The Na+/K+ pump is a hybrid transporter that can also import protons at physiological K+ and Na+ concentrations.
Abstract
A single Na+/K+-ATPase pumps three Na+ outwards and two K+ inwards by alternately exposing ion-binding sites to opposite sides of the membrane in a conformational sequence coupled to pump autophosphorylation from ATP and auto-dephosphorylation. The larger flow of Na+ than K+ generates outward current across the cell membrane. Less well understood is the ability of Na+/K+ pumps to generate an inward current of protons. Originally noted in pumps deprived of external K+ and Na+ ions, as inward current at negative membrane potentials that becomes amplified when external pH is lowered, this proton current is generally viewed as an artifact of those unnatural conditions. We demonstrate here that this inward current also flows at physiological K+ and Na+ concentrations. We show that protons exploit ready reversibility of conformational changes associated with extracellular Na+ release from phosphorylated Na+/K+ pumps. Reversal of a subset of these transitions allows an extracellular proton to bind an acidic side chain and to be subsequently released to the cytoplasm. This back-step of phosphorylated Na+/K+ pumps that enables proton import is not required for completion of the 3 Na+/2 K+ transport cycle. However, the back-step occurs readily during Na+/K+ transport when external K+ ion binding and occlusion are delayed, and it occurs more frequently when lowered extracellular pH raises the probability of protonation of the externally accessible carboxylate side chain. The proton route passes through the Na+-selective binding site III and is distinct from the principal pathway traversed by the majority of transported Na+ and K+ ions that passes through binding site II. The inferred occurrence of Na+/K+ exchange and H+ import during the same conformational cycle of a single molecule identifies the Na+/K+ pump as a hybrid transporter. Whether Na+/K+ pump–mediated proton inflow may have any physiological or pathophysiological significance remains to be clarified.
INTRODUCTION
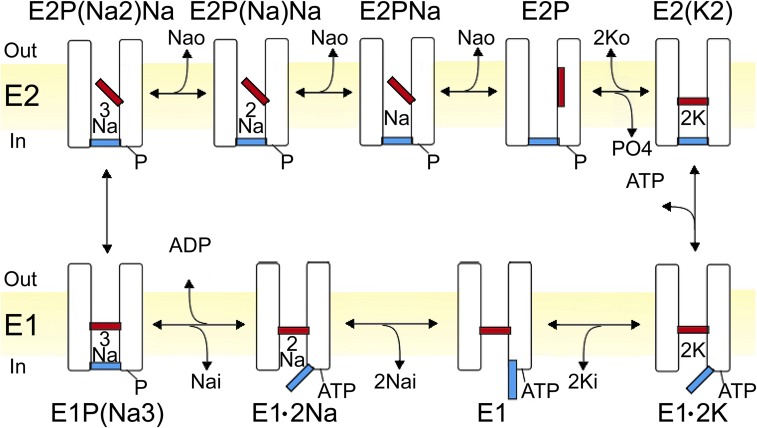
Na+/K+-ATPase pumps expel three Na+ ions and import two K+ ions for each ATP hydrolyzed, generating the ion gradients across the membrane that are essential to the life of all animal cells. Each transport cycle comprises a sequence of conformational transitions that permit extracellular K+ ions to access the binding sites in phosphorylated pumps (E2P conformations; Fig. 1) and cytoplasmic Na+ ions to access the sites after dephosphorylation (E1 conformations; Fig. 1). Binding of the third Na+ ion triggers autophosphorylation, and binding of the second K+ ion prompts auto-dephosphorylation. This coupling of alternating ion access to ATP hydrolysis ensures forward, energetically uphill, progress of the Na+/K+ transport cycle. The larger pumped Na+ efflux than K+ influx constitutes outward current, a direction tending to make the membrane potential more negative. However, because each step in the cycle is reversible (Fig. 1), if the normally transported intracellular Na+ and extracellular K+ are both scarce, the cycle can run backward, thus synthesizing ATP (Garrahan and Glynn, 1967b) and generating inward, depolarizing current (Bahinski et al., 1988; De Weer et al., 2001).
Figure 1.
The Post–Albers transport cycle (e.g., Post et al., 1972) of the Na+,K+-ATPase cartooned as an ion channel with two gates. The gates open strictly alternately to allow access to cation-binding sites from only one side at a time. The extracellular-side gate (red) is closed in all E1 states, and the cytoplasmic-side gate (blue) is closed in E2 states. Parentheses denote occluded ions, and “-P” symbolizes the aspartyl-phosphate formed by autophosphorylation of E1 pumps once three cytoplasmic Na+ ions have bound.
For a fixed 3 Na+/2 K+ transport stoichiometry (Post and Jolly, 1957; Garrahan and Glynn, 1967a; Rakowski et al., 1989), changes in the magnitude of outward (or inward) pump current report alterations of average turnover rate of the cycle. Na+/K+ pump current is conveniently measured as the component of membrane current abolished by specific Na+/K+-ATPase inhibitors such as ouabain. During control assays of ouabain-sensitive current under nonpumping conditions, Na+/K+ pumps stalled by withdrawal of external K+ (K+o) and Na+ (Na+o) were observed to conduct a measurable inward current at negative membrane potentials (Rakowski et al., 1991). The inward current became larger at more negative voltages, and larger still as extracellular [H+] was raised, and so was attributed to proton inflow (Efthymiadis et al., 1993). This suggestion was supported by the negative shifts of the current’s reversal potential that accompanied the ouabain-sensitive intracellular acidification observed after oocytes were held at negative potentials in pH-6.0 solution for several minutes (Wang and Horisberger, 1995). A link between this proton inflow and the third Na+-binding site was inferred when mutations in a region suggested by homology modeling to be selective for Na+ binding (Ogawa and Toyoshima, 2002) diminished both the inward current and inhibition by Na+o of outward Na+/K+ pump current at negative potentials (Li et al., 2006). More recently, the inward current was found to be augmented by mutations at the Na+/K+ pump’s C terminus (Yaragatupalli et al., 2009; Meier et al., 2010; Poulsen et al., 2010; Vedovato and Gadsby, 2010), and in some instances was further enhanced in the presence of Na+o; these findings reinforced an earlier proposal (Vasilyev et al., 2004) that Na+ ions could also flow along what has come to be referred to as the Na+/K+ pump “leak” pathway (Vasilyev et al., 2004; Yaragatupalli et al., 2009; Meier et al., 2010; Poulsen et al., 2010; Azizan et al., 2013; Nyblom et al., 2013).
We demonstrate here, however, that the inward Na+/K+ pump–mediated proton current requires neither the absence of external K+ or Na+ nor extreme negative potentials, and can be observed in native Na+/K+ pumps at physiological K+ and Na+ concentrations and resting potentials. Proton inflow is produced by phosphorylated conformations through which each Na+/K+ pump transits during every Na+/K+ transport cycle. But whether a proton traverses the Na+/K+ pump in any given cycle is determined by several factors. When even a small delay in Na+/K+ pump dephosphorylation postpones completion of the cycle, extracellular protons can exploit rapid reversibility of Na+-releasing transitions among phosphorylated conformations to enter the cell. Protons hop from a protonatable side chain accessible from the extracellular side in one phosphorylated E2 conformation to another protonatable residue with access to the cytoplasm in a kinetically adjacent phosphorylated E2 conformation. By systematic neutralization of carboxylates in the Na+/K+ pump TM domain, we here identify a glutamate and aspartate, and an intervening tyrosine, as forming the core proton route, which traverses the third Na+-binding site observed in the latest Na+/K+ pump crystal structures (Kanai et al., 2013; Nyblom et al., 2013).
In corresponding E2 states of the related SERCA Ca2+-ATPase, reversible protonation of cation-coordinating carboxylates compensates the charge imbalance that would otherwise attend release of the two transported Ca2+ ions into the sarcoplasmic/endoplasmic reticulum (Obara et al., 2005). Release of those protons to the cytoplasm in E1 states, before the next Ca2+ ions bind, results in countertransport of two or three protons each ATPase cycle (Yu et al., 1993). The H+/K+-ATPases, the closest relatives of Na+/K+ pumps, also transport two protons but from the cytoplasm to the cell exterior, analogous to the extrusion of three Na+ ions by a Na+/K+ pump; and, like Na+/K+ pumps, the H+/K+-ATPases countertransport two K+ ions. In H+/K+ pumps, the “third” ion-binding site is modeled to contain the positively charged side chain of a lysine, not found in Na+/K+ pumps (Poulsen et al., 2010); replacing that lysine by alanine makes the electroneutral H+/K+ pump become electrogenic (Burnay et al., 2003). But whether proton transport by H+/K+ pumps occurs by reversible protonation of cation-coordinating carboxylates or by transport of hydronium ions is not yet established (e.g., Law et al., 2008).
The proton inflow through the Na+/K+ pump is energetically downhill and is not obligatory for completion of the forward Na+/K+ transport cycle. But we speculate that it might accompany Na+/K+ pumping at the normal negative resting potentials of neurons, and cardiac and skeletal muscle cells, if extracytoplasmic pH were to fall sufficiently, as might occur, for example, during strenuous muscle exercise or cardiac or cerebral ischemia, and does occur in endosomes.
MATERIALS AND METHODS
Mutagenesis and expression
Substitutions in the Xenopus laevis Na+,K+-ATPase α1 subunit cDNA were introduced using QuickChange (Agilent Technologies) and verified by automated sequencing, as described previously (Reyes and Gadsby, 2006; Takeuchi et al., 2008; Vedovato and Gadsby, 2010). The substitution C113Y (Canessa et al., 1992) rendered the Xenopus Na+,K+-ATPase α1 subunit ouabain resistant and insensitive to extracellular application of hydrophilic, membrane-impermeant MTS reagents; this C113Y construct served as the template for other mutations. After in vitro transcription of cDNA in pSD5 vector, 15–45 ng of wild-type or mutant Na+,K+-ATPase α1 subunit cRNA was coinjected with 5–15 ng of Xenopus β3 subunit cRNA into defolliculated Xenopus oocytes, which were incubated at 18°C for 2–4 d before recording.
Solutions
External solutions contained 125 mM NaOH or tetramethylammonium-OH, 120 mM sulfamic acid, 0–30 mM K+-sulfamate, 5 mM BaCl2, 1 mM MgCl2, 0.5 mM CaCl2, plus 1 µM ouabain to inhibit endogenous Na+/K+ pumps (omitted in experiments to study wild-type Xenopus α1/β3 Na+/K+ pumps), and were buffered to pH 7.6 with 10 mM HEPES or to pH 6.0 with 10 mM Mes; osmolality was 250–260 mosmol/Kg. To inhibit all Na+/K+ pumps (whether wild type or ouabain resistant), 10 mM ouabain was directly dissolved into the appropriate external solution. 2-(Trimethylammonium)ethyl MTS (MTSET+) was diluted from a 100-mM aqueous stock into external solution to a final concentration of 1 mM just before use. A subset of oocytes was injected with 50 nL of 1 mM BeFX (1 mM BeSO4 plus 25 mM NaF) aqueous solution, which was expected to dilute to ∼100 µM in the oocyte intracellular volume. Before recording, [Na+i] was raised by putting oocytes for ≥2 h in K+- and Ca2+-free solution, containing 95 mM NaOH, 90 mM sulfamic acid, 10 mM TEACl, 0.1 mM EGTA, and 5 mM HEPES, pH 7.6; osmolality was ∼210 mosmol/Kg. Pipette (cytoplasmic) solution for outside-out patch recordings contained 125 mM NaOH, 120 mM sulfamic acid, 1 mM MgATP, 1 mM MgCl2, 0.1 mM EGTA, 10 mM HEPES, pH 7.4, plus 10 µM ouabain (to avoid palytoxin-induced currents from endogenous Na+/K+ pumps). Palytoxin (from Palythoa tuberculosa; Wako Pure Chemical Industries, Ltd.) was dissolved in aqueous 0.001% wt/vol BSA solution, and the 100-µM stock solution was stored at −20°C. Just before use, it was diluted to a final concentration of 100 nM in 125 mM Nao solution supplemented with 0.001% BSA and 1 mM Na-borate.
Electrophysiology
Oocytes expressing wild-type and ouabain-resistant mutant Na+/K+ pumps were studied at 22–24°C by two-microelectrode voltage clamp, as described previously (Vedovato and Gadsby, 2010). Whole oocyte currents were acquired with an OC-725A amplifier (Warner Instruments) filtered at 1 kHz and sampled at 5 kHz with an 18-bit ITC-18 A/D-D/A board controlled by Patch Master 2.20 software (Instrutech; HEKA). Steady-state currents (and pre–steady-state transient Na+ currents in 125 mM Na+o, 0 K+o) were elicited with 50-ms voltage steps to potentials between −180 and +60 mV, in increments of 20 mV, from a holding potential of −20 or −50 mV, and were averaged over the last 10 ms of each step. Na+ charge-movement quantities, ΔQ, were obtained directly as integrals of the ouabain-sensitive transient currents at the −20-mV holding potential upon termination of each voltage step. Single-exponential fits (beginning 1–3 ms after the start of the step) to the decay time courses of the transient currents elicited by the ON voltage steps gave relaxation rates of the slow components of pump charge movement. Data were analyzed with IgorPro 6 (WaveMetrics) and Origin 7.0 (OriginLab Corporation). C113Y or D935N(C113Y) mutant Na+/K+ pumps expressed in oocytes were also examined at 22–24°C in outside-out excised patches. Patch current was recorded with an Axopatch 200B amplifier (Molecular Devices), filtered at 500 Hz, and sampled at 5 kHz with a Digidata 1200 A/D-D/A driven by pClamp7 software (Molecular Devices). Palytoxin was applied to outside-out patches held at −50 mV in symmetric 125-mM Na+ solutions until inward Na+/K+ pump–channel current reached a steady level, and was then washed out. Data were analyzed with ClampFit (in pClamp 9; Molecular Devices). Results are reported as mean ± SEM of n measurements.
Online supplemental material
Fig. S1 presents detailed average pump-mediated current–voltage relationships with or without saturating K+o, in 125 mM Na+o or 0 mM Na+o, for E336Q, E788Q, D813N, and D817N mutant Na+/K+ pumps, with each mutant compared side-by-side with the parent C113Y Na+/K+ pump (Xenopus α1 subunit numbering throughout). Fig. S2 similarly compares the pump current–voltage relationships of C113Y pumps under all those conditions with those of Y780F, D935N, E963Q, and double D935N/E963Q mutant Na+/K+ pumps. Fig. S3 summarizes the influence of assorted other mutations (e.g., of TM histidines, of C-terminal basic residues and deletions, and of alternative ouabain-resistance conferring residues) on the ability of lowered extracellular pH (pHo) to amplify pump-mediated inward current at large negative potentials, in comparison to the response of C113Y Na+/K+ pumps. Fig. S4 compares average pump-mediated current–voltage relationships of wild-type Xenopus α1/β3 Na+/K+ pumps with those of C113Y Xenopus α1/β3 Na+/K+ pumps, in 125 mM Na+o, with 0 or 15 mM K+o, all at pHo 7.6 and at pHo 6.0. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201311148/DC1.
RESULTS
Outward Na+/K+ transport current
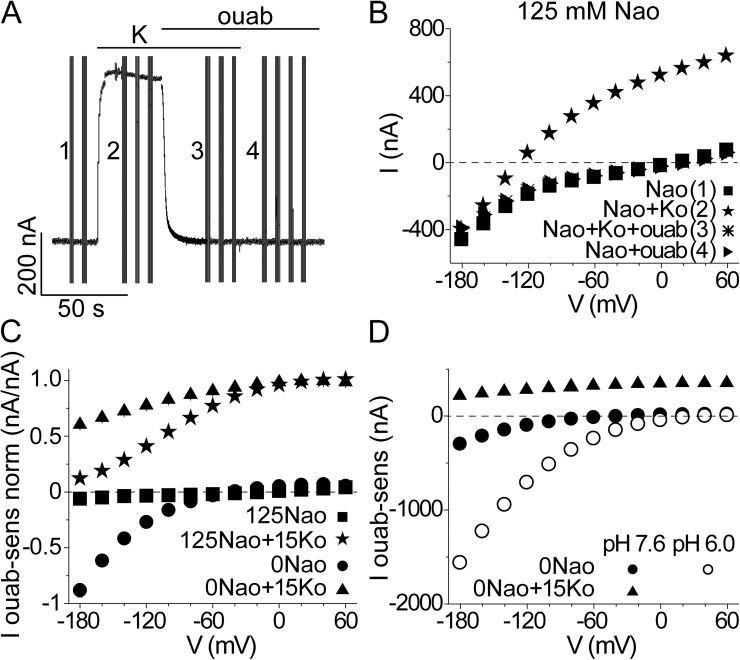
Adding K+o activates the millions of wild-type α1/β3 Xenopus Na+/K+ pumps (believed to be the native isoforms; Verrey et al., 1989; Good et al., 1990) overexpressed in a Xenopus oocyte with elevated intracellular Na+, [Na+i], eliciting a large outward current that is rapidly abolished by ouabain (Fig. 2 A; for consistency, 10 mM ouabain was used in all experiments regardless of whether wild-type or ouabain-resistant pumps were studied). Voltage steps allow current measurements over a range of membrane potentials with and without ouabain (Fig. 2 B), yielding ouabain-sensitive, i.e., Na+/K+ pump-mediated, currents by subtraction (Fig. 2 C). At this saturating, 15 mM, [K+o] outward pump current approaches the same voltage-independent maximum at positive potentials with 125 mM Na+o (Fig. 2 C, stars) or without Na+o (Fig. 2 C, triangles; Nakao and Gadsby, 1989; Vedovato and Gadsby, 2010). This reflects ultimate rate limitation of the forward 3 Na+/2 K+ exchange transport cycle by K+ deocclusion, E2(K2)→E1 · 2K (Fig. 1; Post et al., 1972; Forbush, 1987a), a step known to be voltage insensitive (Bahinski et al., 1988). The maximal turnover rate of the cycle can be calculated from this maximum current amplitude if the number of functional Na+/K+ pumps in each oocyte is known. The latter is obtained from the total quantity of charge moved by transient Na+ currents in response to voltage steps in 125 mM Na+o in the absence of K+o (e.g., Fig. 6, below; Nakao and Gadsby, 1986; Vedovato and Gadsby, 2010). A Boltzmann fit to a plot of Na+ charge, ΔQ, elicited by each step against membrane potential during that step gives total moveable charge, Qtot, and steepness, zq, and hence equivalent charge moved per pump, zq · e0; in each oocyte, the number of participating pumps is then determined as Np = Qtot/zq · e0. For a presumed 3 Na+/2 K+ transport stoichiometry (Post and Jolly, 1957; Garrahan and Glynn, 1967a; Rakowski et al., 1989), and thus net movement across the membrane of a single charge per cycle, the turnover rate at any given pump current amplitude, Ip, at any membrane potential is estimated as Ip/Np · e0 or Ip · zq/Qtot. The maximal turnover rate of wild-type α1/β3 Xenopus Na+/K+ pumps at room temperature is calculated in this way to be ∼45 s−1 (compare to Vedovato and Gadsby, 2010).
Figure 2.
Outward and inward current components in wild-type Na+/K+ pumps. (A) Current in a single Na+-loaded oocyte overexpressing wild-type α1/β3 Xenopus Na+/K+-ATPase pumps, at −20 mV, in 125-mM Na+o solutions. The addition of 15 mM K+o (bar labeled “K”) activated large outward 3 Na+i/2 K+o exchange current that was abolished by 10 mM ouabain (bar labeled “ouab”). Vertical lines are responses to 50-ms jumps to other potentials. (B) Steady currents from the oocyte in A plotted against voltage for the trials identified by numbers 1–4 in A. (C) Average ouabain-sensitive currents (I ouab-sens norm) obtained by subtraction of currents like those in B, at every voltage and presence or absence of K+o, were normalized to the mean amplitude between 0 and 60 mV of ouabain-sensitive current at 15 mM [K+o] in 0 or 125 mM Na+o, as appropriate; normalized currents were then averaged across oocytes; ±SEM is visible where larger than the symbols; n = 8 at 125 mM Na+o; n = 5 at 0 Na+o. In direct comparisons in the same oocyte, maximal outward ouabain-sensitive current at 15 mM [K+o] and positive voltage is the same in the presence or absence of Na+o (Vedovato and Gadsby, 2010). (D) Ouabain-sensitive currents in a representative oocyte at 0 mM Na+o. At pHo 7.6, ouabain-sensitive currents in 15 mM K+o (closed triangles) or in 0 mM K+o (closed circles) are like those in C, but upon lowering pHo to 6.0 in 0 mM K+o (open circles), inward current at negative potentials increased approximately fivefold.
Figure 6.
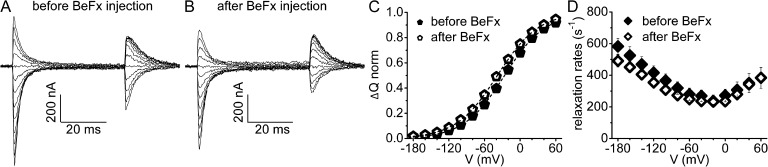
BeFX-bound Na+/K+ pumps mimic conformational transitions of normal phosphorylated pumps. (A and B) C113Y Na+/K+ pump–mediated pre–steady-state Na+ charge movements (e.g., Nakao and Gadsby, 1986; Meier et al., 2010; Vedovato and Gadsby, 2010) elicited by steps from −20 mV to potentials between −180 and +60 mV, in 125 mM Na+o, 0 K+o before (A) and after (B) oocyte injection with 1 mM BeFX (as in Fig. 5 A). (C) Ouabain-sensitive charge, ΔQ, on termination of each step is plotted against step voltage, and ΔQ-V is fitted with the Boltzmann relation to determine total charge moved, Qtot, effective valence, zq, and midpoint voltage; none was altered by BeFX injection. The ratio of Qtot before versus after BeFX was 1.2 for A and B, and averaged 1.1 ± 0.1 (n = 3). The same results were obtained with C113Y-ΔYY mutants (Qtot ratio = 1.15 ± 0.02; n = 4). In contrast, the ratio of K+o-activated outward pump current after versus before BeFX injection was ≤0.2 for C113Y pumps (e.g., outward current was reduced by 88% for the oocyte of A and B, and by ∼90% for C113Y-ΔYY pumps). (D) Mean (n = 3) relaxation rates of the transient currents elicited by the ON voltage steps, plotted against voltage, were not altered by BeFX. The unaltered transient Na+ charge movements demonstrate that BeFX-bound and inhibited Na+/K+ pumps remain capable of a subset of normal conformational changes in 125-mM Na+o solutions.
In the presence of Na+o, the outward Na+/K+ pump current declines at negative membrane potentials (Fig. 2 C, stars). This reflects the strong influence of negative voltage to selectively enhance rebinding of extracellularly released Na+ to the Na+-specific (Schneeberger and Apell, 2001; Li et al., 2005; Ratheal et al., 2010) site III, which lies deep within the pump (Kanai et al., 2013; Nyblom et al., 2013) partway through the membrane’s electric field. As a result, the apparent affinity for Na+o is increased relative to that for K+o at negative voltages (Nakao and Gadsby, 1989; Gadsby et al., 1993; Sagar and Rakowski, 1994; Vedovato and Gadsby, 2010), allowing bound Na+ to more effectively impede K+o binding. Consequently, dephosphorylation of E2P and formation of the occluded E2(K2) state, and hence completion of the Na+/K+ transport cycle, are all delayed. Accordingly, when Na+o is absent, the steep diminution of outward Na+/K+ pump current at negative voltages is greatly attenuated (Fig. 2 C, triangles).
Pump-mediated inward proton current
Without K+o ions to activate Na+/K+ transport, the wild-type Na+/K+ pumps generate little steady current at any voltage at 125 mM Na+o (Fig. 2 C, squares). But in the absence of both K+o and Na+o, there is an easily measured inward current (Rakowski et al., 1991) that increases steeply at more negative potentials (Fig. 2 C, circles). Upon closer inspection, this inward current is discernible even in 125 mM Na+o, although severalfold smaller (Fig. 2 C, squares vs. circles; see also Figs. 8 B and S4 A, closed squares). Its amplification on lowering external pH (pHo), whether Na+o is absent (Fig. 2 D, open circles) or present (see Fig. 8 B), argues that protons carry the current (Efthymiadis et al., 1993; Wang and Horisberger, 1995). Comparison of the size of this inward current with maximal outward Na+/K+ transport current (representing outflow of ∼45 positive charges/s through each pump) in the same pumps indicates that, even at −180 mV in 0 Na+o at pHo 6.0 (Fig. 2 D), the inward current represents inflow of only ∼200 protons s−1 per pump. This meager throughput rate could reflect limitation of proton electrodiffusion through a water-filled pore by the low proton concentration, here 1 µM (DeCoursey, 2003). However, the enthalpic activation energy of the inward current in Na+/K+ pumps lacking the two C-terminal tyrosines (ΔYY) was found to be very high, ∼140 kJ/mol, identical to that of outward Na+/K+ transport current in the same pumps (Meier et al., 2010), suggesting that the inward current is rate limited by substantial changes in pump conformation. A plausible conformational mechanism for inward proton transport could be that a protonatable side chain is exposed to extracellular fluid in one Na+/K+ pump conformation that is in equilibrium with another conformation in which the side chain has cytosolic access (Fig. 3 A); a proton gradient could then drive net proton movement across the membrane. Just such a mechanism has been shown to generate voltage-sensitive proton current in Shaker voltage-gated K+ channels in which a histidine had been substituted for one of the voltage-sensor arginines (Starace and Bezanilla, 2001).
Figure 8.
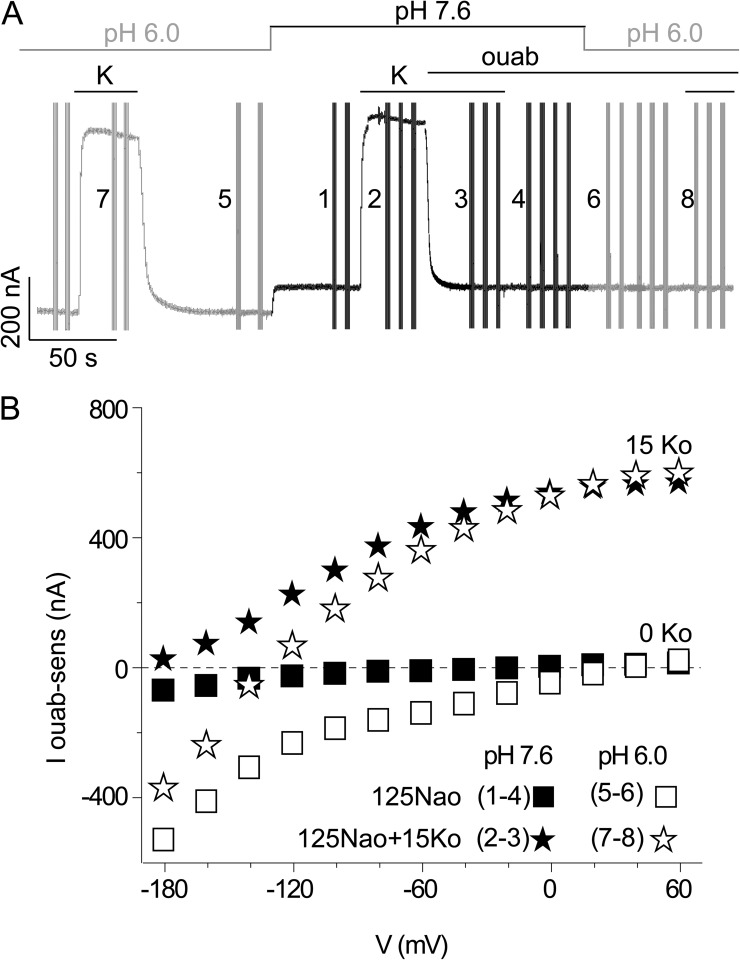
Wild-type Na+/K+ pump currents in 125 mM Na+o at normal and lowered pHo. (A) Current record at −20 mV (same oocyte as in Fig. 2, A and B) during the addition of 15 mM K+o and ouabain, as indicated, at pHo 6.0 (gray current trace) and pHo 7.6 (black trace). (B) Ouabain-sensitive steady currents without (squares) and with (stars) 15 mM K+o, at pHo 7.6 (closed symbols) and 6.0 (open symbols), from subtraction of currents in numbered voltage trials in A (the same results obtained in n = 8 oocytes are shown averaged in Fig. S4 A).
Figure 3.
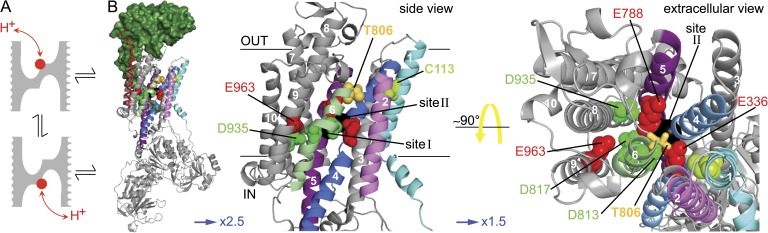
Proposed proton transport mechanism and candidate carboxylates. (A) Cartoon of proposed mechanism indicating protonation/deprotonation of a side chain (red circle) with alternate extracellular and cytoplasmic access in kinetically adjacent pump conformations; gray mass represents protein barrier to proton diffusion in the relevant segment of Na+/K+ pump TM domain structure. (B; from left to right) Increasingly magnified (factors indicated by blue arrows) views of Xenopus α1/β3 Na+/K+ pump homology model based on x-ray crystal structure of the K+-bound E2 · MgF42− Na+/K+-ATPase (Protein Data Bank accession no. 2ZXE; Shinoda et al., 2009), showing the α subunit (gray, with 5 of the 10 TM helices colored), β subunit (green surface), and γ subunit (red helix). (Middle and right) Side and top (from extracellular surface) views of the α-subunit TM domain (helix numbers in white) identifying six candidate carboxylates, three Glu (red spheres) and three Asp (green spheres), at the level of binding sites I and II that contain K+ ions (black balls); the colored TM helices are: pale blue, TM1; magenta, TM2; blue, TM4; purple, TM5; and green, TM6. Gray, TM3 and TM7–TM10; lime spheres, C113 in TM1; yellow spheres (left) or sticks (right) mark T806 at the top of TM6.
Two carboxylates and a hydroxyl required for proton inflow
Appropriate candidates for such protonatable residues in the Na+/K+ pump are six conserved acidic amino acids (Fig. 3 B) in the transmembrane (TM) domain that have been implicated, or demonstrated to be involved, in coordinating bound K+ and/or Na+ ions. Thus, E336 (Xenopus α1 numbering) in TM helix 4 (TM4), E788 in TM5, and D813 and D817 in TM6 are all involved in coordination (directly, or via a water) of K+ or Na+ ions in sites I and II in the crystal structures of K+-bound (E2 · MgF42− · 2 K+; Morth et al., 2007; Shinoda et al., 2009) or Na+-bound (E1 · AlF4− · ADP · 3 Na+; Kanai et al., 2013; Nyblom et al., 2013) Na+/K+ pumps. D935 in TM8 contributes to coordination of the Na+ in site III (Kanai et al., 2013), and E963 in TM9 had previously been linked to a Na+-selective binding site by homology modeling (Ogawa and Toyoshima, 2002) and mutagenesis (Li et al., 2005, 2006). To assess a possible role of each of these residues in the proton current, we neutralized them one at a time and then tested whether a pHo drop from 7.6 to 6.0 in K+o-free, Na+o-free solution caused a large increase in inward current, like that seen in wild-type Na+/K+ pumps in Fig. 2 D. These neutralizing mutations were introduced one at a time into Xenopus α1/β3 Na+/K+ pumps that had been made relatively ouabain resistant by the single α1-subunit mutation C113Y (Canessa et al., 1992), so that 10 mM ouabain could be used to obtain mutant pump currents, whereas the continuous presence of 1 µM ouabain silenced endogenous Xenopus Na+/K+ pumps (e.g., Takeuchi et al., 2008; Vedovato and Gadsby, 2010).
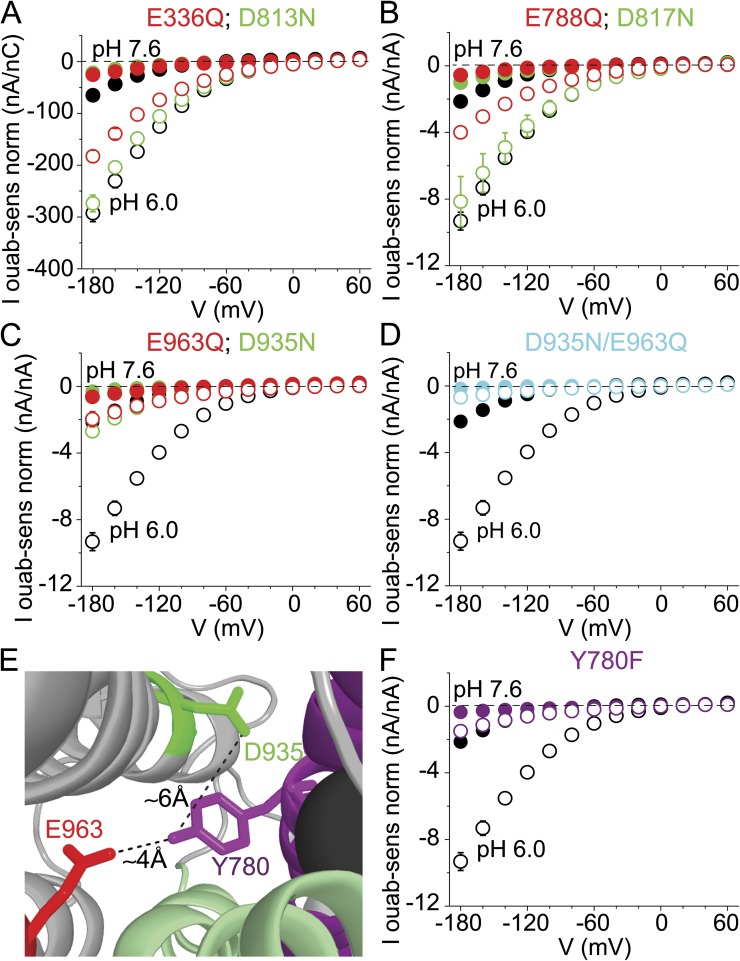
The carboxylate mutants all expressed well, as revealed by the magnitude of pump-mediated transient Na+ charge movements elicited by voltage steps. We found wild-type–like Na+/K+ outward currents, influenced in the usual voltage-dependent manner by Na+o (Fig. 2 C), in the ouabain-resistant C113Y parent Na+/K+ pumps (see Vedovato and Gadsby, 2010), and in E788Q (Fig. S1 F; Peluffo et al., 2000; Koenderink et al., 2003) and E963Q (Fig. S2 D; Li et al., 2006) pumps, whereas 15-mM K+o-activated outward currents in D935N (Fig. S2 B) and double D935N/E963Q (Fig. S2 F) mutants failed to saturate at positive voltage and were insensitive to the presence or absence of Na+o. In contrast, outward Na+/K+ transport currents were absent in E336Q and D813N mutants, with or without Na+o (Fig. S1, B and D; Nielsen et al., 1998; Vilsen and Andersen, 1998; Koenderink et al., 2003), and present in D817N only at 0 Na+o and elevated (30 mM) K+o (Fig. S1 H).
In K+o-free, Na+o-free solution, inward current at large negative potentials increased severalfold on lowering pHo to 6.0 in the ouabain-insensitive background C113Y Xenopus Na+/K+ pumps (Fig. 4, A–D and F, black symbols repeated in each graph), just like in wild-type Xenopus Na+/K+ pumps (Fig. 2 D). Relatively large inward currents at pHo 6.0 were also preserved in E336Q and D813N (Fig. 4 A), and in E788Q and D817N (Fig. 4 B) individual mutant Na+/K+ pumps, but were greatly diminished in D935N (Poulsen et al., 2010) and E963Q (compare to Li et al., 2006) mutants (Fig. 4 C; also in D935C, Fig. S3), and were essentially abrogated in the D935N/E963Q double mutant (Fig. 4 D). Because the residual inward currents in D935N (Fig. 4 C) and D935N/E963Q (Fig. 4 D) pumps are displayed normalized to the observed outward current at positive potentials in those pumps, despite its failure to reach a voltage-insensitive maximum (Fig. S2 B), those currents are overestimates for purposes of comparison with C113Y. Indeed, assessments of D935N expression levels using transient Na+ charge movements or palytoxin-induced currents (see Fig. 9) indicate that the D935N inward currents (Fig. 4 C) are overestimated by about threefold. Given the relative positions of E963 and D935 in the Na+/K+ pump’s TM domain (Fig. 3 B), the apparent requirement of both carboxylates for robust proton inflow that our findings indicate could be explained if extracellular protons bind to E963 and subsequently transfer to D935, from where they access the cytoplasm (compare to Fig. 3 A). Estimates of pKa based on the E2 · MgF42− · 2 K+ Na+/K+ pump crystal structure do imply that E963 and D935 are both protonated in that conformation, and a cytoplasmic route for D935 protonation and deprotonation has been proposed (Poulsen et al., 2010).
Figure 4.
External pH sensitivity of ouabain-sensitive inward current in 0 Na+o and 0 K+o after conservative mutation of each candidate residue in partially ouabain-resistant C113Y pumps. Each Glu (red circles) and Asp (green circles) was neutralized, and the inward current response to lowering pHo (open vs. closed circles) was compared with that of the parent C113Y pumps (black circles). For these comparisons, inward current was normalized to maximally K+o-activated (10–30 mM [K+o]), ouabain-sensitive outward pump current (Figs. S1 and S2) recorded in the same oocyte, averaged between 0 and +60 mV; because E336Q and D813N pumps lacked K+o-activated current (Fig. S1, B and D), inward current was normalized to total pump-mediated Na+ charge movement, Qtot, in 0 K+, 125 mM Na+o solution (Nakao and Gadsby, 1986; Vasilyev et al., 2004; Meier et al., 2010; Poulsen et al., 2010; Vedovato and Gadsby, 2010) in the same oocyte. Inward current of (A) E336Q (n = 3) and D813N (n = 6), and of (B) E788Q (n = 7) and D817N (n = 5), pumps, like that of control C113Y pumps (n = 12–16), increased substantially on lowering pHo from 7.6 to 6.0; inward current at pHo 6.0 and −180 mV in E336Q, D813N, and D817N was >60%, and in E788Q it was 43%, that of C113Y pumps. But inward current of (C) D935N (n = 5) and E963Q (n = 4) pumps was comparatively small at pHo 6.0, and that of (D) D935N/E963Q double mutant (cyan; n = 3) pumps was almost absent compared with C113Y pumps (n = 16); as K+o-activated currents of D935N and D935N/E963Q pumps fail to reach a voltage-independent maximum at positive potentials (Figs. 9 and S2), their normalized inward current magnitude shown here is overestimated (by greater or equal to threefold; Fig. 9). (E) D935 (green sticks) and E963 (red sticks) are ∼10 Å apart in the Xenopus α1 Na+/K+ pump homology model of the E2 · MgF42− conformation (Fig. 3 B), with the hydroxyl of TM5 Y780 (purple sticks) between them; view magnified 2.5 times, from the right-hand image of Fig. 3 B. (F) The response of inward current in Y780F (n = 4) mutant pumps to lowering pHo from 7.6 to 6.0 is small, as in D935N or E963Q pumps, compared with that of C113Y pumps.
Figure 9.
Comparing expression and function of D935N(C113Y) and parent C113Y pumps. (A and B) Representative recordings of pump–channel currents induced by 100 nM palytoxin (PTX), at −50 mV with symmetrical 125-mM [Na+] solutions, in outside-out patches excised from oocytes injected with the same amounts of cRNA (30 ng α subunit plus 10 ng β subunit): (A) after 1 d of expression (black, C113Y: steady current amplitude, −1.2 ± 0.2 nA and n = 4; green, D935N: −1.1 ± 0.2 nA and n = 4), and (B) after 2 d (black, C113Y: −2.1 ± 0.2 nA and n = 15; green, D935N: −2.1 ± 0.1 nA and n = 8). (C) Ouabain-sensitive outward Na+/K+ transport currents at 15 mM K+o in 125 mM Na+o from the very same two C113Y-expressing (black stars) and D35N-expressing (green stars) oocytes from which the patch recordings in B were obtained. (D) Average outward pump currents in 15 mM K+o and 125 mM Na+o for C113Y (black stars; n = 12) and D935N (green stars; n = 9) after 2 d of expression, recorded in oocytes (including those of B and C) from the same batches that yielded the average excised-patch data in B.
However, the E963 and D935 carboxylates are separated by ∼10 Å in the K+-bound E2 · MgF42− structure (Fig. 4 E; Shinoda et al., 2009; also in the Na+-bound E1 structure, Kanai et al., 2013). We therefore examined whether the hydroxyl of the intervening tyrosine Y780 might serve to allow proton relay. The inward current increase on lowering pHo to 6.0 was markedly attenuated by the conservative mutation Y780F (Fig. 4 F), which did not affect outward Na+/K+ currents (Fig. S2 H), confirming that the hydroxyl is also required for proton inflow (Li et al., 2006). In comparison, a substantial amplification of inward current at pH 6.0 was not prevented (Fig. S3) by individual mutation of TM histidines (H292A, H295A, H921A, and H921N; compare to Vasilyev et al., 2004) nor of C-terminal arginine or lysine (R1007N and K1008A), nor by C-terminal truncation (ΔYY and ΔKESYY). Collectively, these results argue that extracellular protons travel from E963 to Y780 to D935, residues in or near Na+-binding site III (Ogawa and Toyoshima, 2002; Poulsen et al., 2010; Kanai et al., 2013; Nyblom et al., 2013). Near abolition of inward current by a conservative mutation at any one of these three positions demonstrates the absence of alternative, parallel, pathways for net proton flow.
The proton route is distinct from the principal pathway for transported Na+ and K+ ions
The proton route we infer, between TM9, TM8, TM5, and TM6 (Fig. 3 B), passes through Na+ site III (Kanai et al., 2013; Nyblom et al., 2013) and differs from the principal pathway proposed for translocation of pumped Na+ and K+ ions, between TM1, TM2, TM4, and TM6, passing through site II (Fig. 3 B; Takeuchi et al., 2008). The latter path, evident as a funnel open to the extracytoplasmic side in crystal structures of the related SERCA Ca-ATPase in a BeF3−-trapped E2P-like conformation (Olesen et al., 2007; Toyoshima et al., 2007) and in related Na+/K+ pump models (Takeuchi et al., 2008), was corroborated by a cysteine scan of TM1–TM6 accessibility to the small positively charged MTS reagent, MTSET+, in palytoxin-bound Na+/K+ pump channels (Reyes and Gadsby, 2006; Takeuchi et al., 2008); palytoxin transforms Na+/K+ pumps into ion channels by disrupting the coordination between their alternating gates (Artigas and Gadsby, 2003).
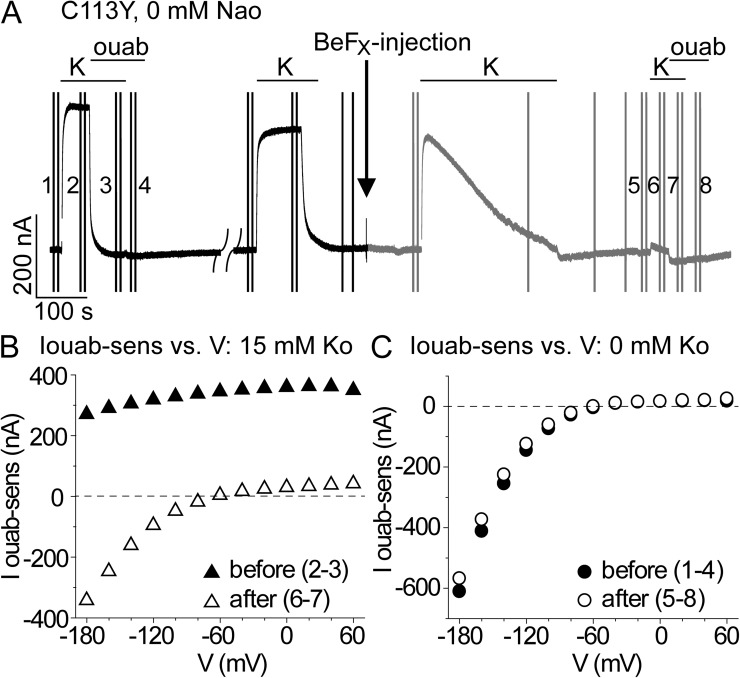
Because cytoplasmic-side access to sites I and II is closed in BeF3−-bound SERCA structures (by a 15–20-Å barrier; Olesen et al., 2007), and Na+/K+ pump treatment with BeFX prevents channel opening by palytoxin (Takeuchi et al., 2008), to further distinguish the proton route from that taken by pumped Na+ and K+, we examined the influence of BeFX on inward proton current. BeFX injection into oocytes (Fig. 5 A) essentially abolished the large K+o-activated outward current at positive potentials in C113Y Na+/K+ pumps (Fig. 5, A and B), but inward proton current in K+o-free, Na+o-free solution remained unaltered (Fig. 5 C). Importantly, after Na+/K+ pump inhibition by BeFX, presumably by formation of relatively stable E2P-like states with tightly bound BeFX, 15 mM K+o failed not only to activate appreciable outward current at positive potentials but also to inhibit inward proton current at negative potentials (Fig. 5 B). This argues that dephosphorylation of E2P, required for K+o occlusion and for completion of the Na+/K+ transport cycle (Fig. 1), is also required for K+o to prevent proton inflow. Unaltered inward proton current in BeFX-inhibited Na+/K+ pumps provides unequivocal evidence that proton import requires only phosphorylated states.
Figure 5.
Closing the cytoplasmic Na+ and K+ access pathway in C113Y pumps by using BeFX to restrict them to phosphorylated-like states does not impair inward proton current. (A) Outward current in C113Y Na+/K+ pumps at −20 mV in Na+o-free solution was twice activated by 15 mM K+o before online injection of 1 mM BeFX into the oocyte (black downward arrow and gray current recording). The slow current decay in 15 mM K+o after BeFX injection reflects accumulation of pumps in stable E2P-like states incapable of Na+/K+ exchange (Olesen et al., 2007; Toyoshima et al., 2007). The numbers mark the voltage trials used to obtain the subtracted currents shown in B and C. (B and C) BeFX abolished ouabain-sensitive outward current in 15 mM K+o at positive voltages (B; open vs. closed triangles) but did not alter inward current in 0 K+o (C; open vs. closed circles). BeFX also prevented K+o inhibition of inward current at negative voltages (B; open vs. closed triangles); two other identical experiments gave the same results, as did nine experiments with C113Y-ΔYY pumps (C-terminal truncation mutant; Vedovato and Gadsby, 2010) in 0 Na+o (n = 6) or in 125 mM Na+o (n = 3).
In addition, BeFX-inhibited C113Y Na+/K+ pumps generated unaltered transient Na+ currents in response to voltage steps in 0 K+o, 125-mM Na+o solution (Fig. 6, A and B; Nakao and Gadsby, 1986; Holmgren et al., 2000; Meier et al., 2010; Vedovato and Gadsby, 2010). The asymptotes of the ΔQ voltage plots (Fig. 6 C) are attributed to Na+/K+ pump accumulation in state E2P, with no bound Na+, at positive potentials, and in E2P and E1P states, with three Na+ ions bound, at negative potentials (Heyse et al., 1994; Hilgemann, 1994; Holmgren et al., 2000; Gadsby et al., 2012). Moreover, the slow (Fig. 6 D) components of Na+ charge movement examined here are believed to be rate limited by the major E1P(Na3)↔E2P(Na2)Na conformational change (Fig. 1; Heyse et al., 1994; Hilgemann, 1994; Holmgren et al., 2000; Gadsby et al., 2012). These unaltered charge movements therefore demonstrate that BeFX-bound Na+/K+ pumps are not locked into a single conformational analogue of the E2P ground state (e.g., Cornelius et al., 2013). Rather, BeFX-bound Na+/K+ pumps are faithful mimics of phosphorylated pumps and, in the presence of Na+o, they can adopt all conformations, and can undergo all transitions, of phosphorylated pumps, from E2P to the occluded E1P(Na3) form.
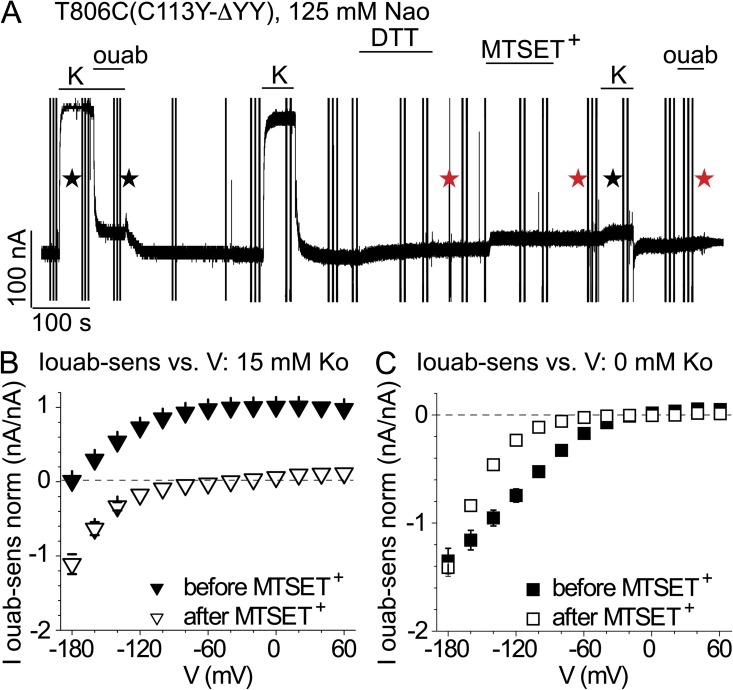
We found previously that modification by MTSET+ of a cysteine substituted for T806, at the extracellular end of TM6 (Fig. 3 B), abolished the large inward Na+ currents (>106 Na+ s−1 per pump; e.g., Fig. 9) that flow through palytoxin-bound T806C Na+/K+ pump channels (Reyes and Gadsby, 2006; Takeuchi et al., 2008). We therefore examined whether that modification of the external cation transport pathway would similarly impair inward proton current (Fig. 7). MTSET+ reaction with T806C (without palytoxin treatment) abolished K+o-activated outward Na+/K+ pump current (C113Y-ΔYY pumps; Fig. 7, A and B), but large inward proton currents persisted (Fig. 7 C, before vs. after), although their voltage dependence was altered somewhat. Strikingly, after MTSET+ treatment, large inward currents were observed even in the presence of 15 mM K+o (Fig. 7 B), implying that MTSET+ modification of T806C abolishes outward Na+/K+ pump current by interfering with the binding and occlusion of K+o ions. These results with MTSET+ further substantiate our above conclusion that the protons travel a pathway distinct from that used by the preponderance of transported Na+ and K+ ions.
Figure 7.
Preventing extracellular access to the principal Na+ and K+ transport pathway does not impair inward proton current in C113Y-ΔYY Na+/K+ pumps. (A) Recording of abolition by 1 mM MTSET+ of K+o-activated outward current in T806C(C113Y-ΔYY) mutant Na+/K+ pumps, at −50 mV in 125-mM Na+o solutions; the ΔYY mutant was chosen as background because that C-terminal truncation amplifies proton currents in 125 mM Na+o (Yaragatupalli et al., 2009; Poulsen et al., 2010; Vedovato and Gadsby, 2010), the condition in which MTSET+ abolished large Na+ currents in palytoxin-bound Na+/K+ pump channels (Reyes and Gadsby, 2006; Takeuchi et al., 2008). 10 mM dithiothreitol (bar labeled “DTT”) was first applied to reverse any spontaneous cysteine oxidation. Outward pump current was assessed before and after the modification by MTSET+ (bar labeled “MTSET+”). Black and red stars, respectively, mark voltage trials that typically yielded the average subtracted currents shown in B (at 15 mM K+o) and C (at 0 K+o). (B) Ouabain-sensitive currents were normalized to the outward pump current between 0 and +60 mV in 15 mM K+o before MTSET+ in each oocyte, and then averaged. In 15 mM K+o, outward current at positive voltages was reduced by 90 ± 0.02% (n = 7 oocytes) by MTSET+, but current was inward at large negative potentials, indicating that K+o could no longer inhibit inward proton current. (C) Inward currents in 0 K+o were normalized to the ouabain-sensitive outward current between 0 and +60 mV in 15 mM K+o before MTSET+ in each oocyte, and then averaged. MTSET+ modification of T806C did not diminish inward current amplitude at −180 mV (n = 7 oocytes), although voltage sensitivity was altered.
Proton inflow results from back-steps during Na+/K+ transport
We chose absence of K+o and Na+o as the experimental condition in the above tests to emphasize the contribution of the inward proton current and thus facilitate its characterization. But does net proton influx occur only in the absence of K+o, as generally stated (Rakowski et al., 1991; Efthymiadis et al., 1993; Wang and Horisberger, 1995; Vasilyev et al., 2004; Li et al., 2006; Yaragatupalli et al., 2009; Meier et al., 2010; Poulsen et al., 2010; Ratheal et al., 2010; Vedovato and Gadsby, 2010; Nyblom et al., 2013), or does it also happen at physiological Na+ and K+ concentrations? If so, does it accompany normal Na+/K+ transport, and might it even be obligatory for Na+/K+ exchange? To address these questions, we determined current generated by wild-type Na+/K+ pumps (i.e., overexpressed Xenopus α1/β3) exposed to 125 mM Na+o both with 0 K+o and with 15 mM K+o, all at pHo 7.6 (as in Fig. 2, A–C), and then repeated the measurements at pHo 6.0 (Fig. 8, A and B). Without K+o, inward current at negative potentials was small but measurable at pHo 7.6 (Fig. 8 B, closed squares; see Fig. S4 A for mean data), and was considerably amplified at pH 6.0 (Figs. 8 B and S4 A, open squares), qualitatively similar to the large amplification of inward current observed on lowering pHo to 6.0 in the same wild-type pumps in 0 Na+o (Fig. 2 D).
But with 15 mM K+o, the asymptotic outward currents at positive potentials were identical at pHo 7.6 and 6.0 (Fig. 8 B, closed vs. open stars), showing that pumps reach the same maximal Na+/K+ transport rate (i.e., forward cycle rate, ∼45 s−1) regardless of pHo level, and hence that proton current is absent at the most positive voltages (proton current is also absent at positive potentials in 0 K+o; Fig. 8 B, closed vs. open squares). Proton influx is therefore not obligatory for Na+/K+ exchange. At potentials more negative than −120 mV, ouabain-sensitive current in 15 mM K+o remains outward at pHo 7.6 (Fig. 8 B, closed stars) but is net inward at pHo 6.0 (Fig. 8 B, open stars). Reversal of the 3 Na+/2 K+ transport cycle (Fig. 1) is thermodynamically infeasible under these conditions, because the Na+/K+ pump equilibrium potential (e.g., Veech et al., 1979; Tanford, 1981; De Weer et al., 1988) is estimated to be at least as negative as −240 mV under physiological conditions (and even more negative at the elevated [K+o] and [Na+i] of these experiments), and it is not expected to vary with pHo. All Na+/K+ transport current must therefore be outward over the entire voltage range explored here. Thus, the net inward current measured at extreme negative voltage means that the inward proton current generated by the entire population of Na+/K+ pumps exceeds any outward Na+/K+ exchange current being generated by that pump population at the same time. As that same population of pumps generates only outward current at positive potentials under these conditions (15 mM K+o at pHo 6.0), if we assume the pumps are all identical, then each pump must be capable of generating both outward Na+/K+ transport current and inward proton current. A pump must complete a forward (clockwise) step through every transition in the Na+/K+ transport cycle (Fig. 1) to generate net outward current, but it needs to visit only the phosphorylated subset of states in that cycle (Figs. 5 and 6) to generate inward proton current (Fig. 5, B and C). We therefore conclude that each pump may generate both inward proton current and outward Na+/K+ current during the same transport cycle. Moreover, proton current prevalence at more negative voltages, where completion of the forward Na+/K+ transport cycle slows (and outward current thus declines), suggests that proton inflow results from reversal of a step that is a normal participant in Na+/K+ transport; i.e., reversal of a transition between phosphorylated states.
Near −120 mV, where net current is zero in 15 mM K+o at pHo 6.0 in this oocyte (Fig. 8 B, open stars), outward Na+/K+ exchange current must just balance inward proton current; i.e., on average, a single proton enters the cell through every Na+/K+ pump during each complete Na+/K+ transport cycle. In other words, at −120 mV and pHo 6.0, approximately one proton-importing back-step occurs in each cycle before K+o binding triggers E2P dephosphorylation and K+ occlusion, thereby preventing any further proton inflow in that 3 Na+/2 K+ transport cycle. At the most negative membrane potentials, each complete Na+/K+ transport cycle at pHo 6.0 must on average include multiple reiterations of the proton-importing back-step (perhaps as many as 10 at −180 mV in this example, from comparison of the amplitude of outward current in 15 mM K+o at pHo 7.6 [Fig. 8 B, closed stars] with that of the net inward current in 15 mM K+o at pHo 6.0 [open stars]). Even between −60 and 0 mV, outward current in 15 mM K+o is somewhat smaller at pHo 6.0 (Fig. 8 B, open stars) than at pHo 7.6 (closed stars), indicating that, also at modest negative potentials, in every Na+/K+ pump an occasional proton-importing back-step interrupts forward progression of the normal Na+/K+ transport cycle at pHo 6.0. At −80 mV, a value between the resting potentials of cardiac and skeletal muscle (−90 mV), and those of neurons (−60 to −70 mV), the normalized (to maximal outward current, corresponding to ∼45 cycles s−1) inward proton current in 0 mM K+o, 125 mM Na+o at pHo 7.6 (Figs. 8 B and S4 A, closed squares) averaged −0.025 ± 0.003 (n = 8), corresponding to ∼1 proton entry s−1, and at pHo 6.0 (Figs. 8 B and S4 A, open squares) was −0.287 ± 0.009 (n = 8), corresponding to ∼13 protons entered s−1. Because outward pump current at −80 mV and at pHo 7.6 (Fig. 8 B, closed stars) corresponded to ∼30 Na+/K+ exchange cycles s−1, we can conclude that, even under these relatively physiological conditions, on average one proton enters the cell through each Na+/K+ pump every ∼30 transport cycles. The results for C113Y Na+/K+ pumps were qualitatively similar to the findings in wild-type Xenopus Na+/K+ pumps (Fig. S4, A vs. B), except that inward currents, and hence proton inflow rates, were consistently larger in the C113Y pumps, as noted previously for both C113Y and RD ouabain-resistant pumps (Vedovato and Gadsby, 2010).
DISCUSSION
Normalization procedures for reliable comparison of Na+/K+ pump mutants
To allow for differences in expression of the same Na+/K+ pumps from oocyte to oocyte, and to permit meaningful comparisons between wild-type and mutant Na+/K+ pumps, it was important to establish reliable procedures for normalizing pump-generated currents and to validate them with estimates of numbers of functional pumps. Relative magnitudes and voltage sensitivities of currents mediated by wild-type or mutant pumps under various conditions are well preserved by normalization to the amplitude of outward Na+/K+ transport current at positive potentials and saturating [K+o] measured in the same oocyte (i.e., to maximal turnover conditions); in practice (Figs. 4, S1, and S2), we normalized to the average current between 0 and +60 mV because, in wild-type and C113Y parent pumps, outward current is constant over that range. But to calculate molecular turnover rates for inward and/or outward pump currents—even in E336Q and D813N pumps that generate no K+o-activated current (although both show robust amplification of inward current on lowering pHo to 6.0)—we used Boltzmann fits to measured transient Na+ charge movements to determine the total charge moved, Qtot, and hence to estimate the number of pumps in the oocyte surface (as described in Results). Although, strictly, two-state Boltzmann fits yield accurate estimates of Qtot only when the charge moves in a single step (Chowdhury and Chanda, 2012; Bezanilla and Villalba-Galea, 2013), that is likely to be a reasonable assumption in this instance. Thus, the slow component of charge measured here is rate limited by the single step E1P(Na3)↔E2P(Na2)Na (Heyse et al., 1994; Hilgemann, 1994; Holmgren et al., 2000; Gadsby et al., 2012), and it amounts to ∼95% of the total moved in the three steps associated with release/rebinding of the three Na+ ions (Gadsby et al., 2012). In addition, we limited estimation of turnover rates to only those mutants for which zq values were little altered.
The maximal voltage-independent turnover rate at saturating [K+o] of C113Y α1 Xenopus pumps determined in this way in these experiments was 18 ± 1 s−1 (n = 13). As reported previously, this is identical to that of RD α1 Xenopus pumps and wild-type rat (RD) α1 pumps, and roughly half that of wild-type α1/β3 Xenopus pumps (Vedovato and Gadsby, 2010). This maximal turnover rate was unaltered in E788Q (18 ± 1 s−1; n = 11) and E963Q (17 ± 0.1 s−1; n = 7) pumps, but the turnover rate at 0 mV was diminished approximately threefold in D935N (6 ± 0.2 s−1; n = 11) and D935N/E963Q (7 ± 1 s−1; n = 4) pumps, and was zero in E336Q and D813N pumps. The ΔQ–voltage plots for these six mutants had Qtot, zq, and midpoint voltage values comparable to those of C113Y, but were somewhat shallower and shifted, by ∼30 mV positive for D817N and by ∼30 mV negative for Y780F, precluding reliable Boltzmann fits and hence turnover rate estimates for those mutants. The principal consequence of using the charge movement values to convert the inward current data at 0 Na+o, 0 K+o, and pHo 6.0 in Fig. 4 to rates, therefore, is diminution of the relative proton import rates of D935N and D935N/E963Q pumps to 12 and 3%, respectively, of parent C113Y proton transport rates under those same conditions.
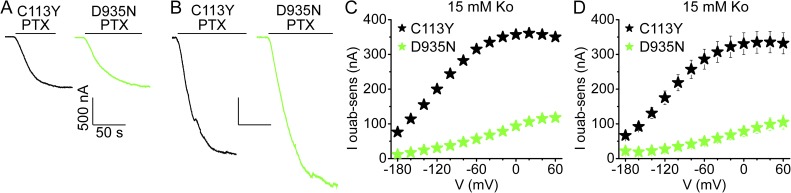
To further corroborate the diminished Na+/K+ transport rates of D935N pumps, we exploited the ability of palytoxin to readily transform even mutant pumps into cation channels (e.g., E336C, D813C, D817C; Reyes and Gadsby, 2006), and made side-by-side comparison of palytoxin-induced current in excised patches and Na+/K+ pump current at saturating [K+o] in intact oocytes injected with the same amount of cRNA encoding either C113Y or D935N pumps (Fig. 9). Palytoxin-induced currents had the same amplitude in patches containing C113Y pumps as in patches containing D935N pumps after 1 d, and after 2 d (when the currents were twice as large), of expression (Fig. 9, A and B). In contrast, however, in the same oocytes after 2 d of expression, outward Na+/K+ transport current at 0 mV was almost fourfold smaller for D935N than for C113Y pumps (Fig. 9, C and D). The same-sized palytoxin-induced currents (D935N, −2.1 ± 0.1 nA and n = 8; C113Y, −2.1 ± 0.2 nA and n = 15) suggest that the surface densities of D935N and of C113Y pumps are the same; this assumes that the D935N mutation has little influence over the single-channel conductance or open probability of palytoxin-bound pump channels (observed to be ≥0.9 in the presence of mM ATP, as used here, for all palytoxin-bound pump channels examined to date; e.g., Artigas and Gadsby, 2003, 2006). That the surface expression levels of D935N and C113Y pumps are indeed similar is also suggested by the similar total charge movements recorded after expression of the same amounts of cRNA for the same time: Qtot for D935N = 8.5 ± 0.5 nC, n = 5; Qtot for C113Y = 9.3 ± 1.2 nC, n = 13. These similar Qtot, and corresponding zq, values, determined in the same experiments that yielded the D935N and C113Y outward Na+/K+ transport currents in Fig. S2 B, resulted in the approximately threefold smaller turnover rate at 0 mV estimated for D935N compared with C113Y pumps, corroborating the impaired pump function of D935N pumps (Einholm et al., 2010; Poulsen et al., 2010).
Structural underpinnings of proton translocation mechanism
SERCA Ca2+-ATPases pump out two cytoplasmic Ca2+ and countertransport two to three protons per cycle (Yu et al., 1993). But close proximity of binding-site oxygens demands protonation of at least two carboxylates, even with two Ca2+ ions bound in sites I and II (Sugita et al., 2005). Similarly, pKa calculations on K+-bound E2 · MgF42− Na+/K+-ATPase suggest that three site I and II carboxylates, equivalent to E336, E788, and D817 (Figs. 3 B and 4, A and B), as well as carboxylates D935 and E963 near site III (Figs. 3 B and 4, C and E), all examined here, are protonated in that conformation (Poulsen et al., 2010; Yu et al., 2011). In fact, disengagement of the Na+/K+ pump’s C terminus, thus uncovering an aqueous pathway that permits D935 protonation from the cytoplasm, was proposed (Poulsen et al., 2010) to rate limit the E1P(Na3)→E2P(Na2)Na deocclusion transition and hence extracellular release of the first of the three Na+ ions. That proton was suggested to return to the cytoplasm after intracellular K+ ion release to allow binding of the next three intracellular Na+ ions (Poulsen et al., 2010). We propose here that once site III becomes vacant upon extracellular release of the first Na+ (whether that Na+ leaves from site II followed by lateral shuffling of the two remaining Na+, or leaves directly from site III), an extracellular proton may bind to E963, within 10 Å of that empty site, and then relay via Y780 to D935 from where it can access the cytoplasm via the aqueous pathway. The hydroxyl of that tyrosine might stabilize a hydrogen-bonded water chain, enabling fast proton transfer by a Grotthuss-type mechanism, as in cytochrome c oxidase (Backgren et al., 2000). In such cases, the dynamics of proton translocation reflect the time taken for waters to enter and establish the chain. Indeed, a bound water bridges E963 and Y780 equivalents in both the K+-containing E2 · MgF42− structure (Shinoda et al., 2009) and the recent 2.8-Å resolution structure of E1 · AlF4− · ADP · 3 Na+, which contains three Na+ ions (Kanai et al., 2013). A 4.3-Å resolution structure of the same E1 · AlF4− · ADP · 3 Na+ conformation (Nyblom et al., 2013) also locates the third Na+ between TM8, TM5, and TM6, coordinated by D935 equivalent, in the site previously called IIIb (Poulsen et al., 2010). It was recently suggested that the originally proposed third Na+ site, containing E963 and Y780 equivalents (Ogawa and Toyoshima, 2002; Li et al., 2005), which has been referred to as site IIIa (Poulsen et al., 2010), might be a transient site for extracellular Na+ release/rebinding (Nyblom et al., 2013); it was also suggested, in accord with the findings presented here, to be a site where extracellular protons might bind and then transfer to the site previously called IIIb and escape to the cytoplasm, generating inward current (Nyblom et al., 2013).
It is not yet possible to specify precisely the phosphorylated states involved in proton import or pinpoint the high activation energy transitions between them that rate limit proton inflow. But the responsible step must have relatively high transition rates (relative to the rate-limiting K+ deocclusion step) because, at large negative membrane potentials and low pHo, multiple proton-importing back-steps seem to occur in each completed (albeit slowed) 3 Na+/2 K+ cycle (Fig. 8 B). Analyses of the three sequential steps responsible for extracellular release of the three Na+ show that after the slowest step, E1P(Na3)→E2P(Na2)Na, enables release of the first Na+, deocclusion and release of the second and third Na+ ions occur relatively rapidly (Heyse et al., 1994; Hilgemann, 1994; Holmgren et al., 2000; Gadsby et al., 2012). The rates of all Na+-releasing steps after the slow E1P→E2P conformational change therefore seem appropriate for allowing several forward and reverse transitions, importing several protons, before the binding of two K+o ions prompts dephosphorylation, precluding further proton entry. Raising the external proton concentration enhances proton inflow whether K+o and/or Na+o are present or absent (e.g., Figs. 2 D and 8), although to a greater extent when both are absent (Fig. 2 D); presumably, lowered pHo raises the probability of the extracellularly accessible E963 being protonated and so increases the frequency of the proton-importing back-step. The similar magnitudes of the inward current increments at negative potentials caused by lowering pHo to 6.0 in 125 mM Na+o, whether K+o is present (15 mM) or absent (Fig. 8 B), support that interpretation, but they also imply that, at least in the presence of Na+o, the steady-state fractional occupancy of the phosphorylated conformation involved in accepting an extracellular proton is independent of that of the K+-occluded conformation. This relatively constant occupancy of the proton-accepting conformation in 125 mM Na+o might be explained if, under those conditions, it were a high energy state with a short lifetime, determined by fast transition rates in either direction, as proposed previously (Hilgemann, 1994) to account for characteristics of the major electrogenic Na+ release that follows the slow E1P→E2P deocclusion.
The observed inhibition of proton current by extracellular Na+ (e.g., Fig. 2 C; 125 vs. 0 mM Na+o; Efthymiadis et al., 1993; Wang and Horisberger, 1995; Vasilyev et al., 2004) could reflect direct competition between Na+o ions and external protons for binding to E963 (e.g., if the first Na+ is released directly from site III), or Na+o-mediated redistribution of Na+/K+ pump states and hence destabilization of the proton-accepting conformation. The latter is more consistent with the recent structural interpretation of single-file binding of three Na+ ions from the cytoplasm (Kanai et al., 2013) and earlier indications of strictly sequential, last-in first-out, external release of two K+ ions (Forbush, 1987b) and three Na+ ions (Wuddel and Apell, 1995; Wagg and Gadsby, 1997). Because the new structure suggests that the last cytoplasmic Na+ resides in site II (Kanai et al., 2013), external release of that Na+ via the principal pathway between TM1, TM2, TM4, and TM6 would allow site III to be vacated by lateral hops of the two residual Na+, enabling proton inflow. In the absence of Na+o, site III would remain vacant and proton inflow would continue. But in the presence of Na+o, rebinding of Na+ to site II would allow occupancy of site I, and thence, occasionally, site III, with resulting temporary interruption of proton inflow. K+o ions, if present, would occupy sites I and II (readily, in the absence of competition from Na+o, and eventually, in its presence) and trigger dephosphorylation, precluding proton inflow.
By the same token, modified responses to Na+o of the inward currents in mutant pumps might reflect altered distribution of pump states or altered interactions with Na+o, or both. Altered consequences of changes in extracellular concentration of Na+ or Na+ substitutes have contributed to suggestions that cations other than protons might carry the inward current through wild-type or mutant pumps (Vasilyev et al., 2004; Yaragatupalli et al., 2009; Meier et al., 2010; Poulsen et al., 2010; Ratheal et al., 2010; Azizan et al., 2013; Nyblom et al., 2013). In no case, however, has modification of proton current caused by redistribution of pump states been ruled out. In particular, the strong inward rectification of the proton current makes measurements of its reversal potential problematic for both wild-type and mutant pumps, and appropriate weighting of small inward current differences observed in mutants will require accurate normalization for numbers of pumps, using methods like those introduced here.
Physiological/pathophysiological implications
This work establishes that, even in the presence of physiological K+o and Na+o concentrations, when Na+/K+ pumps hesitate in E2P states, after releasing the first Na+ ion but before binding and occluding K+o ions—for example, at negative potentials that strengthen effective competition between Na+o and K+o ions—a reverse transition in the cycle can import a proton. Proton inflow occurs at measurable rates when [H+o] is elevated (Figs. 2 D and 8 B; compare to Efthymiadis et al., 1993; Wang and Horisberger, 1995), so pump-mediated proton delivery to the cytoplasm must be considered a possible consequence of Na+/K+ pump exposure to low extracytoplasmic pH. Interestingly, activity of endocytosed Na+/K+ pumps has been reported to limit H+-ATPase acidification of early endosomes to pH ≥6, as treatment with ouabain was found to further lower endosomal pH to ≤5.5 (Cain et al., 1989; Fuchs et al., 1989; Zen et al., 1992). Although H+-ATPase slowing by a more positive endosome interior as a result of electrogenic Na+/K+ transport was initially proposed as the mechanism (Fuchs et al., 1989), direct Na+/K+ pump–mediated flow of protons from endosome interior to cytoplasm now provides a reasonable alternative explanation.
Moreover, in human skeletal muscle, extracellular pH is known to fall rapidly during vigorous exercise (from 7.4 to below 7.0 in 5 min, and still falling; Street et al., 2001), and is lowered because of poor perfusion in tumors (Gillies et al., 1994) and during ischemia in the brain (Vorísek and Syková, 1997) or heart (Yan and Kléber, 1992). Although proton import rates per pump may be relatively low (Fig. 8 B), Na+/K+ pump densities in muscle, nerve, and heart can be very high (≥1,000 µm−2; Nakao and Gadsby, 1986; Anderson et al., 2010). It seems plausible, therefore, that if negative resting potentials are maintained (as measurements in working skeletal muscle imply; Street et al., 2001), and if pHo becomes low enough, proton inflow might become significant. For example, in rabbit heart papillary muscle, ischemia lowered pHo from 7.4 to an average of 6.46 in 18 min (Yan and Kléber, 1992), and a pHo drop from 7.4 to 6.4 resulted in acidification of the interior of guinea pig cardiac myocytes by ∼0.1 pH units min−1 (Sun et al., 1996). Depending on whether the buffer power inside myocytes is taken as 15 mM/pH unit (Bountra et al., 1990) or 60 mM/pH unit (Vaughan-Jones et al., 2009), the observed acidification rate requires a net proton influx of 1.5–6 mM H+ min−1, some 80–85% of which was reported to be mediated by two Cl−o-dependent anion exchangers (Sun et al., 1996); in that case, only the remainder, 0.3–1.2 mM H+ min−1, could possibly enter a myocyte via Na+/K+ pumps. As Na+/K+ pump–mediated Na+ efflux in myocytes beating at 1 Hz is ∼3.5 mM min−1 (e.g., Bers et al., 2003), pump-mediated proton entry of 0.3–1.2 mM H+ min−1 would require a proton to enter every fourth Na+/K+ transport cycle to once every cycle when pHo is 6.4. Our results (Fig. 8 B) indeed show that, at a membrane potential of −80 mV, outward Na+/K+ pump–mediated current in 15 mM K+o and 125 mM Na+o at pH 7.6 (closed stars) becomes smaller by about one quarter when pHo is lowered to 6.0 (open stars). Assuming that the smaller net outward pump current is entirely attributable to increased inward proton current (e.g., compare open with closed squares in Fig. 8 B) summed with unaltered outward Na+/K+ transport current, the implication is that on average, one proton enters the oocyte every four Na+/K+ transport cycles under these conditions (i.e., pHo 6.0). Our data suggest that even at pHo 7.6, one proton enters every 25–30 pump cycles at −80 mV (Fig. 8 B, closed squares vs. closed stars). As most of the myocyte and oocyte data compared here were gathered at room temperature, extrapolation to more physiological conditions should be undertaken only with caution. For at least that reason, it seems safe to conclude that whether Na+/K+ pump–mediated proton uptake plays any physiological or pathological role remains to be established.
Na+/K+-ATPase: A hybrid transporter?
These results show that the Na+/K+-ATPase can behave as a mixed function, or hybrid, transporter, exporting three Na+ and importing two K+ uphill in each ATPase cycle, during which it may also import one or more protons downhill. Other examples of hybrid transporters (Gadsby, 2009) include members of the human excitatory amino acid transporter (EAAT) family. These transporters, in addition to carrying out Na+-coupled stoichiometric glutamate uptake, generate Cl− ion currents that are activated by substrate binding but are energetically uncoupled from its transport (Fairman et al., 1995; Vandenberg et al., 2008). The Cl− ion pathway exists in only a subset of the conformations visited during the substrate transport cycle (Wadiche and Kavanaugh, 1998). A potential structural correlate of the anion-conducting state was recently identified in an intermediate conformation of a prokaryotic homologue (Verdon and Boudker, 2012) as a crevice between the very mobile transport domain and the static trimerization scaffold domain (Reyes et al., 2009). So the Cl− current might reasonably be viewed as the unavoidable byproduct of the evolution of a structure and a mechanism that efficiently cotransports Na+ and glutamate. However, variation in the relationship between substrate transport rate and Cl− current amplitude among EAAT isoforms (Wadiche et al., 1995; Vandenberg et al., 2008), with correlated variation in their physiological role (Veruki et al., 2006; Tzingounis and Wadiche, 2007), implies selective modulation by evolution of the stability and/or physicochemical characteristics of the conformation(s) underlying the Cl− current. Similarly, the possibility that, at sufficiently low pHo, protons may traverse the Na+/K+ pump to reach the cytoplasm can be viewed as the price paid by evolution for efficient export of three Na+ and import of two K+ across the cell membrane. But whether, like EAAT-mediated Cl− current, Na+/K+ pump–mediated proton inflow varies among isoforms of Na+/K+-ATPase α and/or β subunits, or is subject to regulation, has yet to be explored.
Supplementary Material
Acknowledgments
We thank the late R.F. Rakowski for Xenopus α1 and β3 Na+,K+-ATPase cDNAs, A. Gulyás-Kovács for writing acquisition and analysis software, P. Hoff for preparing oocytes, N. Fataliev for molecular biological support, and Mauro Caffarelli for help in preliminary experiments.
This work was supported by National Institutes of Health grant HL36783 to D.C. Gadsby.
The authors declare no competing financial interests.
Merritt C. Maduke served as editor.
Footnotes
Abbreviations used in this paper:
- EAAT
- excitatory amino acid transporter
- MTSET+
- 2-(trimethylammonium)ethyl MTS
- TM
- transmembrane
References
- Anderson T.R., Huguenard J.R., Prince D.A. 2010. Differential effects of Na+-K+ ATPase blockade on cortical layer V neurons. J. Physiol. 588:4401–4414 10.1113/jphysiol.2010.191858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas P., Gadsby D.C. 2003. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc. Natl. Acad. Sci. USA. 100:501–505 10.1073/pnas.0135849100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas P., Gadsby D.C. 2006. Ouabain affinity determining residues lie close to the Na/K pump ion pathway. Proc. Natl. Acad. Sci. USA. 103:12613–12618 10.1073/pnas.0602720103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizan E.A.B., Poulsen H., Tuluc P., Zhou J., Clausen M.V., Lieb A., Maniero C., Garg S., Bochukova E.G., Zhao W., et al. 2013. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 45:1055–1060 10.1038/ng.2716 [DOI] [PubMed] [Google Scholar]
- Backgren C., Hummer G., Wikström M., Puustinen A. 2000. Proton translocation by cytochrome c oxidase can take place without the conserved glutamic acid in subunit I. Biochemistry. 39:7863–7867 10.1021/bi000806b [DOI] [PubMed] [Google Scholar]
- Bahinski A., Nakao M., Gadsby D.C. 1988. Potassium translocation by the Na+/K+ pump is voltage insensitive. Proc. Natl. Acad. Sci. USA. 85:3412–3416 10.1073/pnas.85.10.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D.M., Barry W.H., Despa S. 2003. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc. Res. 57:897–912 10.1016/S0008-6363(02)00656-9 [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Villalba-Galea C.A. 2013. The gating charge should not be estimated by fitting a two-state model to a Q-V curve. J. Gen. Physiol. 142:575–578 10.1085/jgp.201311056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R.D. 1990. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J. Physiol. 424:343–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnay M., Crambert G., Kharoubi-Hess S., Geering K., Horisberger J.-D. 2003. Electrogenicity of Na,K- and H,K-ATPase activity and presence of a positively charged amino acid in the fifth transmembrane segment. J. Biol. Chem. 278:19237–19244 10.1074/jbc.M300946200 [DOI] [PubMed] [Google Scholar]
- Cain C.C., Sipe D.M., Murphy R.F. 1989. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc. Natl. Acad. Sci. USA. 86:544–548 10.1073/pnas.86.2.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa C.M., Horisberger J.-D., Louvard D., Rossier B.C. 1992. Mutation of a cysteine in the first transmembrane segment of Na,K-ATPase α subunit confers ouabain resistance. EMBO J. 11:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Chanda B. 2012. Estimating the voltage-dependent free energy change of ion channels using the median voltage for activation. J. Gen. Physiol. 139:3–17 10.1085/jgp.201110722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F., Kanai R., Toyoshima C. 2013. A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase. J. Biol. Chem. 288:6602–6616 10.1074/jbc.M112.442137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D.C., Rakowski R.F. 1988. Voltage dependence of the Na-K pump. Annu. Rev. Physiol. 50:225–241 10.1146/annurev.physiol.50.1.225 [DOI] [PubMed] [Google Scholar]
- De Weer P., Gadsby D.C., Rakowski R.F. 2001. Voltage dependence of the apparent affinity for external Na+ of the backward-running sodium pump. J. Gen. Physiol. 117:315–328 10.1085/jgp.117.4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey T.E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83:475–579 [DOI] [PubMed] [Google Scholar]
- Efthymiadis A., Rettinger J., Schwarz W. 1993. Inward-directed current generated by the Na+,K+ pump in Na+- and K+-free medium. Cell Biol. Int. 17:1107–1116 10.1006/cbir.1993.1043 [DOI] [PubMed] [Google Scholar]
- Einholm A.P., Toustrup-Jensen M.S., Holm R., Andersen J.P., Vilsen B. 2010. The rapid-onset dystonia parkinsonism mutation D923N of the Na+,K+-ATPase α3 isoform disrupts Na+ interaction at the third Na+ site. J. Biol. Chem. 285:26245–26254 10.1074/jbc.M110.123976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman W.A., Vandenberg R.J., Arriza J.L., Kavanaugh M.P., Amara S.G. 1995. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 375:599–603 10.1038/375599a0 [DOI] [PubMed] [Google Scholar]
- Forbush B., III 1987a. Rapid release of 42K and 86Rb from an occluded state of the Na,K-pump in the presence of ATP or ADP. J. Biol. Chem. 262:11104–11115 [PubMed] [Google Scholar]
- Forbush B., III 1987b. Rapid release of 42K or 86Rb from two distinct transport sites on the Na,K-pump in the presence of Pi or vanadate. J. Biol. Chem. 262:11116–11127 [PubMed] [Google Scholar]
- Fuchs R., Schmid S., Mellman I. 1989. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc. Natl. Acad. Sci. USA. 86:539–543 10.1073/pnas.86.2.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D.C. 2009. Ion channels versus ion pumps: the principal difference, in principle. Nat. Rev. Mol. Cell Biol. 10:344–352 10.1038/nrm2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D.C., Rakowski R.F., De Weer P. 1993. Extracellular access to the Na,K pump: Pathway similar to ion channel. Science. 260:100–103 10.1126/science.7682009 [DOI] [PubMed] [Google Scholar]
- Gadsby D.C., Bezanilla F., Rakowski R.F., De Weer P., Holmgren M. 2012. The dynamic relationships between the three events that release individual Na+ ions from the Na+/K+-ATPase. Nat Commun. 3:669 10.1038/ncomms1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P.J., Glynn I.M. 1967a. The stoicheiometry of the sodium pump. J. Physiol. 192:217–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P.J., Glynn I.M. 1967b. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J. Physiol. 192:237–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R.J., Liu Z., Bhujwalla Z. 1994. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. 267:C195–C203 [DOI] [PubMed] [Google Scholar]
- Good P.J., Richter K., Dawid I.B. 1990. A nervous system-specific isotype of the β subunit of Na+,K+-ATPase expressed during early development of Xenopus laevis. Proc. Natl. Acad. Sci. USA. 87:9088–9092 10.1073/pnas.87.23.9088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H.-J., Stürmer W. 1994. Partial reactions of the Na,K-ATPase: Determination of rate constants. J. Gen. Physiol. 104:197–240 10.1085/jgp.104.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. 1994. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 263:1429–1432 10.1126/science.8128223 [DOI] [PubMed] [Google Scholar]
- Holmgren M., Wagg J., Bezanilla F., Rakowski R.F., De Weer P., Gadsby D.C. 2000. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature. 403:898–901 10.1038/35002599 [DOI] [PubMed] [Google Scholar]
- Kanai R., Ogawa H., Vilsen B., Cornelius F., Toyoshima C. 2013. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 502:201–206 10.1038/nature12578 [DOI] [PubMed] [Google Scholar]
- Koenderink J.B., Geibel S., Grabsch E., De Pont J.J., Bamberg E., Friedrich T. 2003. Electrophysiological analysis of the mutated Na,K-ATPase cation binding pocket. J. Biol. Chem. 278:51213–51222 10.1074/jbc.M306384200 [DOI] [PubMed] [Google Scholar]
- Law R.J., Munson K., Sachs G., Lightstone F.C. 2008. An ion gating mechanism of gastric H,K-ATPase based on molecular dynamics simulations. Biophys. J. 95:2739–2749 10.1529/biophysj.107.128025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Capendeguy O., Geering K., Horisberger J.-D. 2005. A third Na+-binding site in the sodium pump. Proc. Natl. Acad. Sci. USA. 102:12706–12711 10.1073/pnas.0505980102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Geering K., Horisberger J.-D. 2006. The third sodium binding site of Na,K-ATPase is functionally linked to acidic pH-activated inward current. J. Membr. Biol. 213:1–9 10.1007/s00232-006-0035-0 [DOI] [PubMed] [Google Scholar]
- Meier S., Tavraz N.N., Dürr K.L., Friedrich T. 2010. Hyperpolarization-activated inward leakage currents caused by deletion or mutation of carboxy-terminal tyrosines of the Na+/K+-ATPase α subunit. J. Gen. Physiol. 135:115–134 10.1085/jgp.200910301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sørensen T.L., Petersen J., Andersen J.P., Vilsen B., Nissen P. 2007. Crystal structure of the sodium-potassium pump. Nature. 450:1043–1049 10.1038/nature06419 [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. 1986. Voltage dependence of Na translocation by the Na/K pump. Nature. 323:628–630 10.1038/323628a0 [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. 1989. [Na] and [K] dependence of the Na/K pump current–voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 94:539–565 10.1085/jgp.94.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.M., Pedersen P.A., Karlish S.J., Jorgensen P.L. 1998. Importance of intramembrane carboxylic acids for occlusion of K+ ions at equilibrium in renal Na,K-ATPase. Biochemistry. 37:1961–1968 10.1021/bi972524q [DOI] [PubMed] [Google Scholar]
- Nyblom M., Poulsen H., Gourdon P., Reinhard L., Andersson M., Lindahl E., Fedosova N., Nissen P. 2013. Crystal structure of Na+, K+-ATPase in the Na+-bound state. Science. 342:123–127 10.1126/science.1243352 [DOI] [PubMed] [Google Scholar]
- Obara K., Miyashita N., Xu C., Toyoshima I., Sugita Y., Inesi G., Toyoshima C. 2005. Structural role of countertransport revealed in Ca2+ pump crystal structure in the absence of Ca2+. Proc. Natl. Acad. Sci. USA. 102:14489–14496 10.1073/pnas.0506222102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Toyoshima C. 2002. Homology modeling of the cation binding sites of Na+K+-ATPase. Proc. Natl. Acad. Sci. USA. 99:15977–15982 10.1073/pnas.202622299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen C., Picard M., Winther A.M., Gyrup C., Morth J.P., Oxvig C., Møller J.V., Nissen P. 2007. The structural basis of calcium transport by the calcium pump. Nature. 450:1036–1042 10.1038/nature06418 [DOI] [PubMed] [Google Scholar]
- Peluffo R.D., Argüello J.M., Berlin J.R. 2000. The role of Na,K-ATPase α subunit serine 775 and glutamate 779 in determining the extracellular K+ and membrane potential–dependent properties of the Na,K-pump. J. Gen. Physiol. 116:47–60 10.1085/jgp.116.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post R.L., Jolly P.C. 1957. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim. Biophys. Acta. 25:118–128 10.1016/0006-3002(57)90426-2 [DOI] [PubMed] [Google Scholar]
- Post R.L., Hegyvary C., Kume S. 1972. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247:6530–6540 [PubMed] [Google Scholar]
- Poulsen H., Khandelia H., Morth J.P., Bublitz M., Mouritsen O.G., Egebjerg J., Nissen P. 2010. Neurological disease mutations compromise a C-terminal ion pathway in the Na+/K+-ATPase. Nature. 467:99–102 10.1038/nature09309 [DOI] [PubMed] [Google Scholar]
- Rakowski R.F., Gadsby D.C., De Weer P. 1989. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J. Gen. Physiol. 93:903–941 10.1085/jgp.93.5.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R.F., Vasilets L.A., LaTona J., Schwarz W. 1991. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external [K+]. J. Membr. Biol. 121:177–187 10.1007/BF01870531 [DOI] [PubMed] [Google Scholar]
- Ratheal I.M., Virgin G.K., Yu H., Roux B., Gatto C., Artigas P. 2010. Selectivity of externally facing ion-binding sites in the Na/K pump to alkali metals and organic cations. Proc. Natl. Acad. Sci. USA. 107:18718–18723 10.1073/pnas.1004214107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes N., Gadsby D.C. 2006. Ion permeation through the Na+,K+-ATPase. Nature. 443:470–474 10.1038/nature05129 [DOI] [PubMed] [Google Scholar]
- Reyes N., Ginter C., Boudker O. 2009. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 462:880–885 10.1038/nature08616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A., Rakowski R.F. 1994. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. J. Gen. Physiol. 103:869–893 10.1085/jgp.103.5.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger A., Apell H.-J. 2001. Ion selectivity of the cytoplasmic binding sites of the Na,K-ATPase: II. Competition of various cations. J. Membr. Biol. 179:263–273 10.1007/s002320010051 [DOI] [PubMed] [Google Scholar]
- Shinoda T., Ogawa H., Cornelius F., Toyoshima C. 2009. Crystal structure of the sodium-potassium pump at 2.4 Å resolution. Nature. 459:446–450 10.1038/nature07939 [DOI] [PubMed] [Google Scholar]
- Starace D.M., Bezanilla F. 2001. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J. Gen. Physiol. 117:469–490 10.1085/jgp.117.5.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D., Bangsbo J., Juel C. 2001. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J. Physiol. 537:993–998 10.1113/jphysiol.2001.012954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y., Miyashita N., Ikeguchi M., Kidera A., Toyoshima C. 2005. Protonation of the acidic residues in the transmembrane cation-binding sites of the Ca2+ pump. J. Am. Chem. Soc. 127:6150–6151 10.1021/ja0427505 [DOI] [PubMed] [Google Scholar]
- Sun B., Leem C.H., Vaughan-Jones R.D. 1996. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. J. Physiol. 495:65–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Reyes N., Artigas P., Gadsby D.C. 2008. The ion pathway through the opened Na+,K+-ATPase pump. Nature. 456:413–416 10.1038/nature07350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. 1981. Equilibrium state of ATP-driven ion pumps in relation to physiological ion concentration gradients. J. Gen. Physiol. 77:223–229 10.1085/jgp.77.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Norimatsu Y., Iwasawa S., Tsuda T., Ogawa H. 2007. How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump. Proc. Natl. Acad. Sci. USA. 104:19831–19836 10.1073/pnas.0709978104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis A.V., Wadiche J.I. 2007. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 8:935–947 10.1038/nrn2274 [DOI] [PubMed] [Google Scholar]
- Vandenberg R.J., Huang S., Ryan R.M. 2008. Slips, leaks and channels in glutamate transporters. Channels (Austin). 2:51–58 10.4161/chan.2.1.6047 [DOI] [PubMed] [Google Scholar]
- Vasilyev A., Khater K., Rakowski R.F. 2004. Effect of extracellular pH on presteady-state and steady-state current mediated by the Na+/K+ pump. J. Membr. Biol. 198:65–76 10.1007/s00232-004-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R.D., Spitzer K.W., Swietach P. 2009. Intracellular pH regulation in heart. J. Mol. Cell. Cardiol. 46:318–331 10.1016/j.yjmcc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Vedovato N., Gadsby D.C. 2010. The two C-terminal tyrosines stabilize occluded Na/K pump conformations containing Na or K ions. J. Gen. Physiol. 136:63–82 10.1085/jgp.201010407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R.L., Lawson J.W., Cornell N.W., Krebs H.A. 1979. Cytosolic phosphorylation potential. J. Biol. Chem. 254:6538–6547 [PubMed] [Google Scholar]
- Verdon G., Boudker O. 2012. Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nat. Struct. Mol. Biol. 19:355–357 10.1038/nsmb.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F., Kairouz P., Schaerer E., Fuentes P., Geering K., Rossier B.C., Kraehenbuhl J.P. 1989. Primary sequence of Xenopus laevis Na+-K+-ATPase and its localization in A6 kidney cells. Am. J. Physiol. 256:F1034–F1043 [DOI] [PubMed] [Google Scholar]
- Veruki M.L., Mørkve S.H., Hartveit E. 2006. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat. Neurosci. 9:1388–1396 10.1038/nn1793 [DOI] [PubMed] [Google Scholar]
- Vilsen B., Andersen J.P. 1998. Mutation to the glutamate in the fourth membrane segment of Na+,K+-ATPase and Ca2+-ATPase affects cation binding from both sides of the membrane and destabilizes the occluded enzyme forms. Biochemistry. 37:10961–10971 10.1021/bi9802925 [DOI] [PubMed] [Google Scholar]
- Vorísek I., Syková E. 1997. Ischemia-induced changes in the extracellular space diffusion parameters, K+, and pH in the developing rat cortex and corpus callosum. J. Cereb. Blood Flow Metab. 17:191–203 10.1097/00004647-199702000-00009 [DOI] [PubMed] [Google Scholar]
- Wadiche J.I., Kavanaugh M.P. 1998. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J. Neurosci. 18:7650–7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche J.I., Amara S.G., Kavanaugh M.P. 1995. Ion fluxes associated with excitatory amino acid transport. Neuron. 15:721–728 10.1016/0896-6273(95)90159-0 [DOI] [PubMed] [Google Scholar]
- Wagg J., Gadsby D.C. 1997. Ordered interaction of ions with Na/K-pump may confound interpretation of unidirectional fluxes. Ann. NY Acad. Sci. 834:426–431 10.1111/j.1749-6632.1997.tb52290.x [DOI] [PubMed] [Google Scholar]
- Wang X., Horisberger J.-D. 1995. A conformation of Na+-K+ pump is permeable to proton. Am. J. Physiol. 268:C590–C595 [DOI] [PubMed] [Google Scholar]
- Wuddel I., Apell H.-J. 1995. Electrogenicity of the sodium transport pathway in the Na,K-ATPase probed by charge-pulse experiments. Biophys. J. 69:909–921 10.1016/S0006-3495(95)79965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G.X., Kléber A.G. 1992. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ. Res. 71:460–470 10.1161/01.RES.71.2.460 [DOI] [PubMed] [Google Scholar]
- Yaragatupalli S., Olivera J.F., Gatto C., Artigas P. 2009. Altered Na+ transport after an intracellular α-subunit deletion reveals strict external sequential release of Na+ from the Na/K pump. Proc. Natl. Acad. Sci. USA. 106:15507–15512 10.1073/pnas.0903752106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Ratheal I.M., Artigas P., Roux B. 2011. Protonation of key acidic residues is critical for the K+-selectivity of the Na/K pump. Nat. Struct. Mol. Biol. 18:1159–1163 10.1038/nsmb.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Carroll S., Rigaud J.L., Inesi G. 1993. H+ countertransport and electrogenicity of the sarcoplasmic reticulum Ca2+ pump in reconstituted proteoliposomes. Biophys. J. 64:1232–1242 10.1016/S0006-3495(93)81489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Biwersi J., Periasamy N., Verkman A.S. 1992. Second messengers regulate endosomal acidification in Swiss 3T3 fibroblasts. J. Cell Biol. 119:99–110 10.1083/jcb.119.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.