Abstract
Repetitive deformation due to villous motility or peristalsis may support the intestinal mucosa, stimulating intestinal epithelial proliferation under normal circumstances and restitution in injured and inflamed mucosa rich in tissue fibronectin. Cyclic strain enhances Caco-2 and IEC-6 intestinal epithelial cell migration across fibronectin via ERK. However, the upstream mediators of ERK activation are unknown. We investigated whether Src and FAK mediate strain-induced ERK phosphorylation and migration in human Caco-2 intestinal epithelial cells on fibronectin. Monolayers on tissue fibronectin-precoated membranes were subjected to an average 10% repetitive deformation at 10 cycles/min. Phosphorylation of Src-Tyr418, FAK-Tyr397-Tyr576-Tyr925, and ERK were significantly increased by deformation. The stimulation of wound closure by strain was prevented by Src blockade with PP2 (10 (µmol/l) or specific short interfering (si)RNA. Src inhibition also prevented strain-induced FAK phosphorylation at Tyr397 and Tyr576 but not FAK-Tyr925 or ERK phosphorylation. Reducing FAK by siRNA inhibited strain-induced ERK phosphorylation. Transfection of NH2-terminal tyrosine phosphorylation-deficient FAK mutants Y397F, Y576F–Y577F, and Y397F–Y576F–Y577F did not prevent the activation of ERK2 by cyclic strain, but a FAK mutant at the COOH terminal (Y925F) prevented the strain-induced activation of ERK2. Although the Y397F–Y576F–Y577F FAK construct exhibited less basal FAK-Tyr925 phosphorylation under static conditions, it nevertheless exhibited increased FAK-Tyr925 phosphorylation in response to strain. These results suggest that repetitive deformation stimulates intestinal epithelial motility across fibronectin in a manner that requires both Src activation and a novel Src-independent FAK-Tyr925-dependent pathway that activates ERK. This pathway may be an important target for interventions to promote mucosal healing in settings of intestinal ileus or fasting.
Keywords: migration, cyclic strain, intestine, mechanotransduction, signaling
Diverse physical forces including peristalsis (20), villus motility (55), and interaction of the mucosa with luminal contents (43) all repetitively deform the gut mucosa in complex ways. We recently demonstrated that repetitive deformation promotes Caco-2 and IEC-6 intestinal epithelial cell wound closure on tissue fibronectin but not on a collagen I extracellular matrix and intestinal epithelial proliferation on collagen but not fibronectin (58). Thus, repetitive deformation may be trophic for the intestinal mucosa, supporting mucosal proliferation on a collagen-rich basement membrane but promoting migration when inflammatory states and mucosal injury increase tissue fibronectin (16, 22, 47).
ERK activation appears central to the effects of deformation on intestinal epithelial cells. Intestinal epithelial ERK is activated by deformation on both collagen (13) and fibronectin (58) substrates. Furthermore, blockade of ERK blocks both intestinal epithelial proliferation on collagen (13) and intestinal epithelial restitution on fibronectin (58). Many stimuli signal via ERK, including growth factors (29), mechanical forces (12, 13, 39), oxidative stress (31), and integrin-mediated signaling (38). In various cells, such stimuli may mediate their effects on ERK by different intervening signal mediators, including Src (1, 12, 13, 48), FAK (12, 13, 52), and PKC (23). The upstream events that activate intestinal epithelial ERK on fibronectin in response to repetitive strain are unknown. We therefore sought to identify the upstream mediators responsible for ERK activation and migration in Caco-2 intestinal epithelial cells.
We focused on the potential role of focal adhesion signaling via Src and FAK. We used the Flexercell apparatus (Flexcell, McKeesport, PA) to rhythmically deform Caco-2 cell monolayers cultured on tissue fibronectin-coated flexible-bottomed wells at an average 10% repetitive deformation at 10 cycles/ min (5), similar in magnitude and frequency to the irregular repetitive mucosal deformation caused by peristalsis or villous motility in vivo (55). We characterized Caco-2 Src and FAK phosphorylation in response to repetitive deformation and used pharmacological antagonists, short interfering (si)RNA, and dominant negative FAK constructs to trace a mechanotransduced pathway that links these signals into a novel motogenic cascade.
EXPERIMENTAL PROCEDURES
Materials
We obtained DMEM medium, Oligofectamine, Lipofectamine, GAPDH monoclonal antibody, and Plus Reagent from Invitrogen (Carlsbad, CA); Western blot stripping reagent from Chemicon (Temecula, CA); human transferrin and mouse monoclonal hemagglutinin (HA) antibody clone 12C5 from Roche Applied Science (Indianapolis, IN); and trypsin and horseradish peroxidase-conjugated rabbit anti-mouse IgG from Sigma (St. Louis, MO). We purchased phosphospecific polyclonal antibodies to FAK at Tyr397 or Tyr576 from BIOSOURCE (Camarillo, CA). We obtained phosphospecific polyclonal antibodies to FAK at Tyr925, p44/p42 (phospho-ERK1/2), Tyr(P)202/Thr(P)204, Src-Tyr(P)416, which recognized the phosphorylated form of human Src-Tyr(P)418, rabbit polyclonal antibody to p42/44 (total ERK1/2), HA, and horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG from Cell Signaling (Beverly, MA). We obtained mouse monoclonal anti-FAK, rabbit polyclonal anti-Myc tag, and rabbit monoclonal anti-Src from Upstate Cell Signaling Solutions (Charlottesville, VA). 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) and 2′-amino-3′-methoxyflavone (PD-98059) from EMD Biosciences (San Diego, CA) and monoclonal anti-c-Myc (9E10) from Covance (Berkeley, CA). Amersham Biosciences (Piscataway, NJ) provided protein G-Sepharose, and Clontech (Mountain View, CA) provided the pCMV-HA-tagged vector. Dr. S. K. Hanks (Vanderbilt University School of Medicine, Nashville, TN) generously provided HA-tagged FAK mutants with changed codons for phosphoacceptor Tyr397 to Phe397, Tyr576-Tyr577 to Phe576-Phe577, and Tyr397-Tyr576 Tyr577 to Phe397-Phe576 Phe577; Dr. D. D. Schlaepfer (The Scripps Research Institute, La Jolla, CA) provided the HA-tagged FAK mutant with changed codons for phosphoacceptor Tyr925 to Phe925; and Dr. C Marshall (Institute of Cancer Research, London, UK) provided the wild-type Myc-ERK2 expression vector. Dharmacon (Lafayette, CO) provided double-stranded siRNAs targeting human forms of FAK and control nontargeting siRNA 1 (NT1 siRNA). We selected the sequences targeted by siRNA using Dharmacon Smartdesign as follows: human FAK1, NNGCAUGUGGCCUGCUAUGGA; human Src1, NNCUCGGCUCAUUGAAGA-CAA; and human Src2, NNUGGCCUACUACUCCAAACA. We used two different sequences targeted to Src for our initial experiments of the effects of siRNA on Src protein. Because these two sequences yielded similar results, we performed subsequent experiments of the effects of Src reduction on strain-associated motogenicity and signaling using a siRNA pool combining the two Src-targeted sequences.
Cell culture
We studied Caco-2BBE intestinal epithelial cells, a subclone of the original Caco-2 cell line selected for its ability to differentiate in culture as indicated by the formation of an apical brush border and the expression of brush-border enzymes. We maintained the cells at 37°C with 8% CO2 in DMEM with 4,500 mg/l D-glucose, 4 mM glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 µg/ml transferrin, 10 mM HEPES (pH 7.4), and 3.7 g/l NaHCO3 supplemented with 10% heat-inactivated FBS.
Application of mechanical strain
We plated Caco-2 cells on elastomer membranes coated with tissue fibronectin (Flexcell) and exposed them to a continuous cycles of strain/relaxation generated by a cyclic vacuum produced by a computer-driven system (Flexcell 3000, Flexcell) as previously described (13). A 20-kPa vacuum at 10 cycles/min deforms the membranes from below to an average 10% strain with a stretch-to-relaxation ratio of 1:1 (3.0-s deformation alternating with 3.0 s in neutral conformation). The strain is transmitted to adherent cells cultured on the upper surface of the membrane, which experience similar elongation (6, 8). The six-well plates were incubated at 37°C with 5% CO2 during the repetitive strain, and similar control plates were in the same incubator without strain.
Matrix precoating and medium treatments
We precoated six-well amino-coated Flexwell I plates with 12.5 (µg/ml of tissue fibronectin (Sigma Chemical) at saturating concentrations as previously described (58). Cells were seeded at 300,000 cells/well and grown to confluence. We dissolved PD-98059 (a specific inhibitor of the activation of ERK kinase), PP2 (a potent and selective inhibitor of Src family tyrosine kinases), and calphostin C (a PKC inhibitor) in DMSO, aliquoted and stored the inhibitors at − 20°C, and diluted them immediately before use in culture medium. We pretreated Caco-2 cells with PD-98059 (20 (µM), PP2 (10 (µM), light-activated calphostin C (100 nM), or equivalent amounts of DMSO (vehicle control) for 45 min before exposing the cells to cyclic strain to assess wound closure with inhibited signaling.
Migration experiments
We assessed cell migration using a previously described wound healing model (58). Briefly, Caco-2 monolayers on deformable membranes precoated with tissue fibronectin were subjected to 10% deformation at 10 cycles/min for 0–24 h after the induction of a small uniform circular wound. As previously described by other investigators (42, 46), we created wounds in confluent cell monolayers using a 1.0-ml pipette tip (1.5 mm diameter) attached to a vacuum source. We applied the tip gently to the monolayer and applied suction for ~1 s. We photographed each hole at 0 and 24 h after the initiation of strain using a SPOT ADVANCED digital camera (Windows version 3.0.6) attached to a Nikon Eclipse TE 300 microscope (Japan), and calculated wound areas using Kodak 1D software (version 3.6) on a Kodak Image Station (Perkin-Elmer, Boston, MA). To account for variability in the size of the initial holes, we expressed the remaining wound area in each wound at 24 h as a ratio to the initial size of each individual wound. Migration was then expressed as the percentage of wound closure in each wound and calculated as follows: 100 × (Initial wound − Final wound)/Initial wound. Similar procedures were used to calculate migration in control monolayers wounded simultaneously but not subjected to repetitive strain.
Western blot analysis
We cultured cells as previously described (13) to confluence and changed to serum-free media for 24 h. After treatment, we lysed the cells on ice in modified radioimmunoprecipitation buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1% deoxycholic acid, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 2 (µg/ml aprotinin, and 2 (µg/ml leupeptin]. We centrifuged lysates at 15,000 g for 10 min at 4°C and stored supernatants at −80°C. We assayed protein concentrations by bicinchoninic acid analysis (BCA assay, Pierce Chemical, Rockford, IL) and loaded 20 µg protein/well on a SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences). Nonspecific binding sites were blocked with 5% BSA in Tris-buffered saline with 1 ml Tween 20 per liter for 1 h at room temperature. Immunoblots were probed with the appropriate primary and secondary antibodies as indicated above and detected by the ECL method (Amersham Biosciences) with Kodak Image Station 440CF Phosphoimager (Kodak Scientific Imaging Systems).
Transfection with siRNA
Caco-2 cells were plated at 40–50% confluence on Flex I six-well plates precoated with tissue fibronectin 1 day prior to transfection. We combined siRNAs with Plus reagent in Opti-MEM as described previously for plasmid DNA transfection (54). We used Oligofectamine in Opti-MEM for transfection at 10 µg/ml according to the manufacturer’s protocol. The final siRNA concentration was 100 nM unless otherwise indicated. After 6–8 h of transfection, we added 0.5 volumes of DMEM with 20% serum to the cells and continued transfection for 48 h. We serum starved the cells overnight prior to experiments and verified the potency of the siRNA transfection in each experiment by parallel transfections in which we lysed the cells at the end of the study and immunoblotted for the target protein. Parallel experiments using fluorescent-tagged siRNA have demonstrated that ~90% of the cells are transfected with siRNA under these circumstances (not shown).
FAK-ERK cotransfection experiments
To compare the effect of transfection with HA-tagged pCMV empty vector or HA-tagged FAK point mutants with changed codons for phosphoacceptor tyrosine to phenylalanine (Y397F, Y576F–Y577F, Y397F–Y576F–Y577F, and Y925F) on ERK2 activity, we cotransfected 70–80% confluent cells with 4.8 (µg of HA-tagged pCMV vector or HA-tagged FAK mutants Y397F, Y576F–Y577F, Y397F–Y576F–Y577F, and Y925F DNA and 1.2 µg DNA of Myc-tagged ERK2 expression vector before experiments (13). Thus, cells in each well received a total of 1.0 (µg of DNA with vector or FAK and ERK constructs at a 4:1 ratio. We mixed the DNA with 60 µl of Plus reagent in 1 ml of Opti-MEM for 15 min and added Lipofectamine (30.0 µl in 1 ml of Opti-MEM). We incubated this mixture at room temperature for 20 min, diluted it with 6.0 ml of Opti-MEM, and added 1.0 ml/well to cells for 6 h, after first rinsing the cells twice with Opti-MEM. After transfection, we incubated the cells in normal medium for 20–24 h and then with serum-free medium for 18–24 h before initiating cyclic strain; 20–25% of cells were transfected using this procedure (14).
Myc tag monoclonal antibody 9E10 immunoprecipitation and Western blot analysis
We lysed Caco-2 cells cotransfected with FAK mutants and ERK expression constructs in immunoprecipitation buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 10 mM sodium pyrophosphate, 2 (µg/ml aprotinin, and 2 [µg/ml leupeptin] and measured protein by BCA assay (Pierce Chemical). We preblocked 20 µl of protein G-Sepharose with 1% heatin-activated BSA in PBS for 1 h at room temperature for each immunoprecipitation reaction, rinsed twice with cold immunoprecipitation buffer, and incubated 3.0 µg of Myc-tagged monoclonal 9E10 antibody on a rotator in each immunoprecipitation reaction for 2 h at 4°C. We then again rinsed the Myc-tagged antibody-bound protein G-Sepharose twice with immunoprecipitation buffer before adding the protein G-Sepharose-bound Myc antibody complex (50 µl/reaction) to 400 (µg protein-matched samples diluted to equal volumes with immunoprecipitation buffer for each immunoprecipitation reaction. We incubated the resulting mixtures at 4°C overnight on a rotator, rinsed the resultant immunocomplexes four times with 0.5 ml immunoprecipitation buffer by centrifugation at 5,000 rpm for 2 min at 4°C, and resuspended the final pellet of immunocomplexes in 6× sample loading buffer. The immunocomplex suspension was then boiled for 4 min, resolved by 10% SDS-PAGE, and transferred to nitrocellulose Hybond-ECL membranes (Amersham Biosciences). We probed these membranes with polyclonal anti-phospho ERK and Myc-tagged antibodies and detected signals by the ECL method as described in Western blot analysis.
HA tag monoclonal antibody 12C5 immunoprecipitation and Western blot analysis
We lysed Caco-2 cells transfected with wild-type FAK-HA tagged or mutant FAK Y397F-Y576F-Y577F-HA tagged expression constructs in immunoprecipitation buffer as for the Myc tag antibody. We rinsed preblocked protein G-Sepharose twice with 4°C immunoprecipitation buffer and rotated 3.0 µg of anti-HA mouse monoclonal antibody clone 12CA5 in each immunoprecipitation reaction for 2 h at 4°C. We then rinsed the HA-tagged antibody-bound protein G-Sepharose twice with immunoprecipitation buffer and added 50 (µl/reaction of protein G-Sepharose-bound HA antibody complex to 500 (µg protein-matched samples diluted to equal volumes with immunoprecipitation buffer for each immunoprecipitation reaction. We rotated the resulting mixture at 4°C overnight, rinsed the resultant immunocomplexes with immunoprecipitation buffer, and resuspended the final pellet of immunocomplexes in 6× sample loading buffer. We boiled the immunocomplexes for 4 min, resolved them by 10% SDS-PAGE, and transferred them to nitrocellulose Hybond-ECL membranes (Amersham Biosciences) before probing with polyclonal anti-phospho-FAK Tyr925 and anti-HA.
Statistical analysis
We performed all experiments independently at least three times unless otherwise indicated. We expressed all data as means ± SE and analyzed our results using paired or unpaired t-tests using Bonferroni corrections as appropriate and seeking 95% confidence.
RESULTS
Mechanical strain stimulates tyrosine phosphorylation of FAK-Tyr397 and FAK-Tyr576
To determine whether repetitive strain stimulates FAK phosphorylation in Caco-2 intestinal epithelial cells, we subjected confluent serum-deprived cells to repetitive strain for 0–60 min and immunoblotted cell lysates for FAK-Tyr397 and FAK-Tyr576. Repetitive deformation induced FAK phosphorylation at Tyr397 and Tyr576 (Fig. 1). Phosphorylation at Tyr397 and Tyr576 moieties increased as rapidly as 5 min after the initiation of repetitive deformation [166 ± 2%, n = 6, P < 0.05 for Tyr397 (Fig. 1A) and 157 ± 18%, n = 6, P < 0.05 for Tyr576 (Fig. 1B)]. Immunoblots with anti-FAK antibody confirmed equal protein loading.
Fig. 1.
Role of FAK in deformation-induced migration across fibronectin. A and B: deformation stimulated FAK phosphorylation. The effect of cyclic deformation on phosphorylation of FAK at Tyr397 (p-FAKY397; A) and Tyr576 (p-FAKY576; B) was assessed by Western blot analysis of equal protein lysates from cells subjected to repetitive deformation for 0–60 min. Total FAK (t-FAK) served as a protein loading control. Top, typical blots; bottom, densitometric analysis. Strain resulted in rapid and sustained phosphorylation of FAK at Tyr397 and Tyr576. Values are means ± SE; n = 5. *P< 0.05 for each. C: short interfering (si)RNA targeted to FAK reduced FAK protein. Cells were transfected with either a nontargeting siRNA sequence (NT1) or siRNA targeted to FAK. Top, typical blots; bottom, densitometric analysis. GAPDH served as a protein loading control. Transfection with siRNA targeted to FAK reduced FAK protein levels by ~60% compared with cells transfected with NT1 sequences. Values are means ± SE; n = 3. *P< 0.05. D: FAK reduction by siRNA inhibited deformation-induced migration. Circular wounds were made in monolayers of cells transfected with either NT1 or siRNA targeted to FAK, and these wounds were photographed. Cells were then cultured under static conditions (open bars) or conditions of repetitive deformation (solid bars) for 24 h prior to the holes were measured again and wound closure was calculated. Deformation stimulated wound closure in cells transfected with the NT1 sequence but not in cells in which FAK had been reduced. Values are means ± SE; n = 12 from 1 of 3 similar experiments. *P < 0.05.
FAK is essential for the stimulation of migration by cyclic strain
We next sought to determine whether FAK was required for the deformation to stimulate wound closure. We transfected Caco-2 cells with siRNA targeted to FAK or a control nontargeting siRNA sequence (NT1) for 48 h and then assessed migration with or without repetitive strain for 24 h after performing initial experiments to validate the potency of the siRNAs. siRNA targeted to FAK reduced FAK protein by 60 ± 10% (n = 3, P < 0.05; Fig. 1C). Wounds in NT1-transfected Caco-2 monolayers closed more rapidly in response to deformation than NT1-transfected static controls (n = 8, P < 0.05; Fig. 1D). Reduction of FAK by siRNA blocked this motogenic effect (n = 8, P < 0.05; Fig. 1D). Thus, FAK seems necessary for the strain-induced motogenic response in Caco-2 intestinal epithelial cells.
Repetitive deformation stimulates Src phosphorylation
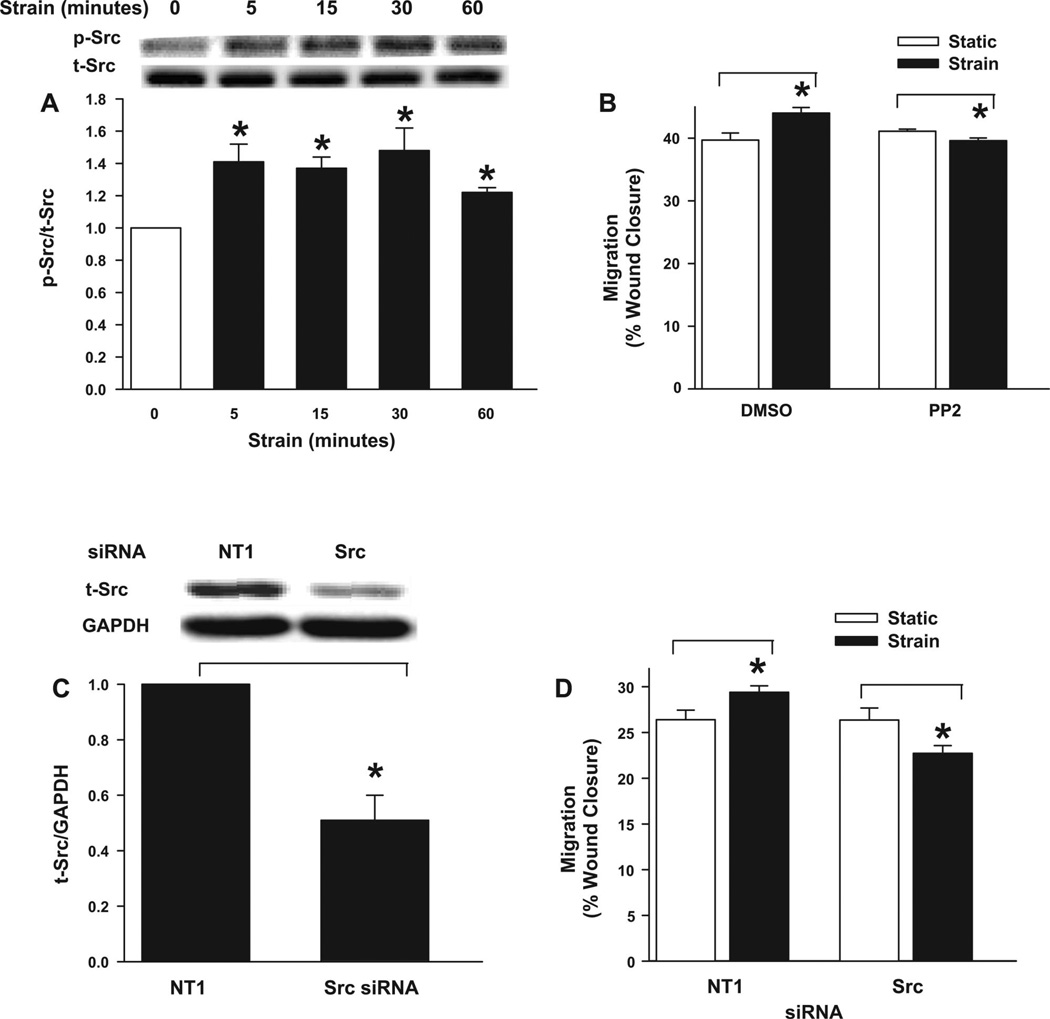
To elucidate the potential role of Src in the deformation-induced motogenic response, we analyzed the effect of repetitive deformation on Src activation, as assessed by the phosphorylation of c-Src at Tyr418 of the activation loop, a critical step leading to full Src activation. Src-Tyr418 phosphorylation increased within 5 min (141 ± 11%, n = 5, P < 0.05; Fig. 2A) after the initiation of repetitive deformation. Phosphorylation was maximal at 30 min (148 ± 13%, n = 5, P < 0.05; Fig. 2A) but remained statistically significantly greater than basal Src phosphorylation even at 60 min.
Fig. 2.
Role of Src in deformation-induced migration. A: strain stimulated Src phosphorylation. The effect of cyclic deformation on time-dependent Src-Tyr418 phosphorylation (p-Src) was assessed by Western blot analysis in Caco-2 cells subjected to cyclic strain for 0–60 min. Total Src (t-Src) served as a protein loading control. Top, typical blots; bottom, densitometric analysis. Strain resulted in rapid and sustained phosphorylation of Src at Tyr418. Values are means ± SE; n = 5. *P < 0.05. B: Src inhibition by PP2 inhibited deformation-induced migration. Although cells pretreated with a 0.1% DMSO (vehicle control) displayed increased migration over 24 h in response to repetitive strain (solid bars) compared with unstretched cells (open bars), pretreatment for 45 min with the Src inhibitor PP2 (10 µM) for 45 min prevented and indeed slightly reversed the stimulation of migration by strain. Values are means ± SE; n = 8. *P < 0.005. C: siRNA targeted to Src reduced Src protein. Cells were transfected with either NT1 or siRNA targeted to Src. Top, typical blots; bottom, densitometric analysis. GAPDH served as a protein loading control. Transfection with siRNA targeted to Src reduced Src protein levels by ~50% compared with cells transfected with NT1 sequences. Values are means ± SE; n = 3. *P < 0.05. D: Src reduction by siRNA inhibited deformation-induced migration. Deformation stimulated cell migration in cells transfected with NT1 sequences. However, reducing Src by specific siRNA resulted in slightly slower wound closure in repetitively deformed monolayers compared with monolayers not subject to deformation. Values are means ± SE; n = 12 from 1 of 3 similar experiments. *P < 0.05.
Src is required for deformation-induced migration
We next sought to determine whether Src was also required for the deformation to stimulate migration. Wounds in Caco-2 monolayers treated with a DMSO vehicle control [0.1% (vol/vol)] closed more rapidly in response to deformation than DMSO-treated static controls (n = 8, P < 0.05; Fig. 2B). Pretreatment with the Src family kinase inhibitor PP2 (10µM) for 45 min before the initiation of repetitive deformation prevented the stimulation of motility by strain without affecting basal migration and indeed slightly but statistically significantly decreased wound closure during deformation compared with wound closure without deformation in PP2-treated cells (Fig. 2B). In parallel experiments, siRNA targeted to Src reduced total Src by 50 ± 4% (n = 3, P < 0.05; Fig. 2C). Deformation also stimulated migration in NT-1-transfected cells, but reducing Src by siRNA again blocked the stimulation of migration by strain without affecting basal migration and with a small but statistically significant decrease in wound closure in Src-reduced cells subjected to repetitive deformation compared with Src-reduced cells without deformation (n = 12, P < 0.05; Fig. 2D).
FAK is required for strain-induced ERK phosphorylation
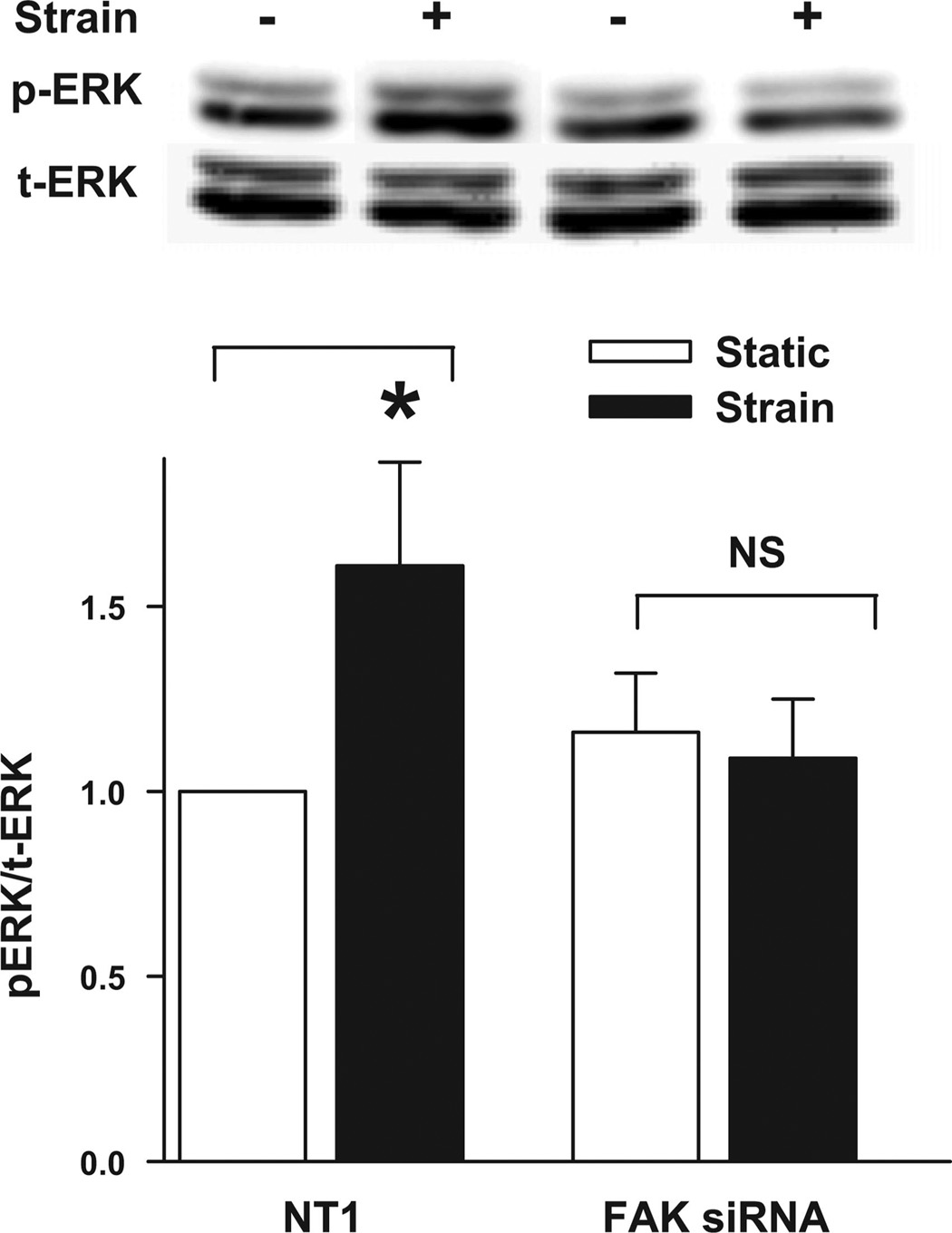
Since FAK and Src were required for strain-stimulated migration, we sought to link them to the ERK activation previously demonstrated to be required for strain to stimulate motility. ERK phosphorylation increased in cells transfected with NT1 siRNA 15 min after the initiation of repetitive strain (n = 5, P < 0.05; Fig. 3). In contrast, cells transfected with FAK siRNA displayed slightly increased basal ERK phosphorylation but did not further increase ERK phosphorylation in response to strain (Fig. 3).These results suggested that FAK is required for ERK activation in response to mechanical strain in Caco-2 cells on tissue fibronectin.
Fig. 3.
Reducing FAK by siRNA inhibited deformation-induced ERK phosphorylation. Cells were transfected with either NT1 or siRNA targeted to FAK. Cells were maintained under static conditions (open bars) or conditions of cyclic strain (solid bars) for 15 min prior to lysis and Western blot analysis for phosphorylated ERK (p-ERK1/2). Total ERK protein (t-ERK1/2) served as a protein loading control. The graph summarizes densitometric analysis from 5 similar experiments. Strain stimulated ERK phosphorylation in NT1-transfected cells but did not significantly affect ERK phosphorylation in FAK-reduced cells. Values are means ± SE. *P < 0.05.
Src inhibition does not prevent deformation-induced ERK phosphorylation but blocks strain-induced FAK-Tyr397 and FAK-Tyr576 phosphorylation
Neither treatment with PP2 nor reducing Src by siRNA blocked deformation-induced ERK phosphorylation 15 min after cyclic strain initiation (not shown). Since FAK is often activated by Src, particularly at Tyr576/577, the observation that FAK but not Src might be required for ERK activation by strain seemed puzzling. We therefore next evaluated whether Src was required for FAK activation by strain. Indeed, Src inhibition by PP2 pretreatment completely blocked the strain-induced FAK-Tyr397 as well as FAK-Tyr576 phosphorylation (Fig. 4, A and B), although PP2 increased basal FAK-Tyr397 phosphorylation slightly (Fig. 4A) without affecting basal FAK-Tyr576 phosphorylation (Fig. 4B). Reducing Src by siRNA similarly prevented strain-induced FAK-Tyr397 and FAK-Tyr576 phosphorylation (n = 5, P < 0.05; Fig. 4, C and D), although reducing Src did not affect basal FAK-Tyr397 phosphorylation.
Fig. 4.
Src inhibition or reduction inhibited deformation-induced FAK-Tyr397 and FAK-Tyr576 phosphorylation. A–D: effects of PP2 on the phosphorylation of FAK at Tyr397 (A) or Tyr576 (B) and of Src reduction by siRNA on the phosphorylation of FAK at Tyr397 (C) or Tyr576 (D) were assessed by Western blot analysis of lysates from cells subjected to cyclic strain for 15 min. t-FAK served as a protein loading control. Top, typical blots; bottom, densitometric analysis. A: cyclic deformation stimulated phosphorylation of FAK at Tyr397 in vehicle-treated cells. PP2 increased basal FAK-Tyr397 phosphorylation slightly, but deformation did not further stimulate FAK at Tyr397 phosphorylation in PP2-treated cells. Values are means ± SE; n = 5. *P < 0.05. B: cyclic deformation stimulated phosphorylation of FAK at Tyr576 in vehicle-treated cells but did not significantly affect FAK at Tyr576 phosphorylation in PP2-treated cells. Values are means ± SE; n = 5. *P < 0.05. C: cyclic deformation stimulated phosphorylation of FAK at Tyr397 in NT1 control siRNA-treated cells but did not significantly affect FAK at Tyr397 phosphorylation in Src-reduced cells. Values are means ± SE; n = 5. *P < 0.05. D: cyclic deformation stimulated phosphorylation of FAK at Tyr576 in NT1 control siRNA-treated cells but did not significantly affect FAK at Tyr576 phosphorylation in Src-reduced cells. Values are means ± SE; n = 5. *P< 0.05.
Neither PKC activity nor FAK phosphorylation of Tyr397-Tyr576-Tyr577 is required for ERK activation in response to strain
The dependence of FAK-Tyr397 and FAK-Tyr576 phosphorylation on Src was consistent with published reports. However, if Src activated FAK, and FAK was required for ERK activation, it seemed unclear why blocking or reducing Src would not also block ERK. PKC also modulates ERK activation in other settings (23), and PKC is activated in intestinal epithelial cells subjected to repetitive deformation on collagen (24). However, neither light-activated calphostin C alone nor the combination of calphostin C and PP2 blocked deformation-induced ERK phosphorylation (not shown). We further investigated whether FAK-Tyr397, FAK-Tyr576, or FAK-Tyr577 phosphorylation were required for ERK activation by transient cotransfection of ERK with FAK constructs unable to be phosphorylated at these sites. We cotransfected cells with Myc-tagged ERK2 and FAK point mutants Y397F, Y576F-Y577F, or Y397F-Y576F-Y577F (13), in which the tyrosine phosphorylation sites of FAK at Tyr397, Tyr576, and Tyr577 are point mutated to phenylalanine to prevent these tyrosine phosphorylation events. Expression of FAK mutants at Y397F, Y576F–Y577F, or Y397F–Y576F–Y577F did not block strain-induced ERK activation (not shown), although Myc-tagged ERK2 was activated by strain after cotransfection with empty vector alone. In parallel experiments, wild-type FAK cotransfection also did not prevent the activation of cotransfected ERK2 (not shown).
Strain stimulates FAK-Tyr925 phosphorylation by a different time course and in a Src-independent manner
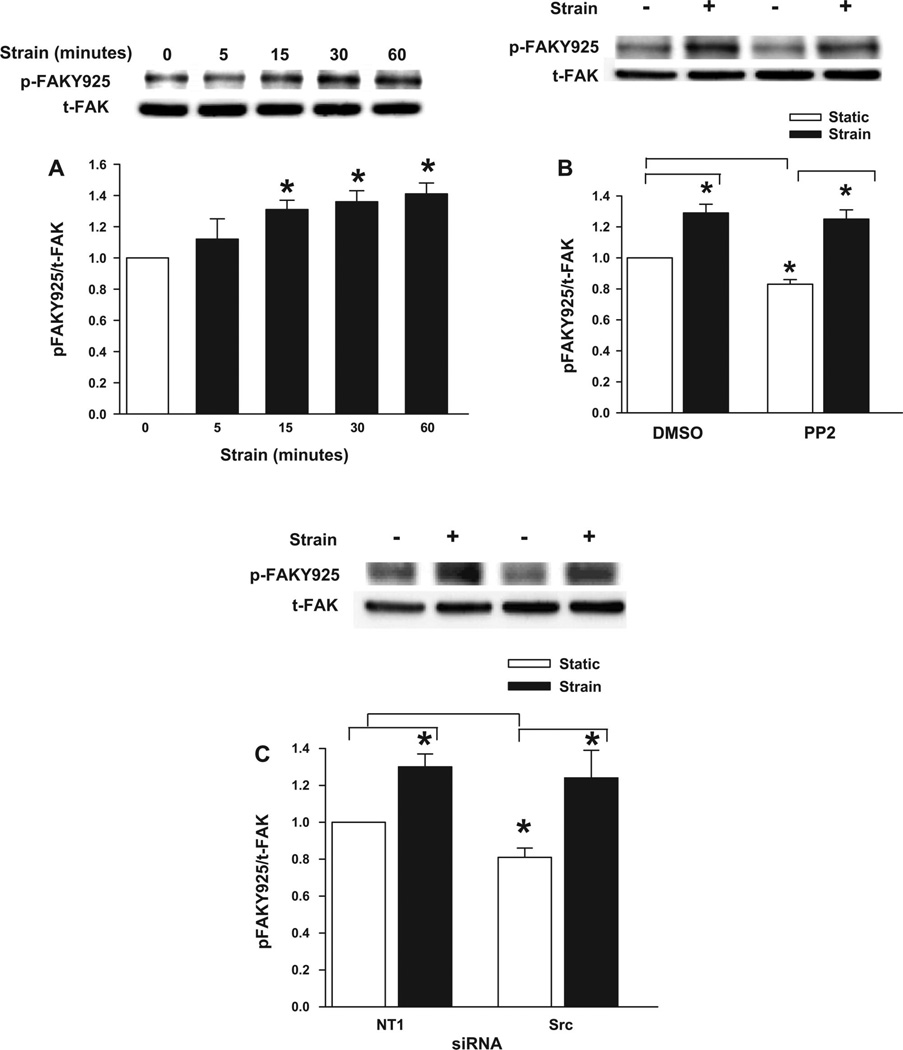
Although FAK phosphorylation at Tyr397, Tyr576, and Tyr577 are among the best characterized FAK phosphorylation sites, FAK is also phosphorylated at Tyr925, within the FAK focal adhesion targeting (FAT) domain. FAK-Tyr925 phosphorylation requires Src in most other settings (2, 48, 49) However, thrombin-stimulated FAK-Tyr925 phosphorylation in vascular endothelial cells is only slightly attenuated by PP2 (50). We therefore examined FAK-Tyr925 phosphorylation in Caco-2 cells subjected to repetitive deformation on a fibronectin substrate. FAK was indeed phosphorylated at Tyr925 in response to strain, apparently over a slightly delayed time course compared with Tyr397 or Tyr576 phosphorylation. FAK-Tyr925 phosphorylation was not increased 5 min after strain initiation, but it did increase over the next 15–60 min (n = 5, P < 0.05; Fig. 5A). Furthermore, although either PP2 (Fig. 5B) or Src reduction by siRNA (Fig. 5C) slightly but statistically significantly decreased basal FAK-Tyr925 phosphorylation (n = 5, P < 0.05 for each), neither PP2 nor Src reduction blocked the increase in FAK-Tyr925 phosphorylation associated with repetitive deformation.
Fig. 5.
Role of FAK-Tyr925 in deformation-induced ERK phosphorylation. A–C: effects of 0–60 min of cyclic deformation (A), PP2 treatment (B), and Src reduction by siRNA (C) on the phosphorylation of FAK-Tyr925 were assessed by Western blot analysis of lysates from cells subjected to static culture or cyclic strain for 15 min. t-FAK served as a protein loading control. Top, typical blots; bottom, densitometric analysis. A: cyclic deformation resulted in rapid and sustained phosphorylation of FAK at Tyr925 (p-FAKY925). Values are means ± SE; n = 5. *P < 0.05. B: cyclic deformation stimulated FAK phosphorylation at Tyr925 in both vehicle-treated cells and cells treated with PP2. A slight but statistically significant decrease in basal FAK-Tyr925 phosphorylation was observed in PP2-treated cells. Values are means ± SE; n = 5. *P < 0.05. C: cyclic deformation stimulated FAK phosphorylation at Tyr925 in cells treated with either NT1 control siRNA or siRNA targeted to Src. A slight but statistically significant decrease in basal FAK-Tyr925 phosphorylation was observed in Src-reduced cells. Values are means ± SE; n = 5. *P< 0.05.
FAK phosphorylation at Tyr925 is required for ERK activation in response to strain
We therefore asked whether FAK-Tyr925 phosphorylation mediates the strain effect on ERK. We compared the effects on strain activation on Myc-tagged ERK2 cotransfected with either empty vector alone or a FAK mutant Y925F construct (13) in which the tyrosine phosphorylation site of FAK at Y925 is point mutated to phenylalanine to prevent this tyrosine phosphorylation. As expected, strain activated Myc-tagged ERK2 after cotransfection with empty vector alone (n = 3, P < 0.05; Fig. 6). However, in contrast to our observations with FAK constructs unable to be phosphorylated at Y397 or Y576/577, the expression of the FAK Y925F mutant completely blocked strain-induced cotransfected ERK activation (Fig. 6).
Fig. 6.
Effect of blockade of FAK-Tyr925 phosphorylation on ERK activation by strain. Cells were cotransfected with hemagglutinin (HA)-tagged empty pCMV vector and Myc-tagged ERK2 expression vectors or HA-tagged FAK mutant F925 and Myc-tagged wild-type ERK2 expression vectors as described in experimental procedures. Cells were then maintained under static conditions or conditions of cyclic strain for 15 min prior to lysis and immunoprecipitation (IP) with monoclonal anti-myc 9E10 antibody. The resulting immunoprecipitates were then probed for p-ERK, stripped, and reprobed for polyclonal Myc tag antibody. Repetitive deformation stimulated ERK activation in cells cotransfected with HA-tagged empty pCMV vector and Myc-tagged ERK2 but not in those cells in which HA-tagged FAK mutant F925 and Myc-tagged ERK2 were cotransfected and immunoprecipitated. Values are means ± SE; n = 5. *P < 0.05.
FAK phosphorylation at Tyr397-Tyr576-Tyr577 is not required for FAK-Tyr925 phosphorylation in response to strain
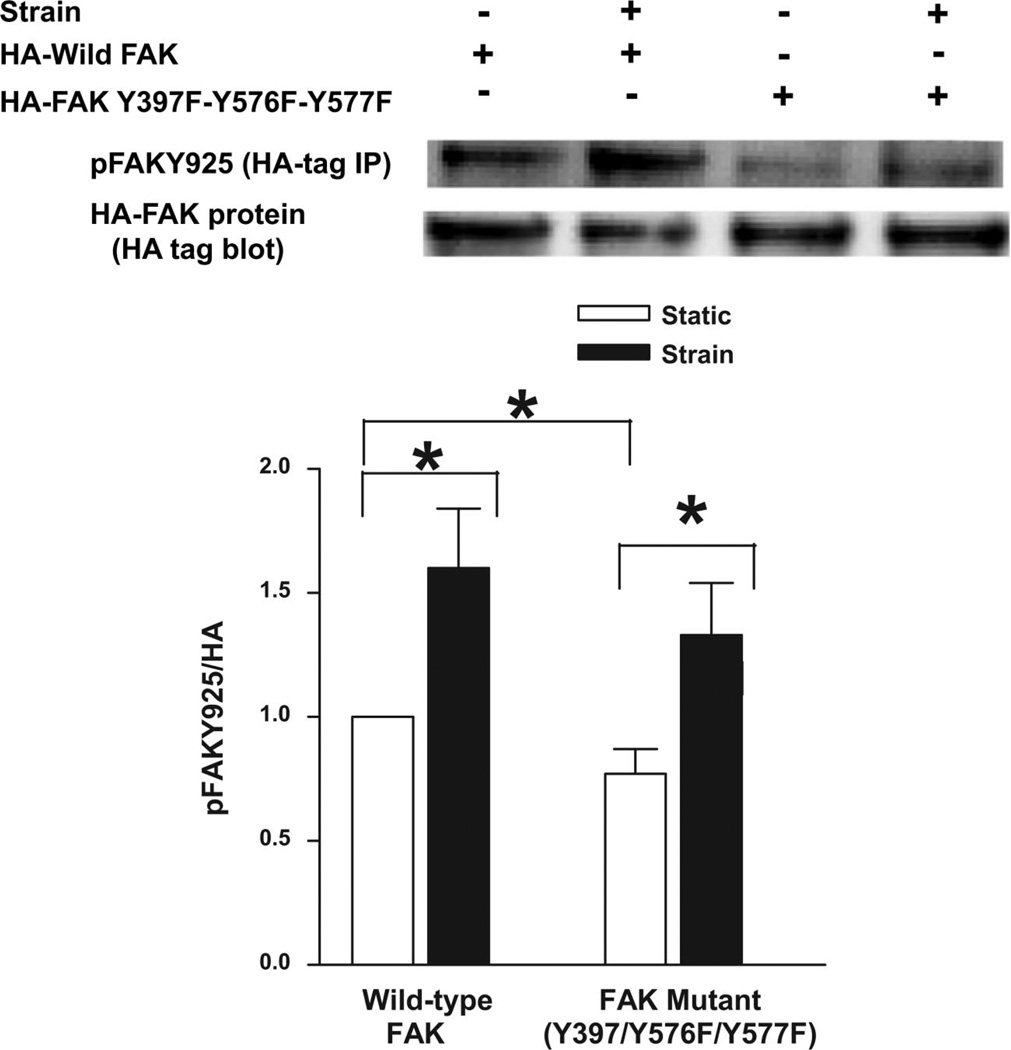
We further investigated whether FAK-Tyr397, FAK-Tyr576, or FAK-Tyr577 phosphorylation are required for Tyr925 phosphorylation. We transiently transfected Caco-2 cells with wild-type HA-tagged FAK or mutant HA-tagged FAK Y397F–Y576F–Y577F. Wild-type HA-tagged FAK derived from cells subjected to repetitive deformation exhibited increased FAK-Tyr925 phosphorylation compared with wild-type HA-tagged FAK derived from cells not subjected to repetitive deformation (n = 6, P < 0.05; Fig. 7). FAK triple mutant Y397F–Y576F–Y577F exhibited less basal FAK-Tyr925 phosphorylation than its wild-type counterpart (n = 6, P < 0.05; Fig. 7) but still responded to strain with increased FAK-Tyr925 phosphorylation (n = 6, P < 0.05; Fig. 7).
Fig. 7.
Effect of blockade of FAK phosphorylation at Tyr397-Tyr576-Tyr577 on FAK-Tyr925 phosphorylation by strain. Cells were transiently transfected with HA-tagged wild-type FAK or HA-tagged tyrosine phosphorylation-deficient FAK triple mutant Y397F–Y576F–Y577F as described in experimental procedures. Cells were then maintained under static conditions or conditions of cyclic strain for 15 min prior to lysis and IP with monoclonal HA 12C5 antibody. The resulting immunoprecipitates were then probed for p-FAK-Tyr925, stripped, and reprobed with a polyclonal HA antibody. HA-tagged tyrosine-deficient FAK triple mutant Y397F–Y576F–Y577F was less Tyr925 phosphorylated under static conditions than tagged wild-type FAK. However, repetitive deformation stimulated Tyr925 phosphorylation in both HA-tagged wild-type FAK and HA-tagged tyrosine phosphorylation-deficient FAK triple mutant Y397F–Y576F–Y577F. Values are means ± SE; n = 6. *P < 0.05.
DISCUSSION
Mechanical forces generated under normal conditions of digestion by intestinal contraction, villous motility, shear stress, and wall stretch due to luminal contents may affect cell signaling, and changes in these rhythms may alter mucosal healing. Indeed, mucosal healing is delayed or abnormal in many disease states that are also characterized by abnormally decreased patterns of repetitive mucosal deformation. Sepsis, postsurgical ileus, and prolonged fasting may be associated with altered contractile rhythms, altered villous motility, and decreased mucosal deformation from interaction with luminal contents. Under these conditions, the mucosa atrophies (32), proliferation slows (10), and mucosal barrier function is impaired, suggesting abnormalities in the mucosal response to injury (17).Our previous work (4, 6, 24, 25, 40, 44, 56, 57), consistent with studies of other organs and tissues (34, 37, 39), suggests that physical forces affect intestinal epithelial cells by flexing the matrix, altering integrin binding, and initiating matrix-dependent signals that regulate enterocyte proliferation and function. Repetitive deformation stimulates tyrosine kinase signaling in the intact mucosa in anesthetized animals as well (4). However, mucosal healing requires epithelial sheet migration as well as proliferation. We therefore sought to investigate the influence of repetitive deformation and extracellular matrix proteins on intestinal epithelial migration.
One concern that might be raised is whether our present observations of increased wound closure across fibronectin substrates in response to deformation might reflect a mitogenic effect of strain. However, in our initial description of the promigratory or motogenic effect of strain on intestinal epithelial cells cultured on fibronectin (58), we demonstrated that cyclic strain actually inhibits intestinal epithelial proliferation on fibronectin substrates. Thus, the more rapid wound closure that occurs in response to strain on fibronectin does not reflect increased proliferation. Indeed, in that same report, we also identified a motogenic effect as early as 6 h after wounding and repetitive deformation, before proliferation would be expected to occur, and demonstrated that the motogenic effect of strain occurs even when proliferation is ablated by mitomycin. The use of the Caco-2 cell model might also be criticized since Caco-2 cells were originally derived from a colonic adenocarcinoma. Any in vitro model has limitations. However, Caco-2 cells are a common model for the study of intestinal epithelial biology, including the regulation of intestinal epithelial migration (11, 28, 41). Our experiments in this study involved Caco-2 cells in confluent monolayers, which resemble normal intestinal epithelial cells in many ways (26), and, although the cells at the edge of a monolayer wound become phenotypically different as they migrate, the same is true for intestinal epithelial cells at the edge of a mucosal wound in vivo (9, 45). Moreover, we have previously demonstrated that both nonmalignant rat IEC-6 cells (58) and low-passage primary human intestinal epithelial cells derived from surgical specimens (56) respond to repetitive deformation similarly to Caco-2 cells.
Although repetitive deformation stimulates intestinal epithelial wound closure across a fibronectin substrate by the activation of ERK (58), the mechanism for this ERK activation was not known. The present study delineates a considerably more complex pathway. A novel Src-independent FAK-Tyr925 signal is required for repetitive deformation to stimulate ERK activation and consequent migration across fibronectin. However, Src itself is also activated by repetitive deformation and is also separately required for the stimulation of migration by cyclic strain, independently of ERK, whether by Src-dependent FAK-Tyr397 and FAK-Tyr576 phosphorylation or via some other downstream signal.
Src has been widely implicated in the regulation of intestinal epithelial migration on nondeforming substrates (12, 19). This potent upstream kinase has numerous downstream effectors, including FAK, ERK, phosphatidylinositol 3-kinase, and small G proteins, which may contribute to its motogenic effect in various settings. Our present results do not allow us to distinguish whether Src promotes motility by phosphorylating FAK at Tyr576–577 or via a completely FAK-independent pathway that might involve other downstream mediators yet to be identified. However, these results do clearly demonstrate that Src is activated by cyclic strain in wounded intestinal epithelial monolayers on fibronectin and that such Src activation is indeed required for the motogenic effect of strain in this setting but in a manner independent of ERK. In contrast, intestinal epithelial cells respond to cyclic strain on collagen substrates via a Src-dependent ERK activation that is mitogenic rather than motogenic (13). These matrix-dependent differences in the consequences of ERK activation may reflect the different subcellular locations of the activated ERK on each matrix (58). The differences in the consequences of Src activation on each matrix may reflect differences in focal adhesion organization and integrin interaction on each substrate. Although adhesion generally results in integrin clustering and focal adhesion formation, cellular interactions with different matrixes by matrix-specific integrins are likely to yield differently organized and activated focal adhesion complexes with different consequences for downstream signaling (35, 53).
The observation that FAK is motogenic in response to cyclic strain is also consistent with observations that FAK influences intestinal epithelial migration on nondeforming substrates (7, 30). Repetitive deformation on collagen stimulates a FAK-dependent ERK activation that ultimately stimulates cell proliferation (13, 40). However, despite this superficial similarity, our present results delineate a novel FAK-dependent strain signaling pathway that contrasts sharply in its details with previous observations. On collagen, FAK-Tyr397/576 phosphorylation is required for downstream ERK activation (13, 40). Moreover, since Src phosphorylates FAK at Tyr576/577, this explains the Src dependence of the ERK signal on collagen. In contrast, although Src is required for FAK-Tyr397 and FAK-Tyr576 phosphorylation in intestinal epithelial cells subjected to repetitive deformation on a fibronectin substrate, neither FAK phosphorylation site is important for ERK activation by strain on fibronectin.
FAK phosphorylation at Tyr925 in response to deformation is independent of Src or FAK-Tyr576/577 phosphorylation, since Tyr925 phosphorylation was not prevented by either PP2 or Src reduction by siRNA at levels that prevented FAK-Tyr576 phosphorylation and since the tyrosine phosphorylation-deficient FAK triple mutant Y397F–Y576F–Y577F still displayed increased Tyr925 phosphorylation in response to strain. FAKTyr925 is generally viewed as a Src substrate (15, 27). Indeed, we observed a tendency toward decreased basal FAK-Tyr925 phosphorylation after Src inhibition by either PP2 or specific Src-targeted siRNA. However, Shikata et al. (50) previously described FAK-Tyr925 phosphorylation independently of Src family kinases in vascular endothelial cells treated with thrombin. Shikata et al. only blocked Src by PP2 treatment, but our present study further supports the model of a Src-independent FAK-Tyr925 phosphorylation by showing similar results with Src reduction by siRNA. Whether the increase in FAK-Tyr925 phosphorylation reflects strain activation of a kinase outside the Src family or strain modulation of a phosphatase that selectively dephosphorylates FAK at Tyr925 awaits further study.
If FAK-Tyr925 phosphorylation is more important than FAK-Tyr397 or FAK-Tyr576/577 in mediating strain-dependent ERK activation on fibronectin, then the different location of this tyrosine within the FAK molecule may provide a clue to its function. Tyr397 is in the NH2-terminal domain of FAK and Tyr576/577 is in its catalytic domain, but Tyr925 is within the FAT domain of FAK. The selective requirement for the phosphorylation of this tyrosine in FAK for the strain motogenic response on fibronectin may suggest that focal adhesion assembly or disassembly may be important in mediating the motogenic effect. The FAT domain, an antiparallel four-helix bundle, exists in alternative conformations that may modulate phosphorylation, ligand binding, and the subcellular localization of focal adhesion kinase. In particular, the conformational dynamic features of helix 1 in the intermediate state of the FAT domain may facilitate Tyr925 phosphorylation, interfere with FAK-paxillin interaction, and promote FAK signaling via the ERK/MAPK pathway and by releasing FAK from focal adhesions (18). Focal adhesion assembly and disassembly are themselves required for cell motility (21), while activated ERK colocalizes with the adaptor protein paxillin in well-defined adhesions (51).
Although the magnitude of wound closure in this study may appear relatively small, the effect is highly statistically significant. We (24, 56) and others (3, 36, 59) have described changes in migration, proliferation, or cell signaling of similar magnitude due to other stimuli in intestinal epithelial cells. For instance, Buffin-Meyer and colleagues (11) studied a 15% stimulation of Caco-2 intestinal epithelial wound closure by the α2-adrenoreceptor agonist 5-bromo-6-(2-imidazolin-2-ylamino) quinoxaline (UK-14304), and Itoh and colleagues (33) characterized effects of IL-8 on Caco-2 wound closure of similar magnitude. Here, wound closure increased an average 10–18% on fibronectin compared with cells not subjected to mechanical strain. Such apparently modest alterations may well be biologically important in determining whether a mucosal wound heals or fails to heal in vivo.
These results substantially extend previous studies using this system. Although we previously traced a FAK-Tyr397-dependent Src-dependent ERK pathway activated by deformation on collagen that stimulates proliferation and slightly inhibits cell migration, we now trace a radically different pathway by which FAK phosphorylation at the Tyr925 position independent of Src stimulates ERK-dependent migration on fibronectin. Indeed, the Src-independent phosphorylation of FAK-Tyr925 in response to repetitive deformation of intestinal epithelial cells is itself unusual within the broad context of how FAK phosphorylation is usually understood. Thus, this signal pathway differs markedly from that which has previously been described.
Taken together, these results suggest that the same kinases can interact very differently in response to the same stimulus in different settings. Such variation may reflect fundamental differences in the organization of focal adhesion complexes associated with different integrins binding to different matrix substrates. In addition to providing a valuable contrasting model to dissect differences in focal adhesion function, these results delineate a novel pathway by which repetitive deformation stimulates intestinal epithelial migration across a fibronectin substrate. Moreover, although the previously described collagen-dependent strain signal pathway leads to increased proliferation and slightly inhibits motility, the fibronectin-dependent strain signal pathway promotes epithelial cell motility. This seems particularly important because fibronectin is deposited into inflamed, infected, or chronically injured tissues. Such fibronectin deposition may be a critical switch that shifts the effect of repetitive deformation on the gut mucosa. There are clearly substantial differences between cultured epithelial cells in vitro and an intact intestine in vivo. Nevertheless, these data suggest that on a healthy basement membrane, repetitive deformation may be a mitogenic and differentiating stimulus that teleologically may serve to maintain normal gut homeostasis and absorptive function. However, in the setting of chronic inflammatory mucosal injury, fibronectin deposition may switch the intestinal epithelial response to strain to a migratory effect adapted to seal mucosal wounds and maintain the mucosal barrier critically important to prevent bacterial translocation and allow the organism to survive without sepsis. This pathway may be an important target for interventions designed to promote mucosal healing, particularly in settings in which inflammation or infection increases tissue fibronectin levels or in which patterns of repetitive intestinal epithelial deformation are altered.
ACKNOWLEDGMENTS
We thank Dr. Steven K. Hanks (Vanderbilt University School of Medicine, Nashville, TN) for kindly providing the hemagglutinin (HA)-tagged dominant negative FAK mutant Y397F, Y576F/Y577F, and Y397F/Y576F/Y577F expression plasmids. We also thank Dr. D. D. Schlaepfer (The Scripps Research Institute, La Jolla, CA) for providing the HA-tagged FAK mutant Y925F and Dr. Christopher Marshall (Institute of Cancer Research, London, UK) for providing the wild-type myc-ERK2 expression vector for our research experiments. We also thank Dr. Mary F. Walsh, Dr. Rajakrishanan Veluthukal, and Samira A. Saad (Wayne State University, Detroit, MI) for technical assistance.
GRANTS
This research work was supported in part by a Veterans Affairs Merit Research Award and by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-067257 (to M. D. Basson).
REFERENCES
- 1.Albinsson S, Hellstrand P. Integration of signal pathways for stretch-dependent growth and differentiation in vascular smooth muscle. Am J Physiol Cell Physiol. 2007;293:C772–C782. doi: 10.1152/ajpcell.00622.2006. [DOI] [PubMed] [Google Scholar]
- 2.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH2-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol. 2006;207:553–561. doi: 10.1002/jcp.20604. [DOI] [PubMed] [Google Scholar]
- 4.Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism. 2002;51:1525–1527. doi: 10.1053/meta.2002.36303. [DOI] [PubMed] [Google Scholar]
- 5.Basson MD, Hong F. Regulation of human Caco-2 intestinal epithelial brush border enzyme activity by cyclic nucleotides. Cancer Lett. 1996;99:155–160. doi: 10.1016/0304-3835(95)04058-7. [DOI] [PubMed] [Google Scholar]
- 6.Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168:476–488. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Basson MD, Sanders MA, Gomez R, Hatfield J, Vanderheide R, Thamilselvan V, Zhang J, Walsh MF. Focal adhesion kinase protein levels in gut epithelial motility. Am J Physiol Gastrointest Liver Physiol. 2006;291:G491–G499. doi: 10.1152/ajpgi.00292.2005. [DOI] [PubMed] [Google Scholar]
- 8.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301–305. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- 9.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown HO, Levine ML, Lipkin M. Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol. 1963;205:868–872. doi: 10.1152/ajplegacy.1963.205.5.868. [DOI] [PubMed] [Google Scholar]
- 11.Buffin-Meyer B, Crassous PA, Delage C, Denis C, Schaak S, Paris H. EGF receptor transactivation and PI3-kinase mediate stimulation of ERK by α2A-adrenoreceptor in intestinal epithelial cells: a role in wound healing. Eur J Pharmacol. 2007;574:85–93. doi: 10.1016/j.ejphar.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol. 2007;292:C1701–C1713. doi: 10.1152/ajpcell.00529.2006. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem. 2007;282:14–28. doi: 10.1074/jbc.M605817200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HL, Steinway ML, Russell JW, Feldman EL. GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem. 2000;275:27197–27204. doi: 10.1074/jbc.M002534200. [DOI] [PubMed] [Google Scholar]
- 15.Choma DP, Milano V, Pumiglia KM, DiPersio CM. Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol. 2007;127:31–40. doi: 10.1038/sj.jid.5700505. [DOI] [PubMed] [Google Scholar]
- 16.Dammeier J, Brauchle M, Falk W, Grotendorst GR, Werner S. Connective tissue growth factor: a novel regulator of mucosal repair and fibrosis in inflammatory bowel disease? Int J Biochem Cell Biol. 1998;30:909–922. doi: 10.1016/s1357-2725(98)00046-6. [DOI] [PubMed] [Google Scholar]
- 17.Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987;205:681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon RD, Chen Y, Ding F, Khare SD, Prutzman KC, Schaller MD, Campbell SL, Dokholyan NV. New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate. Structure. 2004;12:2161–2171. doi: 10.1016/j.str.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Emami S, Le Floch N, Bruyneel E, Thim L, May F, Westley B, Rio M, Mareel M, Gespach C. Induction of scattering and cellular invasion by trefoil peptides in Src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J. 2001;15:351–361. doi: 10.1096/fj.00-0355com. [DOI] [PubMed] [Google Scholar]
- 20.Friedman HI, Cardell RR., Jr Alterations in the endoplasmic reticulum and Golgi complex of intestinal epithelial cells during fat absorption and after termination of this process: a morphological and morphometric study. Anat Rec. 1977;188:77–101. doi: 10.1002/ar.1091880109. [DOI] [PubMed] [Google Scholar]
- 21.Gallant ND, Garcia AJ. Model of integrin-mediated cell adhesion strengthening. J Biomech. 2007;40:1301–1309. doi: 10.1016/j.jbiomech.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Goke M, Zuk A, Podolsky DK. Regulation and function of extracellular matrix intestinal epithelial restitution in vitro. Am J Physiol Gastrointest Liver Physiol. 1996;271:G729–G740. doi: 10.1152/ajpgi.1996.271.5.G729. [DOI] [PubMed] [Google Scholar]
- 23.Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem. 2002;277:18440–18446. doi: 10.1074/jbc.M200468200. [DOI] [PubMed] [Google Scholar]
- 24.Han O, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol Gastrointest Liver Physiol. 1998;275:G534–G541. doi: 10.1152/ajpgi.1998.275.3.G534. [DOI] [PubMed] [Google Scholar]
- 25.Han O, Sumpio BE, Basson MD. Mechanical strain rapidly redistributes tyrosine phosphorylated proteins in human intestinal Caco-2 cells. Biochem Biophys Res Commun. 1998;250:668–673. doi: 10.1006/bbrc.1998.9372. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 27.Hiscox S, Jordan NJ, Morgan L, Green TP, Nicholson RI. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin Exp Metastasis. 2007;24:157–167. doi: 10.1007/s10585-007-9065-y. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins AM, Bruewer M, Brown GT, Pineda AA, Ha JJ, Winfree LM, Walsh SV, Babbin BA, Nusrat A. Epithelial cell spreading induced by hepatocyte growth factor influences paxillin protein synthesis and posttranslational modification. Am J Physiol Gastrointest Liver Physiol. 2004;287:G886–G898. doi: 10.1152/ajpgi.00065.2004. [DOI] [PubMed] [Google Scholar]
- 29.Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 2005;167:1587–1597. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iizuka M, Sasaki K, Hirai Y, Shindo K, Konno S, Itou H, Ohshima S, Horie Y, Watanabe S. Morphogenic protein epimorphin protects intestinal epithelial cells from oxidative stress by the activation of EGF receptor and MEK/ERK, PI3 kinase/Akt signals. Am J Physiol Gastrointest Liver Physiol. 2007;292:G39–G52. doi: 10.1152/ajpgi.00181.2006. [DOI] [PubMed] [Google Scholar]
- 32.Inoue Y, Grant JP, Snyder PJ. Effect of glutamine-supplemented total parenteral nutrition on recovery of the small intestine after starvation atrophy. J Parenter Enteral Nutr. 1993;17:165–170. doi: 10.1177/0148607193017002165. [DOI] [PubMed] [Google Scholar]
- 33.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, Kubota H, Mori Y, Ohara H, Nomura T, Higashiyama S, Itoh M. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31:186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- 35.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25:713–726. doi: 10.1038/sj.emboj.7600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-κB transcription factor. FASEB J. 2004;18:1524–1535. doi: 10.1096/fj.04-2414com. [DOI] [PubMed] [Google Scholar]
- 38.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, Dostal DE. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–147. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–25280. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G75–G87. doi: 10.1152/ajpgi.2001.280.1.G75. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 42.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 44.Murnin M, Kumar A, Li GD, Brown M, Sumpio BE, Basson MD. Effects of glutamine isomers on human (Caco-2) intestinal epithelial proliferation, strain-responsiveness, and differentiation. J Gastrointest Surg. 2000;4:435–442. doi: 10.1016/s1091-255x(00)80025-6. [DOI] [PubMed] [Google Scholar]
- 45.Numata M, Ido A, Moriuchi A, Kim I, Tahara Y, Yamamoto S, Hasuike S, Nagata K, Miyata Y, Uto H, Tsubouchi H. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm Bowel Dis. 2005;11:551–558. doi: 10.1097/01.mib.0000164192.71381.5c. [DOI] [PubMed] [Google Scholar]
- 46.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi FG, Leaphart C, Cetin S, Li J, Grishin A, Watkins S, Ford HR, Hackam DJ. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012–1022. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 48.Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via α1β1- and α2β1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G547–G557. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- 49.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate-and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 51.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–381. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takai E, Landesberg R, Katz RW, Hung CT, Guo XE. Substrate modulation of osteoblast adhesion strength, focal adhesion kinase activation, and responsiveness to mechanical stimuli. Mol Cell Biomech. 2006;3:1–12. [PubMed] [Google Scholar]
- 54.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology. 2004;126:8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 55.Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol Gastrointest Liver Physiol. 1987;252:G250–G256. doi: 10.1152/ajpgi.1987.252.2.G250. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926–928. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Li W, Sumpio BE, Basson MD. Fibronectin blocks p38 and jnk activation by cyclic strain in Caco-2 cells. Biochem Biophys Res Commun. 2003;306:746–749. doi: 10.1016/s0006-291x(03)01044-1. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology. 2006;131:1179–1189. doi: 10.1053/j.gastro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Zhong J, Gencay MM, Bubendorf L, Burgess JK, Parson H, Robinson BW, Tamm M, Black JL, Roth M. ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect cell migration: a comparison between mesothelioma and mesothelial cells. J Cell Physiol. 2006;207:540–552. doi: 10.1002/jcp.20605. [DOI] [PubMed] [Google Scholar]