin. We used 125I-insulin to trace native insulin movement in vivo. As shown in Figure 5, gAd administration significantly increased muscle clearance of insulin from blood (Figure 5A) and uptake of insulin (Figure 5B), and this effect was again completely abolished by the inhibition of NO production with L-NAME. These changes were clearly not caused by differential degradation of 125I-insulin as evidenced by comparable fractions of intact 125I-insulin in both blood and muscle across all 3 groups (Figure 5C).
Figure 5. Globular adiponectin (gAd ) increases muscle 125I-insulin uptake.
Blood and skeletal muscle were collected 5 minutes after bolus injection of 125I-insulin (1.5 μCi) administered intravenously. Intact 125I-insulin was determined after trichloroacetic acid (30%) precipitation. A, Muscle clearance of insulin. B, Muscle insulin uptake. C, Fraction of 125I-insulin in blood and in muscle. n=5 to 6. Compared with saline group, *P<0.05. L-NAME indicates NG-nitro-l-arginine methyl ester; and dpm, disintegrations per minute.
Adiponectin Increases Muscle Microvascular Recruitment Without Affecting Endothelial Insulin Uptake
The increased muscle uptake of insulin could be secondary to increased insulin delivery to the muscle via microvascular recruitment and transendothelial insulin transport. Figure 6 showed that combination of adiponectin and insulin recruited significantly more muscle microvasculature than insulin alone. As found in a previous report,21 insulin infusion alone increased both muscle MBV and microvascular blood flow by ≈60% (P<0.05) without affecting MFV. Administration of gAd before the initiation of insulin recruited significantly more microvasculature by expanding MBV (P<0.05) without altering MFV.
Figure 6. Globular adiponectin (gAd) enhances insulin-mediated muscle microvascular recruitment.
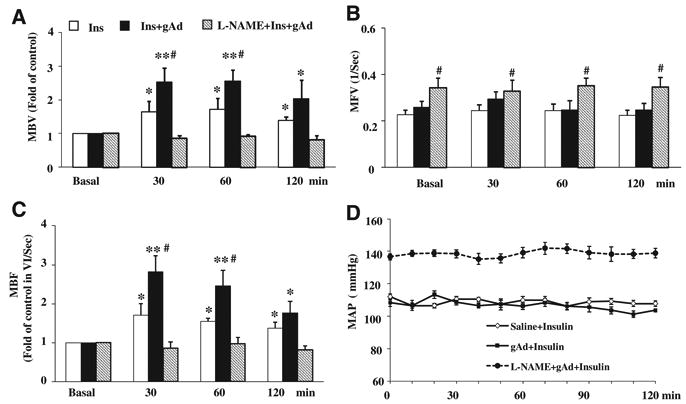
Each rat received an IP injection of saline or gAd (0.4 μg/g body weight) followed by a 2-hour euglycemic hyperinsulinemic clamp (3 mU/kg per minute). Contrast-enhanced ultrasound measurements were done at the set time points. A, Microvascular blood volume (MBV). B, Microvascular blood flow velocity (MFV). C, Microvascular blood flow (MBF). D, Mean arterial blood pressure (MAP). n=5 to 7. Compared with basal, *P<0.05, **P<0.05; compared with insulin group, #P<0.05. L-NAME indicates NG-nitro-l-arginine methyl ester.
We finally assessed whether gAd treatment alters the uptake of insulin in cultured endothelial cells, which is the first and rate-limiting step in transendothelial insulin transport, and signaling proteins that could contribute to this process. As shown in Figure 7, similar to insulin (10 nmol/L), gAd significantly increased the phosphorylation of both Akt (Ser473) and eNOS (Ser1177; P<0.05, ANOVA). Treatment of cells with both insulin and gAd further increased Akt and eNOS phosphorylation over insulin alone (P<0.05), suggesting an additive effect. There was no additive effect on AMPK phosphorylation. However, gAd had no effect on endothelial uptake of 125I-insulin (P=0.217, ANOVA).
Figure 7. Globular adiponectin (gAd) increases endothelial nitric oxide synthase (eNOS) phosphorylation but not endothelial 125I-insulin uptake in cultured bovine aortic endothelial cells.
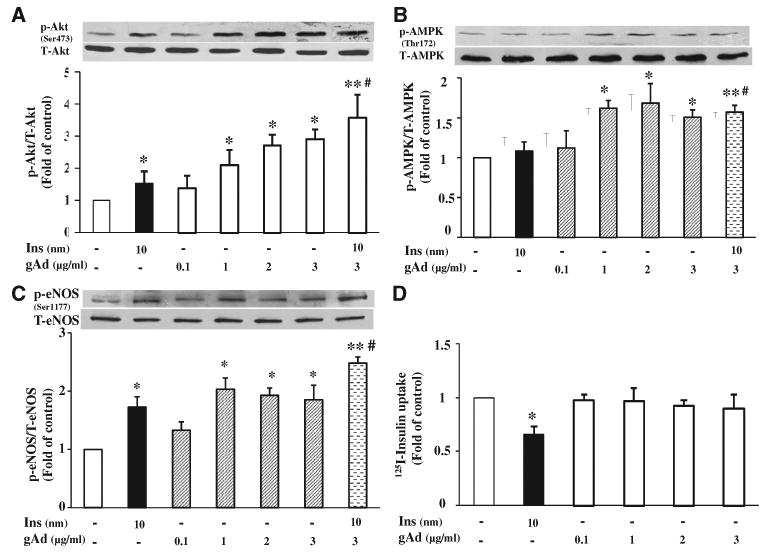
A, Changes in Akt phosphorylation. B, Changes in AMP-activated protein kinase (AMPK) phosphorylation. C, Changes in eNOS phosphorylation. D, Changes in endothelial 125I-insulin uptake. n=3 to 4. Compared with control, *P<0.05, **P<0.01. Compared with insulin #P<0.05.
Discussion
Diabetes mellitus is associated with hypoadiponectinemia, and adiponectin has been shown to improve insulin sensitivity. Muscle microvasculature provides endothelial exchange surface area to facilitate nutrient and hormonal exchanges between plasma and muscle interstitium. In the current study, we used a combination of the insulin clamp and contrast-enhanced ultrasound techniques and found that gAd potently recruited muscle microvasculature, which was associated with increased muscle insulin uptake and action. Inasmuch as adiponectin has both insulin-sensitizing and vasodilatory effects (in the conduit arteries and resistance arterioles), we tested whether adiponectin's vascular actions contributed directly to its insulin-sensitizing actions. Our results show for the first time that adiponectin vasodilates precapillary arterioles to increase muscle microvascular perfusion and thereby endothelial exchange surface area. This action improved insulin and glucose delivery to muscle capillary exchange surfaces and allowed increased glucose and insulin uptake. These findings provide the first demonstration that a significant component of the insulin-sensitizing effect of adiponectin derives from its vascular effects.
For insulin to act on muscle cells, it has to be first delivered to muscle microcirculation and then muscle interstitium via transendothelial transport, 2 discrete steps that limit insulin's action.36,37 We and others have shown that multiple interventions that increase muscle's endothelial exchange surface area also increase muscle insulin uptake.20,21,24,38 Our current findings again indicate that the muscle microvasculature plays an important role in the regulation of muscle insulin action. We found a close coupling of gAd-induced microvascular recruitment and increased insulin uptake and action in muscle. Inasmuch as both insulin delivery to the microcirculation and insulin transendothelial transport can limit muscle insulin uptake and action, the absence of an effect of gAd on insulin uptake of endothelial cells suggests that gAd's insulin-sensitizing action in muscle is caused by recruiting microvasculature and expanding the exchange surface area.
Adiponectin exerts pleiotrophic actions on the vasculature via direct actions on almost all the major cell types present in blood vessels and protects against endothelial dysfunction, atherosclerosis, and hypertension.39 Unlike previous reports of adiponectin vasodilating larger vessels (eg, aorta and mesenteric arteries),12,13 we found that adiponectin has a potent vasodilatory effect on the microvasculature even in the absence of increases in total leg blood flow. This is of particular significance as this microvascular effect can account for some of the effects of adiponectin to regulate tissue/organ substrate metabolism.3,39–43 It is thus not surprising that hypoadiponectinemia is not only associated with endothelial dysfunction and hypertension, but also insulin resistance and diabetes mellitus.
Our study suggests that gAd is more potent than fAd in recruiting muscle microvasculature. Indeed, at a dose of 0.2 μg/g body weight, fAd only increased muscle MBV at 60 minutes despite persistently elevated plasma concentrations whereas gAd potently increased muscle MBV at 30 minutes, and this effect lasted for 90 minutes. At 0.4 μg/g body weight, gAd's microvascular effect lasted for the entire 2 hours. This is not surprising as both AdipoR1 and AdipoR2 exhibit 3- to 4-fold higher binding affinity for gAd than for fAd.1 Although endothelial cells and microvessels express both AdipoR1 and AdipoR2,16,44 it is possible that gAd may have acted via the AdipoR1 as it is the most abundant isoform in the skeletal muscle and AdipoR2 is more prevalent in the liver.1 It is of interest to note that in cultured muscle cells gAd has also been shown to directly increase GLUT4 translocation and glucose uptake.45 Taken together, our in vivo study results and the previously reported in vitro muscle cell findings suggest that gAd may act on both endothelium (to increase muscle perfusion and insulin uptake) and muscle cells (to directly increase GLUT4 translocation) to enhance muscle glucose utilization.
The microvascular effect of gAd was clearly NO-dependent as plasma NO levels increased promptly after gAd administration and simultaneous infusion of NO synthase inhibitor L-NAME completely abolished gAd-stimulated microvascular perfusion and insulin uptake. This is consistent with our in vitro observation that gAd potently increased eNOS phosphorylation at Ser1177 and previous reports that adiponectin directly increases NO production by promoting eNOS phosphorylation (Ser1177) and eNOS association with heat-shock protein 9014–16 in cultured endothelial cells, an effect that is mediated through a multiple-domain adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif.16 Consistent with these in vitro findings, treatment of diet-induced obese rats with recombinant adiponectin increased eNOS activity, NO production, and endothelium-dependent relaxation of aortic rings.46 Similarly, in an isolated mesenteric artery preparation, adiponectin-induced vasodilation was completely inhibitable by NOS inhibition,13 and administration of adiponectin to db/db diabetic mice reversed the impairment in endothelium-dependent relaxations in isolated coronary arterioles and aortae.47
It seems that insulin and adiponectin have additive effect on recruiting muscle microvasculature. Although insulin alone increased muscle MBV by ≈60% and gAd increased MBV by 100%, the combination of gAd and insulin increased MBV by >150%. This is not surprising as insulin and adiponectin act via different receptors to increase NO production, and neither insulin nor gAd seems to have maximal vasodilatory effect on the precapillary arterioles. We and others have previously shown that administration of either angiotensin II type 1 receptor losartan or glucagon-like peptide 1, or low-frequency electric stimulation can increase muscle MBV by >3-fold.20–24 This is also consistent with our in vitro cell studies demonstrating that insulin and gAd exert an additive effect on eNOS phosphorylation. Whereas insulin acts via its receptors to activate Akt and eNOS and recruit muscle microvasculature, gAd increases NO production via AdipoR1 and AdipoR2. However, there is clearly a crosstalk between the 2 systems, and adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif has been shown to counteract obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of NO and endothelial-1.48 This is potentially very important in the state of insulin resistance as insulin facilitates its own delivery and action in the insulin-sensitive state and insulin resistance blunts this action. Thus, gAd could increase insulin action in the insulin-resistant state by acting on AdipoR1 and AdipoR2 to increase insulin delivery, uptake, and action in muscle.
In conclusion, gAd recruits muscle microvasculature and expands endothelial exchange surface area via an NO-dependent mechanism, which contributes to increased muscle insulin delivery, uptake, and action. Our results provide new understanding of the mechanisms underlying the insulin-sensitizing action of gAd, and suggest that both hypoadiponectinemia and muscle microvasculature could be important therapeutic targets in the prevention and treatment of insulin resistance and diabetes mellitus.
Novelty and Significance.
What Is Known?
Adiponectin enhances insulin action and induces nitric oxide-dependent vasodilatation.
Insulin delivery to muscle microcirculation and transendothelial transport are 2 discrete steps that limit insulin's action.
Expansion of muscle microvascular surface area promotes muscle insulin delivery and action.
What New Information Does This Article Contribute?
Adiponectin expands muscle microvascular blood volume and endothelial exchange surface area by dilating the precapillary arterioles thus recruiting muscle microvasculature via a nitric oxide-dependent mechanism.
Globular adiponectin enhances insulin action in muscle by increasing muscle delivery of insulin.
The actions of globular adiponectin and insulin on muscle microvasculature are additive.
Although adiponectin has been shown to enhance the metabolic action of insulin and induce nitric oxide–dependent vasodilation, evidence linking these 2 effects is lacking. It has been shown previously that muscle microvasculature is a critically important target for insulin's metabolic action as it provides the principal endothelial surface area for substrate exchange between plasma and muscle interstitium. We examined the effects of adiponectin on muscle microvasculature and insulin's metabolic action. We found that globular adiponectin recruits muscle microvasculature and expands endothelial exchange surface area via a nitric oxide– dependent vasodilation of the precapillary arterioles. This action contributes to increased muscle insulin delivery, uptake, and action. These results provide new understanding of the mechanisms underlying the insulin-sensitizing action of globular adiponectin, and suggest that both hypoadiponectinemia and muscle micro-vasculature could be important therapeutic targets in the prevention and treatment of insulin resistance and diabetes mellitus.
Acknowledgments
Sources of Funding: This work was supported by American Diabetes Association grants 1-11-CR-30 and 9-09-NOVO-11 (to Z. Liu), and National Institutes of Health Grant R01HL094722 (to Z. Liu).
Nonstandard Abbreviations and Acronyms
- fAd
full-length adiponectin
- gAd
globular adiponectin
- MBV
microvascular blood volume
- MFV
microvascular blood flow velocity
Footnotes
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at: http://www.lww.com/reprints
Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/
Disclosures: None.
References
- 1.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 7.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 10.Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991–7996. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]
- 12.Fésüs G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, Schach C, Buechler C, Riegger GA, Luchner A, Endemann DH. Globular and full-length adiponectin induce NO-dependent vasodilation in resistance arteries of Zucker lean but not Zucker diabetic fatty rats. Am J Hypertens. 2011;24:270–277. doi: 10.1038/ajh.2010.239. [DOI] [PubMed] [Google Scholar]
- 14.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng KKY, Lam KSL, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 18.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 19.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 20.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011;60:2939–2946. doi: 10.2337/db10-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes. 2009;58:2457–2463. doi: 10.2337/db08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes. 2007;56:2194–2200. doi: 10.2337/db07-0020. [DOI] [PubMed] [Google Scholar]
- 25.Chai W, Wu Y, Li G, Cao W, Yang Z, Liu Z. Activation of p38 mitogen-activated protein kinase abolishes insulin-mediated myocardial protection against ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2008;294:E183–E189. doi: 10.1152/ajpendo.00571.2007. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Barrett EJ, Long W, Liu Z. Glucocorticoids differentially modulate insulin-mediated protein and glycogen synthetic signaling downstream of protein kinase B in rat myocardium. Endocrinology. 2004;145:1161–1166. doi: 10.1210/en.2003-1429. [DOI] [PubMed] [Google Scholar]
- 27.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 28.Hoffstedt J, Arvidsson E, Sjölin E, Wåhlén K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- 29.Dernovsek KD, Bar RS. Processing of cell-bound insulin by capillary and macrovascular endothelial cells in culture. Am J Physiol. 1985;248:E244–E251. doi: 10.1152/ajpendo.1985.248.2.E244. [DOI] [PubMed] [Google Scholar]
- 30.Hachiya HL, Takayama S, White MF, King GL. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. J Biol Chem. 1987;262:6417–6424. [PubMed] [Google Scholar]
- 31.Hachiya HL, Halban PA, King GL. Intracellular pathways of insulin transport across vascular endothelial cells. Am J Physiol. 1988;255:C459–C464. doi: 10.1152/ajpcell.1988.255.4.C459. [DOI] [PubMed] [Google Scholar]
- 32.Bertelsen M, Anggård EE, Carrier MJ. Oxidative stress impairs insulin internalization in endothelial cells in vitro. Diabetologia. 2001;44:605–613. doi: 10.1007/s001250051667. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007;148:3356–3363. doi: 10.1210/en.2006-1441. [DOI] [PubMed] [Google Scholar]
- 35.Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–E263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007;56:2958–2963. doi: 10.2337/db07-0670. [DOI] [PubMed] [Google Scholar]
- 39.Xu A, Vanhoutte PM. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;302:H1231–H1240. doi: 10.1152/ajpheart.00765.2011. [DOI] [PubMed] [Google Scholar]
- 40.Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubota N, Yamauchi T, Tobe K, Kadowaki T. Adiponectin-dependent and -independent pathways in insulin-sensitizing and antidiabetic actions of thiazolidinediones. Diabetes. 2006;55:S32–38. [Google Scholar]
- 42.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(suppl 7):S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 44.Spranger J, Verma S, Göhring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschöp M, Banks WA. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55:141–147. [PubMed] [Google Scholar]
- 45.Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132–139. doi: 10.1007/s00125-004-1609-y. [DOI] [PubMed] [Google Scholar]
- 46.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Park Y, Zhang C. Coronary and aortic endothelial function affected by feedback between adiponectin and tumor necrosis factor α in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2010;30:2156–2163. doi: 10.1161/ATVBAHA.110.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Cheng KK, Lam KS, Wu D, Wang Y, Huang Y, Vanhoutte PM, Sweeney G, Li Y, Xu A. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes. 2011;60:3044–3054. doi: 10.2337/db11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]