Abstract
Background
Fetuin-A, a protein secreted primarily by the liver, has been associated with non-alcoholic fatty liver disease and insulin resistance. In a recent study, higher circulating fetuin-A was associated with cardiovascular events, particularly ischemic stroke. However, these data have not been replicated.
Methods
A nested case-control design was utilized to examine the relationship between fetuin-A and ischemic stroke among female participants of the Nurses’ Health Study. Fetuin-A was measured in blood samples collected and stored between 1989–1990. A total of 459 incident cases of ischemic stroke were identified and confirmed by medical records according to the National Survey of Stroke criteria between 1990–2006 and matched to 459 controls by age, menopausal status, postmenopausal hormone use, and smoking status. The association between fetuin-A and ischemic stroke was modeled using conditional logistic regression.
Results
Circulating Fetuin-A was higher in women (P<0.01), who reported increased body mass index (BMI≥25 kg/m2), total cholesterol ≥200 mg/dL, high-sensitivity C-reactive protein ≥3 mg/L and current hormone use at baseline. Significant partial Spearman correlations (P<0.001), adjusted for matching factors, were found between measured concentrations of fetuin-A and triglycerides (r=0.20), C-reactive protein (r=0.14), and BMI (r=0.15). Fetuin-A quartiles were not significantly associated with increased risk of incident ischemic stroke when adjusted for matching factors (RR=1.03; 95% CI: 0.69–1.54, extreme quartiles); additional adjustment for lifestyle factors or CVD risk factors and biomarkers did not alter results.
Conclusions
In this sample of women, fetuin-A was not significantly associated with risk of ischemic stroke. Further research is needed to explore this association.
Keywords: Ischemic stroke, fetuin-A, α2-Heremans-Schmid glycoprotein
Introduction
Stroke is the fourth leading cause of death and the leading cause of long term disability in the U.S.(1) Further, the economic burden associated with stroke is estimated at 73.7 billion dollars,(2) largely due to loss of earnings. The best known risk factors for stroke such as hypertension, diabetes, smoking and hypercholesterolemia, account for only less than 50% of stroke incidence.(3) Hence, there is great need to identify additional risk factors that might help identify people at risk for stroke.
Fetuin-A (α2-Heremans-Schmid glycoprotein), a protein secreted primarily by the liver, has been associated with subclinical markers of cardiovascular disease (CVD),(4, 5) insulin resistance,(6) type 2 diabetes(7) and incidence of total CVD,(8) particularly ischemic stroke.(9) However, the concentrations at which fetuin-A has a deleterious effect remain unclear. Low concentrations of fetuin-A have been associated with increased vascular calcification(5, 10) and CVD mortality.(8, 11) However, high concentrations have been associated with increased incidence of CVD.(9) Of note, several of the studies evaluating the association between fetuin-A and CVD incidence or mortality have been conducted among chronically ill populations.(11, 12) In a generally healthy middle-aged population, Weikert and colleagues(9) reported that individuals in the highest quintile of fetuin-A had a 3-fold higher multivariable-adjusted risk of total CVD (RR=3.12, 95% CI:2.15–4.52) and an even greater risk of ischemic stroke (RR=3.66; 95% CI:1.99–6.71) when compared to those in the lowest quintile. Further, Laughlin et al.(8) reported that the association between fetuin-A and CVD mortality varied by diabetes status, with a positive association among those without diabetes and an inverse association among those with diabetes. However, these associations have not been replicated by specific CVD endpoints, such as ischemic stroke.
We aimed to examine whether circulating fetuin-A was associated with risk of ischemic stroke among a cohort of women after adjustment for important cardiovascular risk factors. Furthermore, we sought to evaluate whether the association varied by key CVD risk factors including age, body mass index (BMI), smoking, history of diabetes, hypertension, and postmenopausal hormone use.
Materials and Methods
Nurses’ Health Study Cohort
The Nurses’ Health Study (NHS) enrolled 121,700 primarily White female registered nurses living in 11 US states aged 30–55 years who completed a mailed questionnaire in 1976. Follow-up questionnaires have been mailed biennially, with a semi-quantitative food frequency questionnaire (FFQ) mailed approximately every 4 years since 1980. Detailed descriptions of the NHS have been published previously.(13) Over 90% of the baseline population has responded to follow-up questionnaires and mortality follow-up is greater than 98% complete.(14) Between 1989 and 1990, 32,826 participants, age 43–69 years, provided blood samples in collection tubes with sodium heparin. Additionally, approximately 10 years later (2000–2001) 18,743 of these participants provided a second blood sample. Women had their blood drawn and shipped it to our laboratory via overnight courier for processing. Samples were processed and archived and have been continuously stored in monitored liquid nitrogen freezers.(15)
A nested case control study of ischemic stroke was conducted among those women with blood samples available. Among those, stroke cases were defined as women free of known prior stroke or cancer at the time of the blood collection, but with confirmed incident ischemic stroke during follow-up. For each stroke case, one control subject was selected from those women who were free of known prior stroke or cancer at the time of the blood collection and who did not have a stroke by the time of the index case. Controls were randomly selected eligible participants and matched to the index case by age (±2 years), race/ethnicity (Caucasian/African-American/Asian/Hispanic/Other/Unknown), smoking (current/past/never at blood sample collection), menopausal status, postmenopausal hormone use at time of blood draw (yes/no), date of sample collection. Approximately 75% of the women in this sample were fasting at the time of blood collection.
Blood Sample Assay
Case-control pair samples were handled identically and shipped together to the laboratory in the same batch and assayed in the same run. Each batch included replicate, blinded plasma samples to assess laboratory precision and ‘drift samples’ standardized to a particular level for the biomarker to track and correct for laboratory assay drift.
Fetuin-A was measured by solid-phase enzyme-linked immunosorbent assay (BioVendor,) in the Clinical and Epidemiological Research Laboratory at Children’s Hospital (Boston, MA) for 457 cases-controls pairs. Reproducibility of fetuin-A concentrations was assessed among 44 samples at 3 different processing times (0, 24 and 48 hrs). Plasma fetuin-A demonstrated a mean intra-assay coefficient of variation (CV) of 7%. Fetuin-A measures were reproducible even with delayed time from blood draw to processing (with samples kept on ice); the mean CV across processing times was 8% (0–24h processing time spearman correlation coefficient=0.94, P-value<0.001 and 0–48h processing time spearman correlation coefficient=0.88, P-value<0.001) and an intra-class correlation (ICC) of 0.93 (95% CI: 0.82–0.97). Fetuin-A concentrations were not found to vary significantly by fasting status (results not shown). Furthermore, we conducted a within person stability pilot for 88 samples collected an average of 1 year apart and demonstrated a mean intra-assay CV of 15.7% and an ICC of 0.88 (95% CI: 0.76–0.94) between draws. Additionally, we estimated long-term within-person stability of measures among the 102 case-controls pairs with 2 measurements obtained approximately 10 years apart. An ICC of 0.52 (95% CI: 0.42–0.62) indicated moderate reproducibility for samples collected 10 years apart.
Total cholesterol, low density lipoprotein (LDL-C) and high density lipoprotein (HDL-C) cholesterol, high-sensitivity C-reactive protein (hs-CRP) and glycosylated hemoglobin (HbA1C) were measured in the Clinical and Epidemiological Research Laboratory at Children’s Hospital (Boston, MA) for all cases and controls. Total cholesterol was measured enzymatically, with a mean intra-assay CV 4%. LDL-C was be determined by a homogenous direct method from Roche Diagnostics with a mean intra-assay CV of 3%. HDL-C concentration was determined using a direct enzymatic colorimetric assay, with a mean intra-assay CV of 3%. CRP was assayed using a latex-enhanced immunonephelometric assay on a BN II analyzer (Dade-Behring) with a mean intra-assay CV of 2%. HbA1C levels were determined by turbidimetric immunoinhibition using packed red blood cells with an Hitachi 911 analyzer (Roche Diagnostics) with an mean intra-assay CV of 1.2%.
Cerebrovascular Disease Assessment
We included confirmed ischemic strokes that occurred from the return of the blood sample (1989–1990) through 2006, resulting in up to 17 years of follow-up. Nonfatal stroke was reported on biennial questionnaires and confirmed by medical records. Deaths were detected through information provided by the next of kin, postal authorities or by systematic searches of the National Death Index. In addition to death certificates, fatal stroke was also confirmed by review of hospital records or autopsy. Women (or next-of-kin for decedents) reporting stroke on follow-up questionnaires were asked for permission to review medical records, which were reviewed by a physician blinded to exposure status. Stroke was classified according to criteria established by the National Survey of Stroke(16) requiring evidence of a neurologic deficit with sudden or rapid onset that persisted for >24 hours or until death. Strokes were classified as ischemic stroke due to thrombotic or embolic occlusion of a cerebral artery with imaging data from CT or MRI or data on autopsy available for >92% of events. Reproducibility of our classification system is high.(17)
Statistical Analysis
Descriptive analyses for baseline characteristics were conducted comparing cases and controls and participants above and below the median concentration of fetuin-A. Fetuin-A, cholesterol/HDL-C, triglycerides and CRP displayed skewed distributions; therefore, nonparametric statistical approaches were used in these analyses and covariates were log-transformed. Fetuin-A was modeled as continuous (in spline models), in quartiles based on the control distribution, as ordinal quartiles to estimate linear trend and dichotomized at the median value. In unadjusted analyses, median fetuin-A concentrations were compared across key characteristics. Spearman correlations coefficients adjusted for matching factors were calculated between fetuin-A concentrations, age, BMI and CVD biomarkers. Conditional logistic regression models were used to evaluate the multivariable adjusted association of fetuin-A with ischemic stroke. Odds ratios (ORs) with 95% confidence intervals (CI) were used to approximate the relative risk (RR). Lifestyle and dietary covariates were utilized from the 1990 questionnaire or the closest year prior to 1990, with the exception of height (collected only in 1976).
We estimated 3 multivariable models: Model 1 adjusted for matching factors (age, race/ethnicity, smoking, menopausal status, hormone therapy use and date of sample collection), Model 2 additionally adjusted for BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), aspirin use (<1 tablet/wk, 1–5 tablets/wk, >6 tablets/wk), alcohol intake (0, >0–4.9, 5–14.9, ≥15 g/d), physical activity (METs/wk-tertiles), alternate Healthy Eating Index 2010 (aHEI 2010 - score based on a diet low in trans-fat, red and processed meats, sodium and sugar sweetened beverages, while high in fruits and vegetables, nuts and legumes, polyunsaturated and omega-3 fats and whole grains). Model 3 was further adjusted for history of diabetes (yes/no), hypertension (yes/no), coronary heart disease (CHD) or revascularization (yes/no), HbA1c, total/HDL-C and triglycerides (mg/dL). Physical activity, diet, hormonal status and chronic disease outcomes (hypertension, diabetes and heart disease) have been previously validated in this or similar populations.(18–21) A missing indicator variable was used to model missing values for smoking and aspirin intake. HbA1c was missing in 16 case control pairs and the median value was imputed by case control status. We examined the possibly non-linear relation between fetuin-A and ischemic stroke non-parametrically with restricted cubic and linear splines based on 4 knots located at: 0.30, 0.40, 0.49, 0.66 g/L.(22–24) Tests for non-linearity used the likelihood ratio test, comparing a model with a linear term to a model with a linear and cubic spline terms.
Several sensitivity analyses were conducted. A priori we proposed to evaluate effect modification by selected risk factors, age (<65/≥65 years), BMI (<25/≥25 kg/m2), smoking (nonsmoker/current), hypertension (yes/no), diabetes status (yes/no), postmenopausal hormone use (yes/no) and time period of event occurrence (<8 years from baseline blood collection versus ≥8 years). A likelihood ratio test was used to assess the significance of interactions, comparing an unconditional main effects model adjusted for matching factors and covariates to one with interaction terms included. We conducted sensitivity analyses to calculate adjusted RRs and 95% CIs for measurement error correction in the fetuin-A measurements, by using the fetuin-A samples collected approximately 10 years apart. Additionally, we estimated the association between fetuin-A and ischemic stroke subtypes (thrombotic and embolic strokes). All P-values were two-sided. Analyses were conducted with SAS for UNIX statistical software (version 9.1.3; SAS Institute).
Statement of Ethics
This study was approved by the Institutional Review Board of Brigham and Women’s Hospital and all procedures followed were in accordance with institutional guidelines. Participants provided informed consent to participate.
Results
The baseline descriptive characteristics of the 457 complete case-control pairs are presented in Table 1. The mean age was 61 years. As expected, women who later developed ischemic stroke were more likely to be hypertensive, diabetic, report a family history of heart disease and had borderline significantly higher CRP concentrations compared to controls. Median fetuin-A (g/L) concentrations were not significantly different between cases and controls.
Table 1.
Baseline Characteristics by Case Control Status in 1990
| Cases (n=457) | Controls (n=457) | p-value | |
|---|---|---|---|
| Age (yrs) | 61 ± 5.9 | 61 ± 6.0 | - |
| Fetuin-A (g/L) | 0.46 ± 0.12 (median=0.44) | 0.46 ± 0.12 (median=0.45) | 0.82 |
| BMI (kg/m2) | 25.9 ± 5.1 | 25.4 ± 4.8 | 0.14 |
| Smoking, % | - | ||
| Never | 50 | 50 | |
| Past | 49 | 51 | |
| Current | 52 | 48 | |
| Alcohol, g/day | 5.7 ± 10.7 | 5.3 ± 10.2 | 0.53 |
| Physical Activity, METs/Week | 15.1 ± 19.5 | 16.2 ± 18.5 | 0.15 |
| Hypertension, % | 47 | 34 | <0.0001 |
| High Cholesterol, % | 48 | 46 | 0.55 |
| History of Heart Disease, % | 5 | 6 | 0.55 |
| Family History of Heart Disease, % | 12 | 7 | 0.01 |
| Diabetes, % | 12 | 6 | 0.001 |
| HbA1c ≥6, % | 16 | 11 | 0.01 |
| hs-CRP ≥ 3 (mg/L) | 40 | 34 | 0.07 |
Values are means ± SD (except where noted) or percentages
Normally distributed continuous variables compared using t-test, skewed variables compared using Wilcoxon rank-sum test and categorical variables compared with Chi-squared test
In univariate analyses, median fetuin-A was significantly higher among participants with BMI≥30 kg/m2, total cholesterol ≥200 mg/dL, CRP ≥3 mg/L and women with postmenopausal hormone use (Table 2). In partial Spearman correlations, adjusted for matching factors, fetuin-A was significantly and positively correlated with total cholesterol, total cholesterol/HDL ratio, triglycerides, hs-CRP, and BMI (Table 3); with the strongest correlations observed for triglycerides followed by hs-CRP.
Table 2.
Fetuin-A levels (g/L) by key cardiovascular risk factors
| Characteristics | Case/Control | Mean ± SD | Median | p-value | |
|---|---|---|---|---|---|
| Case/Control Status | Case | 457 | 0.46 ± 0.12 | 0.44 | 0.82 |
| Control | 457 | 0.46 ± 0.12 | 0.45 | ||
| Age | <65 | 303/306 | 0.46 ± 0.11 | 0.44 | 0.51 |
| ≥65 | 154/151 | 0.46 ± 0.13 | 0.44 | ||
| BMI (kg/m2) | <25 | 226/251 | 0.44 ± 0.12 | 0.43 | <0.001 |
| ≥25 | 231/206 | 0.48 ± 0.12 | 0.45 | ||
| Smoking Status | Never | 190/189 | 0.46 ± 0.13 | 0.45 | 0.21 |
| Former | 185/192 | 0.46 ± 0.12 | 0.44 | ||
| Current | 82/76 | 0.45 ± 0.11 | 0.43 | ||
| Hypertension | No | 241/302 | 0.45 ± 0.11 | 0.44 | 0.26 |
| Yes | 216/155 | 0.47 ± 0.13 | 0.45 | ||
| Diabetes | No | 400/430 | 0.46 ± 0.12 | 0.44 | 0.68 |
| Yes | 57/27 | 0.47 ± 0.12 | 0.44 | ||
| History of Heart Disease | No | 435/431 | 0.46 ± 0.12 | 0.45 | 0.10 |
| Yes | 22/26 | 0.45 ± 0.19 | 0.42 | ||
| Total Cholesterol (mg/dL) | <200 | 108/127 | 0.44 ± 0.12 | 0.43 | 0.004 |
| ≥200 | 349/330 | 0.47 ± 0.12 | 0.45 | ||
| LDL Cholesterol (mg/dL) | <160 | 333/344 | 0.46 ± 0.12 | 0.44 | 0.55 |
| ≥160 | 124/113 | 0.46 ± 0.13 | 0.45 | ||
| HbA1c(%) | <6 | 382/409 | 0.46 ± 0.12 | 0.44 | 0.50 |
| ≥6 | 75/48 | 0.46 ± 0.11 | 0.45 | ||
| CRP (mg/L) | <28.6 | 276/302 | 0.45 ± 0.12 | 0.43 | <0.001 |
| ≥28.6 | 181/155 | 0.48 ± 0.13 | 0.47 | ||
| Postmenopausal | No | 214/216 | 0.44 ± 0.11 | 0.43 | <0.0001 |
| Hormone use | Yes | 243/241 | 0.48 ± 0.13 | 0.46 |
Fetuin-A levels (g/L) compared across binary variables using Wilcoxon rank-sum test and multiple categorical variables compared with Kruskal Wallis Test.
Total and LDL cholesterol mg/dL can be converted to SI units (mmol/L) by dividing values by 38.67.
Table 3.
Adjusted Spearman-rank Correlation Coefficients for fetuin-A (g/L) and CVD risk markers
| CVD Risk Marker | β* | p-value |
|---|---|---|
| Total Cholesterol (mg/dL) | 0.08 | 0.02 |
| HDL (mg/dL) | −0.05 | 0.12 |
| LDL (mg/dL) | 0.04 | 0.25 |
| Total cholesterol/HDL ratio | 0.08 | 0.02 |
| Triglycerides (mg/dL) | 0.20 | <0.001 |
| CRP (mg/L) | 0.14 | <0.0001 |
| HbA1c (%) | 0.004 | 0.91 |
| BMI (kg/m2) | 0.15 | <0.0001 |
Adjusted for matching factors
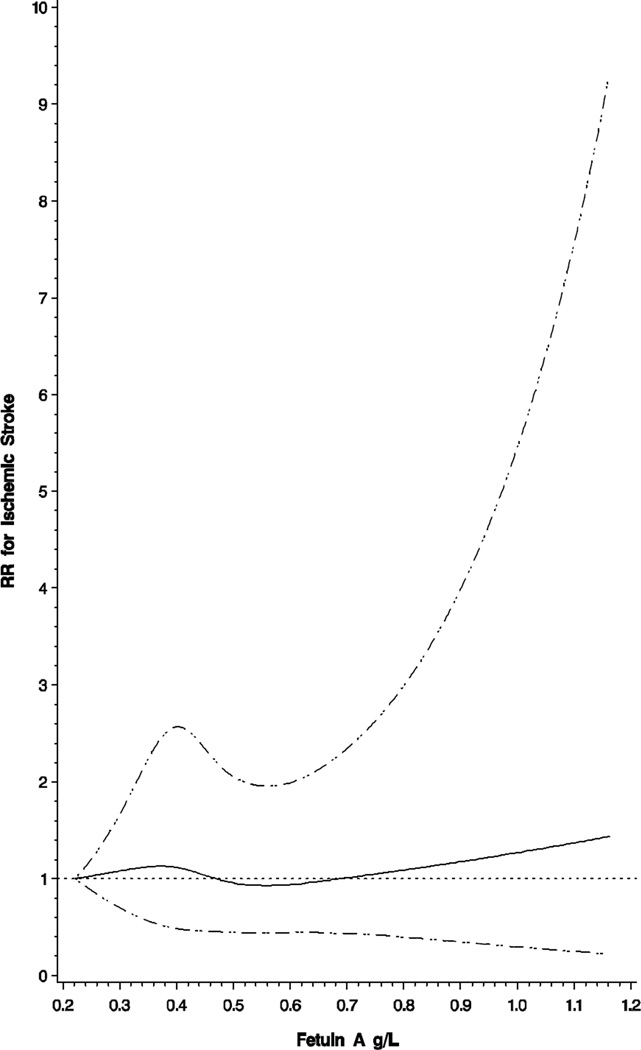
In multivariable analyses, quartiles of fetuin-A did not exhibit a significant association with risk of ischemic stroke (Table 4). When adjusted for matching factors, the association between extreme quartiles was null (RR=1.03; 95% CI: 0.69–1.54). Results were virtually unchanged upon further adjustment for lifestyle factors (RR=0.98; 95% CI: 0.65–1.49, extreme quartiles) or additional adjustment for chronic disease and biomarkers associated with stroke risk (RR=1.03; 95% CI: 0.66–1.60, extreme quartiles). We additionally modeled continuous fetuin-A concentrations and risk of ischemic stroke to examine any potential non-linear association utilizing restricted cubic splines; however, deviation from linearity was non-significant (Figure 1).
Table 4.
Multivariable adjusted relative risk (RR) and 95% CIs for ischemic stroke by fetuin-A quartiles.
| Quartiles of Fetuin-A | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 |
P for linear trend |
|
| Range (g/L) | <0.38 | 0.38–<0.45 | 0.45–<0.53 | 0.53–1.2 | |
| Cases/Controls | 108/114 | 136/115 | 102/115 | 111/113 | |
| Model 1 | 1.00 | 1.24 (0.86–1.78) | 0.93 (0.63–1.37) | 1.03 (0.69–1.54) | 0.75 |
| Model 2 | 1.00 | 1.18 (0.81–1.72) | 0.86 (0.57–1.28) | 0.98 (0.65–1.49) | 0.59 |
| Model 3 | 1.00 | 1.30 (0.87–1.93) | 0.98 (0.64–1.51) | 1.03 (0.66–1.60) | 0.72 |
Model 1: Conditional on matching factors (age, race/ethnicity, menopausal status, hormone use, smoking)
Model 2: Model 1 + aspirin use, alcohol intake, physical activity, alternate healthy eating index 2010, current post-menopausal hormone use
Model 3: Model 2 + history of diabetes, elevated cholesterol, high blood pressure, and CHD or revascularization, HbA1c, ln(total cholesterol/HDL-C), ln(triglycerides) and ln(hs-CRP)
Figure 1.
Multivariable association between fetuin-A (g/L) and risk of ischemic stroke modeled using a linear spline with 4 knots located at 0.30, 0.40, 0.49, 0.66, adjusted for covariates in Model 2 (see Table 3). Dashed lines represent 95% confidence bands, dotted line represents null value.
There was no evidence of effect modification with the relationship between fetuin-A and stroke by age, BMI, hypertension, diabetes, postmenopausal hormone use and time period of event occurrence (all Pinteraction>0.05) (See Supplemental Table 1). Additional analyses were conducted to correct for measurement error. These suggested that a larger sample size might be needed to determine the underlying association, as indicated by a wider confidence interval in the corrected estimate (1 sd increase in fetuin-A: uncorrected RR=1.00, 95% CI: 0.86–1.16; corrected RR=1.01, 95% CI: 0.64–1.59). Estimates by ischemic stroke subtype were not materially different from the aggregate estimates Table 5.
Table 5.
Multivariable adjusted relative risk (95% CI) of ischemic stroke subtypes by fetuin A quartiles
| Quartiles of Fetuin-A | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 |
P for linear trend |
|
| Range (g/L) | <0.38 | 0.38–<0.45 | 0.45–<0.53 | 0.53–1.2 | |
| Thrombotic Stroke | |||||
| Cases/Controls | 75/74 | 92/70 | 65/84 | 74/78 | |
| Model 1 | 1.00 | 1.30 (0.82–2.06) | 0.74 (0.46–1.19) | 0.92 (0.56–1.51) | 0.31 |
| Model 2 | 1.00 | 1.26 (0.78–2.04) | 0.69 (0.42–1.14) | 0.90 (0.53–1.52) | 0.27 |
| Model 3 | 1.00 | 1.44 (0.85–2.42) | 0.89 (0.52–1.53) | 1.05 (0.60–1.84) | 0.65 |
| Embolic Stroke | |||||
| Cases/Controls | 31/31 | 33/41 | 32/26 | 34/32 | |
| Model 1 | 1.00 | 0.84 (0.43–1.63) | 1.24 (0.60–2.59) | 1.08 (0.53–2.22) | 0.59 |
| Model 2 | 1.00 | 0.70 (0.32–1.53) | 1.12 (0.48–2.65) | 1.03 (0.46–2.29) | 0.63 |
| Model 3 | 1.00 | 0.76 (0.33–1.73) | 1.26 (0.49–3.22) | 1.11 (0.48–2.58) | 0.55 |
Model 1: Conditional on matching factors (age, race/ethnicity, menopausal status, hormone use, smoking)
Model 2: Model 1 + aspirin use, alcohol intake, physical activity, alternate healthy eating index 2010
Model 3: Model 2+history of diabetes, elevated cholesterol, high blood pressure, and CHD or revascularization, HbA1c, ln(Total cholesterol/ HDL-C), ln (triglycerides) and ln(hs-CRP)
Discussion
In this sample of women, who were free of stroke at the time of blood collection and followed for up to 17 years, we observed that fetuin-A was positively associated with biomarkers of cardiovascular risk (i.e. blood lipids, triglycerides and hs-CRP). In contrast to previous studies, we did not observe a significant association between fetuin-A concentrations and risk of ischemic stroke.
Fetuin-A has shown complex and divergent associations with cardiovascular outcomes, with reports of increased risk of subclinical cardiovascular outcomes and CVD events for both high and low fetuin-A concentrations.(4, 5, 8, 10, 25, 26) Only two previous studies have examined the association between fetuin-A and incident CVD in healthy populations, with inconsistent results.(8, 9) Higher fetuin-A was associated with a significantly greater risk of ischemic stroke among participants of the EPIC-Potsdam study, a European prospective cohort (highest vs. lowest quintile: RR=3.66, 95% CI: 1.99–6.71), which was a stronger association than that observed for total CVD (highest quintile vs. lowest: RR=3.12, 95% CI=2.15–4.52). Laughlin et al.,(8) reported a significantly increased risk of CVD mortality with low fetuin-A and observed significant variation by diabetes status among participants of the Rancho Bernardo study, a community based cohort of older adults. CVD mortality was significantly increased among non-diabetics in the lowest quartile of fetuin-A compared to quartiles 2–4 (hazard ratio [HR]=1.76; 95% CI: 1.34–2.31); whereas among diabetics low concentrations were associated with significantly lower risk (HR=0.43; 95CI%:0.19–0.98). However, our results did not support either of these findings.
We did not observe significant effect modification by diabetes status; however, our sample was younger (mean age=61 yrs) than those in the Rancho Bernardo Study (mean age=72 yrs) and exhibited a lower prevalence of diabetes (6% controls, 12% cases) which was assessed by validated self-report rather than clinical measures as compared to the prevalence (14% overall) seen in the Rancho Bernardo study. Inconsistencies between study findings may be a result of variations in endpoints (CVD composite vs. endpoint specific outcomes), evaluation of fatal vs. non-fatal events, and differences in population characteristics (i.e. age, underlying chronic disease).
Lower circulating fetuin-A concentrations have been associated with vascular calcification and cardiovascular mortality(4, 5, 10, 27) in both animal and human models. In contrast, high concentrations have been associated with carotid intima medial thickness,(28) an adverse atherogenic lipid profile,(6, 29) increased risk of insulin resistance,(6) diabetes (7, 30) and metabolic syndrome.(29) Although our results did not support a direct association between fetuin-A and ischemic stroke, we found evidence of an association with CVD risk factors, consistent with previous studies.(9, 29–31) These associations may suggest an indirect role of fetuin-A in the pathogenesis of ischemic stroke through an influence on stroke risk factors, although not supported in these analyses. There may be a complex balance in the influence of fetuin-A concentrations on cardiometabolic outcomes owing to either insufficiency or excess; with the effects potentially dependent upon underlying subclinical disease (e.g. atherosclerosis or insulin resistance).(32) Our results however, cannot confirm these hypotheses. Furthermore, we are unable to rule out the role of overall liver function since biomarkers of standard liver enzymes were not available for analysis.
Notable strengths of this work include the nested case control design with 17 years of follow-up and fetuin-A samples collected prior to stroke events. Furthermore, we observed a moderate number of events allowing for sensitivity analyses by key cardiovascular risk factors. However, we had limited power to evaluate ischemic stroke subtypes, particularly embolic strokes. Fetuin-A concentrations have been shown to be higher among women; therefore generalizability to men or ethnically diverse populations may be limited. While main analyses utilized a single measure of fetuin-A, we observed a moderate correlation between measures of fetuin-A over 10 years (ICC=0.52; 95% CI: 0.42–0.62) and from our pilot study observed a high correlation between measures collected on average 1 year apart (ICC=0.88; 95% CI: 0.76–0.94). Furthermore, there was no significant evidence that the association between fetuin-A and ischemic stroke varied by time.
We did not find evidence of an association between fetuin-A and risk of ischemic stroke in this sample of older women, in contrast to previous reports. Furthermore, we did not find evidence of significant variation of the association between fetuin-A and ischemic stroke by key CVD risk factors. Further research is needed to explore these associations in other populations.
Supplementary Material
Acknowledgments
Nurses’ Health Study Participants for their invaluable participation. HL088521-S1 (Rexrode), HL34594 (Manson) and K99HL098459 (Sun) from the National Heart, Lung and Blood Institute of the National Institutes of Health and CA87969 (Brigham and Women’s Hospital, NHS cohort follow-up) and CA49449 (Brigham and Women’s Hospital, NHS biomarker repository) from the National Cancer Institute.
Abbreviations
- BMI
body mass index
- RR
relative risk
- CI
confidence interval
- CVD
cardiovascular disease
- NHS
Nurses’ Health Study
- FFQ
food frequency questionnaire
- CV
coefficient of variation
- ICC
intraclass correlation
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- HbA1c
glycosylated hemoglobin
- OR
odds ratio
- METs
metabolic equivalents
- aHEI 2010
Alternate Healthy Eating Index 2010
- CHD
coronary heart disease
- HR
hazard ratio
- SD
standard deviation
- Q1-Q4
quartiles 1–4
Footnotes
Abstract Presentation: Jiménez M, Sun Q, Hu FB, Manson JE, Rexrode KM. Fetuin-A and risk of ischemic stroke among women. Circulation. 2012; 125: AMP075. American Heart Association Nutrition, Physical Activity and Metabolism and Cardiovascular Disease Epidemiology and Prevention 2012 Scientific Sessions; March 13–16, 2012; San Diego, CA.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Endres M, Heuschmann PU, Laufs U, Hakim AM. Primary prevention of stroke: blood pressure, lipids, and heart failure. European heart journal. 2011;32:545–552. doi: 10.1093/eurheartj/ehq472. [DOI] [PubMed] [Google Scholar]
- 4.Ix JH, Barrett-Connor E, Wassel CL, Cummins K, Bergstrom J, Daniels LB, Laughlin GA. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: the Rancho Bernardo Study. J Am Coll Cardiol. 2011;58:2372–2379. doi: 10.1016/j.jacc.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ix JH, Katz R, de Boer IH, Kestenbaum BR, Peralta CA, Jenny NS, et al. Fetuin-A is inversely associated with coronary artery calcification in community-living persons: the Multi-Ethnic Study of Atherosclerosis. Clin Chem. 2012;58:887–895. doi: 10.1373/clinchem.2011.177725. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi A, Ikeda Y, Ohguro T, Kumon Y, Yamanaka S, Takata H, et al. Serum fetuin-A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb. 2010;17:925–933. doi: 10.5551/jat.3830. [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, et al. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laughlin GA, Cummins KM, Wassel CL, Daniels LB, Ix JH. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol. 2012;59:1688–1696. doi: 10.1016/j.jacc.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 10.Emoto M, Mori K, Lee E, Kawano N, Yamazaki Y, Tsuchikura S, et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism: clinical and experimental. 2010;59:873–878. doi: 10.1016/j.metabol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int. 2005;67:2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 12.Mehrotra R. Emerging role for fetuin-A as contributor to morbidity and mortality in chronic kidney disease. Kidney Int. 2007;72:137–140. doi: 10.1038/sj.ki.5002355. [DOI] [PubMed] [Google Scholar]
- 13.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 16.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12:I13–I44. [PubMed] [Google Scholar]
- 17.Iso H, Rexrode K, Hennekens CH, Manson JE. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol. 2000;10:81–87. doi: 10.1016/s1047-2797(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126:319–325. doi: 10.1093/aje/126.2.319. [DOI] [PubMed] [Google Scholar]
- 20.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 23.Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5 doi: 10.2202/1557-4679.1104. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–208. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 25.Bilgir O, Kebapcilar L, Bilgir F, Bozkaya G, Yildiz Y, Pinar P, Tastan A. Decreased serum fetuin-A levels are associated with coronary artery diseases. Intern Med. 2010;49:1281–1285. doi: 10.2169/internalmedicine.49.3223. [DOI] [PubMed] [Google Scholar]
- 26.Eraso LH, Ginwala N, Qasim AN, Mehta NN, Dlugash R, Kapoor S, et al. Association of lower plasma fetuin-a levels with peripheral arterial disease in type 2 diabetes. Diabetes Care. 33:408–410. doi: 10.2337/dc09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittig K, Thamer C, Haupt A, Machann J, Peter A, Balletshofer B, et al. High plasma fetuin-A is associated with increased carotid intima-media thickness in a middle-aged population. Atherosclerosis. 2009;207:341–342. doi: 10.1016/j.atherosclerosis.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ix JH, Biggs ML, Mukamal KJ, Kizer JR, Zieman SJ, Siscovick DS, et al. Association of Fetuin-A with Incident Diabetes in Community-Living Older Adults: The Cardiovascular Health Study. Circulation. doi: 10.1161/CIRCULATIONAHA.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefan N, Fritsche A, Weikert C, Boeing H, Joost HG, Haring HU, Schulze MB. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore CE, Celotta G, Politi GG, Di Pino L, Castelli Z, Mangiafico RA, et al. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195:110–115. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.