The analgesic α-conotoxin Vc1.1 inhibits Cav2.3 channels through a GABAB receptor–dependent pathway involving c-Src.
Abstract
Neuronal Cav2.1 (P/Q-type), Cav2.2 (N-type), and Cav2.3 (R-type) calcium channels contribute to synaptic transmission and are modulated through G protein–coupled receptor pathways. The analgesic α-conotoxin Vc1.1 acts through γ-aminobutyric acid type B (GABAB) receptors (GABABRs) to inhibit Cav2.2 channels. We investigated GABABR-mediated modulation by Vc1.1, a cyclized form of Vc1.1 (c-Vc1.1), and the GABABR agonist baclofen of human Cav2.1 or Cav2.3 channels heterologously expressed in human embryonic kidney cells. 50 µM baclofen inhibited Cav2.1 and Cav2.3 channel Ba2+ currents by ∼40%, whereas c-Vc1.1 did not affect Cav2.1 but potently inhibited Cav2.3, with a half-maximal inhibitory concentration of ∼300 pM. Depolarizing paired pulses revealed that ∼75% of the baclofen inhibition of Cav2.1 was voltage dependent and could be relieved by strong depolarization. In contrast, baclofen or Vc1.1 inhibition of Cav2.3 channels was solely mediated through voltage-independent pathways that could be disrupted by pertussis toxin, guanosine 5′-[β-thio]diphosphate trilithium salt, or the GABABR antagonist CGP55845. Overexpression of the kinase c-Src significantly increased inhibition of Cav2.3 by c-Vc1.1. Conversely, coexpression of a catalytically inactive double mutant form of c-Src or pretreatment with a phosphorylated pp60c-Src peptide abolished the effect of c-Vc1.1. Site-directed mutational analyses of Cav2.3 demonstrated that tyrosines 1761 and 1765 within exon 37 are critical for inhibition of Cav2.3 by c-Vc1.1 and are involved in baclofen inhibition of these channels. Remarkably, point mutations introducing specific c-Src phosphorylation sites into human Cav2.1 channels conferred c-Vc1.1 sensitivity. Our findings show that Vc1.1 inhibition of Cav2.3, which defines Cav2.3 channels as potential targets for analgesic α-conotoxins, is caused by specific c-Src phosphorylation sites in the C terminus.
INTRODUCTION
Presynaptic voltage-gated Cav2.1 (P/Q-type), Cav2.2 (N-type), and Cav2.3 (R-type) voltage-gated calcium channels (VGCCs) mediate nerve-evoked transmitter release. Their modulation by G protein–coupled receptors (GPCRs) is a key factor in controlling neuronal excitability at central and peripheral synapses (Luebke et al., 1993; Takahashi and Momiyama, 1993; Wu et al., 1998; Gasparini et al., 2001). Multiple GPCR-mediated pathways converge on VGCCs, but Cav2.3 channels are less susceptible to direct G protein βγ dimer modulation than Cav2.1 or Cav2.2 (Shekter et al., 1997), a finding attributed to differences between the N terminus, domain I, and the I–II intracellular linker of Cav2.3 and Cav2.2 channels (Stephens et al., 1998; Simen and Miller, 2000). Nevertheless, carbachol, somatostatin, ATP, and adenosine inhibit exogenous Cav2.3 channels via endogenous receptors in human embryonic kidney (HEK) cells (Mehrke et al., 1997). Interestingly, carbachol, a muscarinic receptor agonist, stimulates or inhibits Cav2.3 currents by distinct signaling pathways in HEK cells (Bannister et al., 2004), whereas the D2 dopamine receptor agonist quinpirole (Page et al., 1998) and µ opioid receptor agonist DAMGO (Ottolia et al., 1998) inhibit Cav2.3 currents in the Xenopus laevis oocyte system. Electrophysiological data suggest that baclofen, a derivative of γ-aminobutyric acid (GABA), inhibits R-type currents in the rat medial nucleus (Wu et al., 1998) and locus coeruleus neurons (Chieng and Bekkers, 1999).
VGCCs are associated with a wide range of pathologies, including pain, and the value of selectively targeting Cav2 channels for neuropathic pain treatment is recognized (Altier et al., 2007; Pexton et al., 2011). We have shown that α-conotoxin Vc1.1, a small venom peptide from Conus victoriae, inhibits Cav2.2 channels via GABA type B (GABAB) receptors (GABABRs) in rodent dorsal root ganglion (DRG) neurons (Callaghan et al., 2008; Callaghan and Adams, 2010) and the HEK expression system (Cuny et al., 2012). We also demonstrated that Vc1.1 can be used as an analgesic in rat models of neuropathic pain (Klimis et al., 2011). Cav2.3 channels are also present in various nociceptors (Fang et al., 2007, 2010) and contribute to pain behavior control by spinal and supraspinal mechanisms (Saegusa et al., 2000; Terashima et al., 2013). However, Cav2.3 modulation via GABABRs is incompletely characterized and has not been reconstituted in any heterologous expression system. Moreover, few drugs or toxins have specific Cav2.3 inhibitory effects (Schneider et al., 2013).
In this study, we hypothesized that α-conotoxin Vc1.1 can modulate Cav2.1 and Cav2.3 channels via GABABR activation. We designed experiments to examine the mechanisms of VGCC Ba2+ current (IBa) inhibition by baclofen and Vc1.1, with emphasis on voltage-dependent (VD) and voltage-independent (VI) pathways, which may be present in these cells. Our data show that Vc1.1 only inhibits Cav2.3 channels, despite baclofen efficiently inhibiting both Cav2.1 and Cav2.3 channels. Using site-directed mutagenesis in combination with functional expression in HEK cells, we demonstrate that c-Src phosphorylation of specific tyrosine residues in the α1 subunit C terminus is sufficient to mediate Vc1.1 inhibition of Cav2.3 channels. A preliminary report of these results, in part, has been presented in abstract form (Berecki, G., J.R. McArthur, and D.J. Adams. 2013. Australian Neuroscience Society Inc. 33rd Annual Meeting. Abstr. ORAL-03-03).
MATERIALS AND METHODS
Cell culture, clones, and transfections
HEK cells containing the SV40 large T antigen (HEK-293T) were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen), 50 IU/ml penicillin, and 50 µg/ml streptomycin (Invitrogen). HEK-293 cells, stably expressing human Cav2.1 (P/Q-type) channel α1A-2 splice variant (GenBank accession no. AF004883) or human Cav2.3c (R-type) channel α1E-3 splice variant (also called α1E-c; GenBank accession no. L29385), were obtained from Merck and cultured according to procedures described previously (Dai et al., 2008). Both cell lines express human α2bδ-1 (GenBank accession no. M76559) and human β3a (RefSeq accession no. NM_000725) auxiliary subunits, and the human KCNJ4 (Kir2.3; GenBank accession no. U07364) channel (Dai et al., 2008).
The human Cav2.1 (P/Q-type) channel, α1A, transcript variant 5 (RefSeq accession no. NM_001174080), cloned into pCMV plasmid, was provided by J. Striessnig (University of Innsbruck, Innsbruck, Austria). The human Cav2.3d (R-type) channel (fetal brain α1E-d, splice variant L27745) was provided by T. Schneider (University of Cologne, Cologne, Germany). The wild-type human Cav2.3c (α1E-c) channel (GenBank accession no. L29385) and mutant Cav2.3c channels, α1E-c (Y1761F) and α1E-c (Y1765F), all cloned into pCDNA3.1 vectors, were purchased from GenScript USA Inc. The α1E-d splice variant is identical in amino acid sequence to α1E-c, except for a 43–amino acid segment (insert III or exon 46) at the C terminus of the α1E-d channel (Pereverzev et al., 2002). Human α2bδ-1 (RefSeq accession no. NM_000722) and human β3 channel subunits, transcript variant 1 (RefSeq accession no. NM_000725), were purchased from OriGene Technologies, Inc. Site-directed mutagenesis of the wild-type human Cav2.3d (α1E-d) channel, resulting in α1E-d (Y1761F) or α1E-d (Y1765F), and site-directed mutagenesis of the wild-type human Cav2.1 (α1A-5) channel, resulting in α1A-5 (L1852T) or α1A-5 (Q1852E), was performed with the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies), using the following oligonucleotides: Cav2.3(Y1761F)-for, GCATGTGGCCGCATCCATTTCACTGAGATGTATGAAATG; Cav2.3(Y1761F)-rev, CATTTCATACATCTCAGTGAAATGGATGCGGCCACATGC; Cav2.3(Y1765F)-for, CATTACACTGAGATGTTTGAAATGCTGACTCTC; Cav2.3(Y1765F)-rev, GAGAGTCAGCATTTCAAACATCTCAGTGTAATG; Cav2.1(L1852T)-for, GGCCGCATGCCTTACACGGACATGTATCAGATG; Cav2.1(L1852T)-rev, CATCTGATACATGTCCGTGTAAGGCATGCGGCC; Cav2.1(Q1856E)-for, CTTACCTGGACATGTATGAGATGCTGAGACACATGTC; Cav2.1(Q1856E)-rev, GACATGTGTCTCAGCATCTCATACATGTCCAGGTAAG.
For all primers, sense and antisense orientations are denoted as “for” and “rev,” respectively. The names reflect the position of the tyrosine to be mutated to a phenylalanine (Cav2.3), leucine to threonine (Cav2.1), or glutamine to glutamic acid (Cav2.1). All mutations were verified by automated DNA sequencing (Australian Genome Research Facility).
HEK cells stably expressing Cav2.1 or Cav2.3c channels were transiently cotransfected with plasmid cDNAs encoding human GABABR1 (RefSeq accession no. NM_001470; 3 µg; OriGene Technologies, Inc.), human GABABR2 (RefSeq accession no. NM_005458; 3 µg; OriGene Technologies, Inc.), and enhanced green fluorescent protein (eGFP) reporter gene construct (1 µg; provided by J.W. Lynch, The University of Queensland, Brisbane, Australia), using the calcium phosphate precipitation method (Jordan et al., 1996). In separate experiments, pRC-CMV/Src encoding wild-type mouse c-Src or pRC-CMV/Src(K295R/Y527F) double mutant mouse c-Src cDNA (provided by J. Ulrich, University of Iowa, Iowa City, IA) was also included in the above transfection mixture. The K295R mutation in the ATP-binding site inactivates the kinase, whereas the Y527F mutation abolishes intramolecular interactions between the C-terminal tail and the SH2 domain (Gao et al., 1997).
HEK-293T cells were transiently cotransfected with plasmid cDNAs encoding human Cav2.1 channel transcript variant 5 (5 µg) or wild-type or mutant human Cav2.3d channels (5 µg), human α2bδ-1 (5 µg) and human β3 (5 µg) auxiliary subunits, human GABABR1 (3 µg), human GABABR2 (3 µg), and eGFP (1 µg). In a separate series of experiments, HEK-293T cells were transiently cotransfected with plasmid cDNAs encoding rabbit Cav2.1 channel (RefSeq accession no. NM_001101693; 5 µg; provided by F. Meunier, The University of Queensland, St. Lucia, Australia), rat α2δ-1 (5 µg; provided by G.W. Zamponi, University of Calgary, Calgary, Canada) and rat β3 (5 µg; provided by D. Lipscombe, Brown University, Providence, RI) auxiliary subunits; human GABABR1 (3 µg) and human GABABR2 (3 µg); and eGFP (1 µg). After transfection, cells were plated on glass coverslips and incubated at 37°C in 5% CO2 for 6 h. Transfection medium was then replaced with culture medium, and cells were incubated at 30°C in 5% CO2.
Electrophysiology
Experiments were performed 3–5 d after transfection, using the whole-cell patch-clamp technique. Currents through calcium channels were recorded using barium (Ba2+) as the charge carrier. Cells expressing the proteins of interest were superfused with a solution containing (mM): 110 NaCl, 10 BaCl2, 1 MgCl2, 5 CsCl, 30 TEA-Cl, 10 d-glucose, and 10 HEPES, pH 7.4 with TEA-OH, at ∼600 µl/min. Fire-polished borosilicate patch pipettes with tip resistance values of 2–3 MΩ were filled with an intracellular solution containing (mM): 125 K-gluconate, 2 MgCl2, 5 EGTA, 5 NaCl, 4 MgATP, and 10 HEPES, pH 7.25 with CsOH. In a series of experiments, EGTA was included in the intracellular solution at a concentration of 0.5 or 10 mM. GTP was not used in the intracellular solution to prevent IBa rundown caused by activation of signaling pathways when the whole-cell recording configuration was established (Raingo et al., 2007). To minimize endogenous currents, the osmolarity of solutions was adjusted with sucrose (310-mOsm extracellular, slightly hypertonic with respect to the 295-mOsm intracellular solution).
Electrophysiological recordings were performed at room temperature (23–25°C) using Multiclamp 700B amplifiers (Molecular Devices) controlled by Clampex 9.2/DigiData 1332 acquisition systems. I-V relationships were recorded from a holding potential (HP) of −80 mV using 100-ms depolarizations from −45 to +50 mV, in 5-mV increments. Peak IBa was measured for each step and normalized to the cell’s maximal current. Normalized currents were averaged across cells and plotted (mean ± SEM) as a function of voltage. Test depolarizations to 10 mV (in cells coexpressing Cav2.3 channels and GABABRs) or 15 mV (in cells coexpressing Cav2.1 channels and GABABRs) of 150-ms duration were applied at a frequency of 0.1 Hz from an HP of −80 mV, where IBa was evaluated in the absence and presence of various compounds.
VD relief of the inhibition was assessed from an HP of −80 mV, using a protocol with a 20-ms prepulse to +80 mV, a 5-ms interpulse to −80 mV, and a 40-ms test pulse to +10 mV. The percentage of IBa inhibited in the absence of a prepulse (−PP IBa) or presence of a +80-mV prepulse (+PP IBa) was determined according to [(I0−PP − I−PP)/I0−PP] × 100, or [(I0+PP − I+PP)/I0+PP] × 100, respectively, where I0+PP and I0−PP represent current amplitudes (controls) obtained with or without a prepulse in the absence of a compound, respectively. I0−PP was normalized to I0+PP. I+PP and I−PP represent current amplitudes obtained with or without a prepulse in the presence of a compound, respectively. The VI fraction was defined as (+PP IBa) × 100/(−PP IBa), whereas the VD fraction was calculated as (−PP IBa) − VI.
Membrane currents were filtered at 3 kHz and sampled at 10 kHz. Leak and capacitive currents were subtracted using a −P/4 pulse protocol. Peptides and various drugs were prepared from stock solutions, diluted to appropriate final concentration, and applied via perfusion in the bath solution. Data were stored digitally on a computer for further analysis. Current densities were calculated by dividing the normalized current amplitude by the cell capacitance measured at the start of each experiment.
In successive transfections, the magnitude of baclofen inhibition of IBa was routinely tested in HEK cells stably expressing Cav2.1 or Cav2.3 channels and coexpressing GABABRs. In ∼5% of all cells tested, IBa inhibition by baclofen was ≤25%. In such cases, the results were not included in the analysis or the experiment was discontinued. When evaluating the Vc1.1 concentration dependence of IBa inhibition, only a maximum of three different Vc1.1 concentrations per cell were tested because of the relatively long time needed to reach maximum inhibition with each Vc1.1 concentration.
Peptides, chemicals, and drugs
α-Conotoxins Vc1.1, cyclized-Vc1.1 (c-Vc1.1), and PeIA were synthesized as described previously (Clark et al., 2006, 2010; Daly et al., 2011). Synthetic Vc1.1 and PeIA are 16–amino acid residue peptides with a characteristic helical region and two disulfide bonds in a I–III, II–IV arrangement (Clark et al., 2006, 2010; Daly et al., 2011). c-Vc1.1 exhibits better properties than the linear Vc1.1 (also known as ACV1), including high chemical stability, resistance to cleavage by proteases, and improved potency to inhibit N-type VGCCs (Clark et al., 2010). Most data on α-conotoxin effects on various Cav2 channels were obtained using c-Vc1.1, unless otherwise noted. GABA, baclofen, guanosine 5′-[β-thio]diphosphate trilithium salt (GDP-β-S), and pertussis toxin (PTX) were purchased from Sigma-Aldrich. (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid hydrochloride (CGP55845) and pp60c-Src peptide (521–533) were purchased from Tocris Bioscience.
c-Src phosphorylation site prediction
A publicly available catalog of phosphorylation motifs (http://www.hprd.org/PhosphoMotif_finder; Amanchy et al., 2007) was used to identify Src kinase substrate motifs within Cav2.1 and Cav2.3 C-terminal regions corresponding to exon 37 (e37) of Cav2.2 channel. This catalog does not use algorithms or computational strategies to predict phosphorylation but reports the presence of any literature-derived motifs.
Curve fitting and statistical analysis
Data analysis was performed in Clampfit 9.2 (Molecular Devices) and Origin 9.0 (Microcal Software Inc.). The voltage dependence of IBa activation was determined from I-V curves fitted to the following transform of a Boltzmann function: IBa = Gmax(V − Vrev)/{1 + exp[(V − V0.5,act)/k]}, where Vrev is the extrapolated reversal potential, V is the membrane potential, IBa is the peak current elicited by the voltage pulse, Gmax is the maximum conductance, V0.5,act is the voltage for half-maximal current activation, and k is the slope factor (Favre et al., 1995). Current amplitudes obtained in the presence of a compound (I) were normalized to current amplitudes obtained under control conditions (I0). Concentration–response curves were obtained by plotting averaged relative peak current amplitude (I/I0) against compound concentration and fitting the Hill equation I = I0{[D]h/(IC50h + [D]h)} to resulting data, where I0 is the maximum peak current amplitude, [D] is the concentration of the compound (drug), IC50 is the half-maximal inhibitory concentration, and h is the Hill coefficient (slope). Concentration–response curves are interpreted as functional responses by a ligand (baclofen or c-Vc1.1) against a change in ligand concentration. Results shown in Fig. 2 (B and C) and Table 2 were obtained by applying increasing concentrations of baclofen to the extracellular solution. Because baclofen inhibition of Cav2.3 channels is irreversible, these experiments do not represent equilibrium steady-state measurements (Christopoulos and Kenakin, 2002).
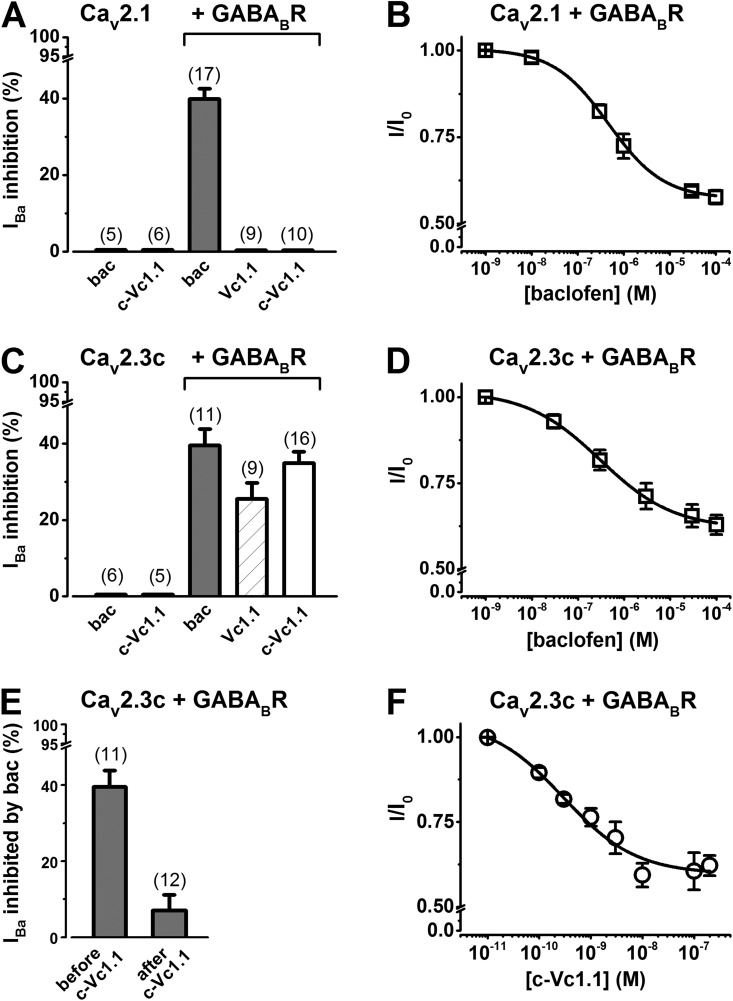
Figure 2.
Stably expressed human Cav2.1 (α1A-2) or human Cav2.3c (α1E-c) channel inhibition by baclofen (bac) and α-conotoxin Vc1.1 in the absence and presence of transiently expressed human GABABR. (A and C) Bar graphs showing average IBa inhibition through Cav2.1 (A) or Cav2.3c (C) channels by 50 µM baclofen, 200 nM Vc1.1, or 200 nM c-Vc1.1. Numbers in parentheses indicate the number of experiments. (E) Average IBa inhibited by 50 µM baclofen applied before or after 200 nM c-Vc1.1; “before c-Vc1.1” data are replotted from C. (B, D, and F) Concentration-dependent inhibition of IBa through Cav2.1 and Cav2.3c channels by baclofen (B and D) and Cav2.3c channels by c-Vc1.1 (F). See IC50 values in Table 2.
Table 2.
Summary of half-maximal inhibitory concentration (IC50) and Hill slope values in HEK cells stably expressing human Cav2.1 or Cav2.3c channels and transiently coexpressing GABABRs
| Agonist | Cav2.1 and GABABR | Cav2.3c and GABABR | ||
| IC50 | Hill slope | IC50 | Hill slope | |
| nM | nM | |||
| Baclofen | 470 ± 34 (5) | 0.73 ± 0.05 | 330 ± 72 (7) | 0.75 ± 0.14 |
| GABA | 388 ± 17 (4) | 0.76 ± 0.20 | 328 ± 102 (4) | 0.75 ± 0.24 |
| c-Vc1.1 | — | — | 0.29 ± 0.08 (4–7)a | 0.61 ± 0.14 |
Values represent mean ± SEM; n, number of experiments in parentheses; see details of the fitting procedure in Materials and methods.
Each data point (Fig. 2 F) was obtained from four to seven individual experiments.
Data are mean ± SEM (n, number of experiments). Statistical analyses were performed in Sigma Plot 11.0 (Systat Software, Inc.) using Student’s t test for two groups or one-way ANOVA with Bonferroni post-hoc testing for multiple comparisons. When one-way ANOVA failed, Kruskal–Wallis one-way ANOVA on ranks with Tukey test for multiple comparisons was used. Differences were considered statistically significant at P < 0.05.
Online supplemental material
Table S1 shows the parameters of the Boltzmann fits to I-V and G-V curves in Cav2.1/GABABR cells in the presence of 0.5 or 10 mM EGTA in the intracellular recording solution. Fig. S1 shows the voltage dependence of baclofen inhibition of Cav2.3d channels in the presence of 0.5 or 10 mM EGTA in the intracellular recording solution. Whole-cell IBa was recorded from HEK cells transiently coexpressing wild-type Cav2.3d or mutant Cav2.3d (Y1765F) channels and GABABRs. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201311104/DC1.
RESULTS
Differential inhibition of Cav2.3 and Cav2.1 channels by α-conotoxin Vc1.1 via G protein–coupled GABABRs
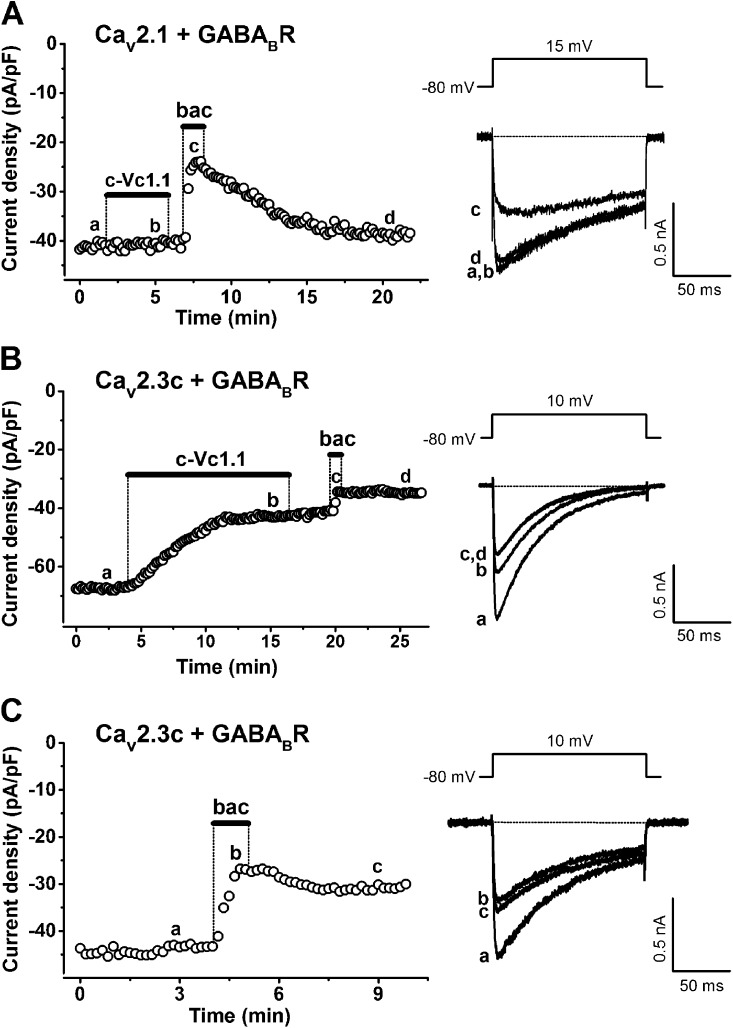
We investigated VGCC modulation by baclofen and α-conotoxin Vc1.1 in HEK cells stably expressing Cav2.1 (α1A-2) or Cav2.3c (α1E-c) channels and transiently expressing GABABRs (Cav2.1/GABABR cells or Cav2.3/GABABR cells, respectively). Fig. 1 (A–C) shows typical examples of depolarization-activated whole-cell IBa in the absence or presence of 200 nM c-Vc1.1 or 50 µM baclofen. In Cav2.1/GABABR cells, c-Vc1.1 did not modulate IBa but inhibited IBa in Cav2.3/GABABR cells. The effect of c-Vc1.1 developed relatively slowly, reached maximum inhibition 3–7 min after the response started, and was irreversible (Fig. 1 B). The “linear” α-conotoxin Vc1.1 and α-conotoxin PeIA also inhibited depolarization-activated IBa in Cav2.3/GABABR cells (Table 1).
Figure 1.
Effects of α-conotoxin c-Vc1.1 and baclofen (bac) on stably expressed human Cav2.1 (α1A-2) or human Cav2.3c (α1E-c) channels in the presence of transiently expressed human GABABR subunits R1 and R2 (GABABR). (A and B) 50 µM baclofen inhibits Cav2.1 or Cav2.3c channel currents, whereas 200 nM c-Vc1.1 only inhibits Cav2.3c currents. Bars indicate c-Vc1.1 or baclofen application. IBa was evoked by 150-ms depolarizations to 10 mV (Cav2.3c) or 15 mV (Cav2.1), applied every 10 s from an HP of −80 mV (voltage inset). Peak current amplitudes were plotted as a function of time. Representative IBa traces (right) are shown at the times indicated by lowercase letters. Horizontal dotted line represents zero-current level. Note that Cav2.1 current inhibition by baclofen is reversible on washout (A), whereas baclofen or c-Vc1.1 irreversibly inhibits Cav2.3c currents (B). (C) 50 µM baclofen inhibits IBa in Cav2.3/GABABR cells. Experimental procedures are similar to those in A or B.
Table 1.
Summary of IBa inhibition by baclofen, GABA, or α-conotoxins in the presence or absence of GABABRs in HEK cells stably expressing human Cav2.1 or Cav2.3c channels
| Agonist | IBa inhibition (%) | |||
| Cav2.1 and GABABR | Cav2.1 alone | Cav2.3c and GABABR | Cav2.3c alone | |
| Baclofen (50 µM) | 39.9 ± 2.6 (17) | 0 (5) | 39.5 ± 4.3 (11) | 0 (6) |
| GABA (50 µM) | 38.4 ± 2.7 (7) | ND | 39.3 ± 4.1 (9) | ND |
| Vc1.1 (200 nM) | 0 (9) | ND | 25.5 ± 4.2 (9) | ND |
| c-Vc1.1 (200 nM) | 0 (10) | 0 (6) | 34.8 ± 2.9 (16) | 0 (5) |
| PeIA (200 nM) | 0 (3) | ND | 27.0 ± 3.0 (3) | ND |
Values represent mean ± SEM; n, number of experiments in parentheses; ND, not determined.
These peptides have been shown to selectively inhibit high voltage–activated N-type calcium channels by acting as G protein–coupled GABABR agonists in rat DRG neurons (Callaghan et al., 2008; Daly et al., 2011). Cav2.1/GABABR or Cav2.3/GABABR cells typically responded to baclofen, with relatively fast IBa inhibition that was completely reversible or weakly reversible/irreversible, respectively (Fig. 1, A and C, and Table 1). In most experiments, applying baclofen after c-Vc1.1 exposure further suppressed a small fraction (<10%) of IBa in Cav2.3/GABABR cells. We determined the baclofen concentration dependence of IBa inhibition for Cav2.1 and Cav2.3 channels (Fig. 2, B and D), resulting in relationships described by the Hill equation (Table 2). 50 µM GABA also inhibited ∼40% of IBa in Cav2.1/GABABR and Cav2.3/GABABR cells and exhibited IC50 values similar to those obtained with baclofen (Table 2). The c-Vc1.1 concentration dependence of IBa inhibition in Cav2.3/GABABR cells (Fig. 2 F) resulted in IC50 and Hill coefficient values of 290 ± 0.8 pM and 0.61 ± 0.1, respectively, and defined c-Vc1.1 as a potent Cav2.3 channel inhibitor (Table 2). Fig. 2 (A and C) and Table 1 summarize the average IBa inhibition by baclofen, GABA, Vc1.1, c-Vc1.1, and PeIA in the absence and presence of GABABRs. These results demonstrate that GABABR expression is needed for baclofen to inhibit Cav2.1 and Cav2.3 channels, and for c-Vc1.1 to inhibit Cav2.3 channels. Moreover, the decreased response to baclofen after c-Vc1.1’s effect is consistent with an overlap between the intracellular signaling mechanisms induced by these two compounds (Figs. 1 B and 2 E).
Voltage dependence of GABABR-mediated inhibition of Cav2.1 and Cav2.3 channels
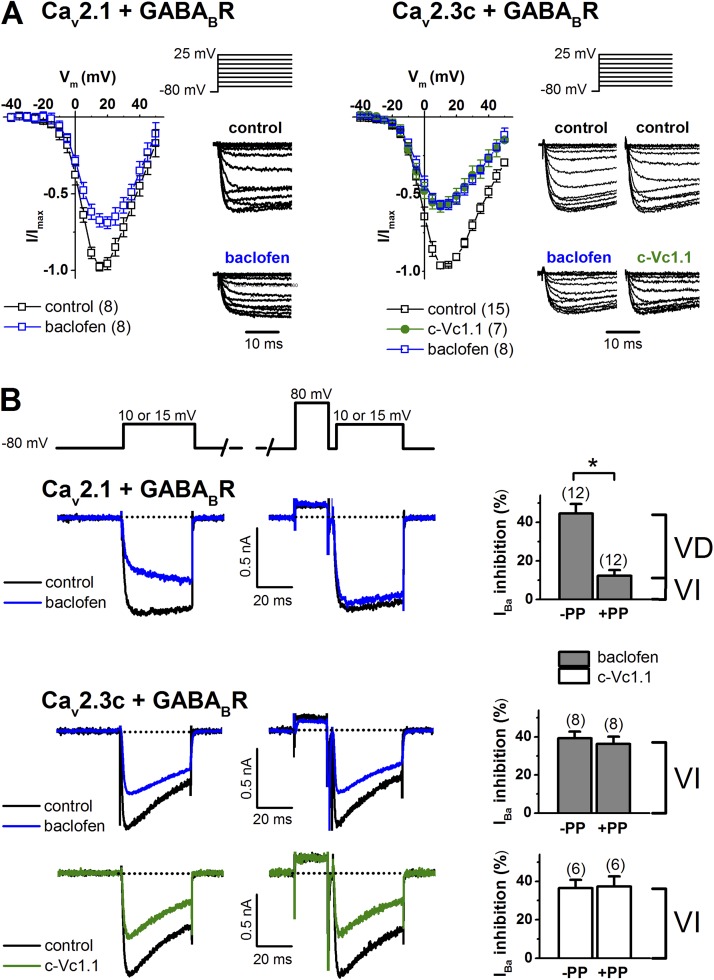
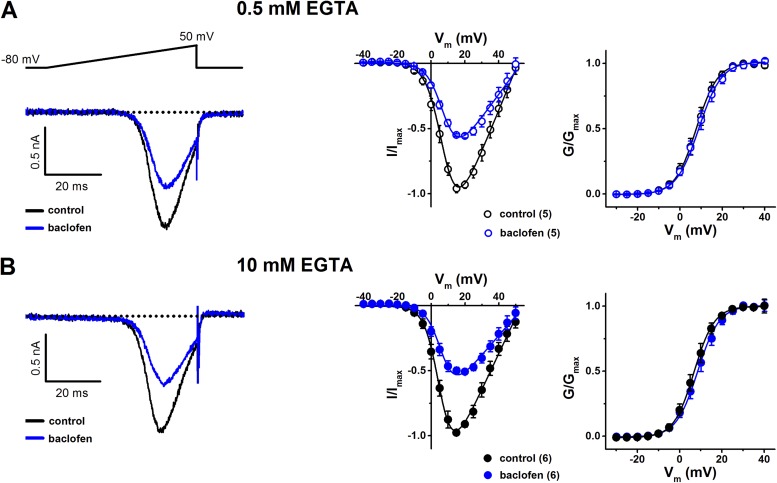
Direct VGCC inhibition by G protein–dependent inhibitory pathways involves VD Gβγ binding to the pore-forming subunit (Bean, 1989; Kasai and Aosaki, 1989; Lipscombe et al., 1989). I-V relationships were recorded in the absence and presence of baclofen in Cav2.1/GABABR cells and Cav2.3/GABABR cells, or c-Vc1.1 in Cav2.3/GABABR cells. The biophysical properties of ion permeation through Cav2.1 and Cav2.3 channels stably expressed in HEK cells have been characterized previously (Dai et al., 2008). We evaluated any depolarizing shift in the midpoint of activation (V0.5,act) of these channels, which may indicate the presence of direct Gβγ modulation, by fitting I-V relationships to a modified Boltzmann function (see Materials and methods; Fig. 3 A). Fits of the normalized I-Vs revealed that V0.5,act shifted slightly from 6.18 ± 0.3 mV (n = 8; control) to 7.40 ± 0.58 mV (n = 8; baclofen), but the difference was not statistically significant (P = 0.083) in Cav2.1/GABABR cells. However, it should be noted that the inhibition of Cav2.1/GABABR cells by baclofen (31.1 ± 2.6%) is slightly less than the inhibition shown in Fig. 2 A and Table 1. This is probably because of spontaneous IBa recovery from inhibition that occurred even in the continuous presence of baclofen and could cause the underestimation of the V0.5,act positive shift in these experiments. To reduce the contribution of recovery in this process, we also investigated baclofen inhibition of IBa in Cav2.1/GABABR cells, evoked by voltage ramps. To assess any effect of divalent cation buffering on I-V relationships, we included 0.5 or 10 mM EGTA in the intracellular solution (Fig. 4 and Table S1). Voltage ramps in the absence and presence of baclofen resulted in V0.5,act values similar to those obtained with voltage steps (Fig. 3 A). In Cav2.3/GABABR cells, the V0.5,act values were −3.44 ± 0.67 mV (n = 8) in the presence of baclofen and −0.44 ± 0.46 mV (n = 7) for c-Vc1.1 compared with 0.67 ± 0.24 mV (n = 15; control). In these experiments, baclofen caused a significant hyperpolarizing shift of V0.5,act (P < 0.001 vs. control; one-way ANOVA). However, V0.5,act was not altered by c-Vc1.1 (P = 0.223).
Figure 3.
Voltage dependence of Cav2.1 (α1A-2) and stably expressed Cav2.3c channel inhibition via GABABR activation. (A) I-V relationships in Cav2.1/GABABR cells (left) in the absence (control) and presence of 50 µM baclofen, and in Cav2.3/GABABR cells (right) in the absence (control) or presence of 50 µM baclofen or 200 nM c-Vc1.1. (Insets) Voltage protocol and representative normalized current traces (only 23 ms of the 100-ms traces are shown). See Table S1 for V0.5,act values. (B) Cav2.1 channel inhibition via GABABR is VD, whereas that of Cav2.3c is VI. Representative 15-mV depolarization-activated inward IBa from Cav2.1/GABABR cells (top) in the absence (control) or presence of 50 µM baclofen, without (−PP) or after the application of a depolarizing prepulse to +80 mV (+PP). (Bottom) Representative 10-mV depolarization-activated IBa from Cav2.3/GABABR cells in the absence (control) or presence of 50 µM baclofen or 200 nM c-Vc1.1. Dotted lines indicate zero-current level. The voltage protocol (top inset) is described in Materials and methods. (Right) Summary of IBa inhibition in the absence or presence of a prepulse. Data are mean ± SEM (paired Student’s t test; *, P < 0.001 vs. control [−PP] in Cav2.1/GABABR cells). The number of experiments is in parentheses. VD, voltage dependent; VI, voltage independent.
Figure 4.
Effects of baclofen on stably expressed human Cav2.1 (α1A-2) channels in the presence of transiently expressed human GABABRs (Cav2.1/GABABR cells). (A) Baclofen-inhibition of IBa in the presence of 0.5 mM EGTA in the intracellular recording solution. 50 µM baclofen reversibly inhibited IBa by 38.5 ± 3.9% (n = 5). (Left) Representative currents in the absence (control) and presence of baclofen, elicited by voltage ramps to +50 mV from an HP of −80 mV at 0.1 Hz. Dotted line represents zero-current level. (Middle) I-V relationships in the absence and presence of baclofen. Current amplitudes were determined from voltage ramps at selected membrane potentials (Vm). Solid lines are fits of the modified Boltzmann equation to normalized I-V relationships (see Materials and methods). (Right) Voltage dependence of activation determined from G-V relationships. Relative conductance (G/Gmax) was calculated as IBa/(Vm − Vrev), where Vrev is the reversal potential of the whole-cell current and plotted as a function of Vm. The normalized G-V relationships were fitted with a Boltzmann function, G = Gmax/(1 + exp((V0.5,act − Vm)/k)), where V0.5,act is the potential at which the conductance is half-maximally activated, and k is the slope factor. (B) Similar experimental procedures and data representation as shown in A, with 10 mM EGTA in the intracellular recording solution. Baclofen reversibly inhibited IBa by 41.8 ± 4.7% (n = 6). See Table S1 for V0.5,act (voltage for half-maximal current activation) and k (slope factor) values resulting from experiments shown in A and B.
We evaluated if baclofen inhibition of IBa in Cav2.1/GABABR and Cav2.3/GABABR cells, and c-Vc1.1 inhibition of IBa in Cav2.3/GABABR cells, could be reversed by strong depolarization. A +80-mV prepulse of 20-ms duration was applied before the test pulse to relieve any VD component of G protein–mediated IBa inhibition (Fig. 3 B). In both cells, shortening (10 ms) or prolonging (50 ms) the prepulse or interpulse (10 ms) did not change IBa facilitation. Applying +120-mV prepulses only added ∼5% facilitation in Cav2.1/GABABR cells, without changing IBa relief with Cav2.3/GABABR cells (not depicted). The inhibitory effect of baclofen was associated with a large (73 ± 4%) VD component in Cav2.1/GABABR cells. In contrast, the effect of baclofen and Vc1.1 was solely mediated by a VI pathway in Cav2.3/GABABR cells, which clearly indicates that intracellular signaling does not involve the classical G protein βγ dimer (Gβγ) binding to the pore-forming Cav2.3 channel subunit. Alternatively, Gβγ could bind with high affinity to the Cav2.3 channel in a VI manner.
c-Vc1.1 inhibition of Cav2.3 channels involves Gαi/o subunits and c-Src kinase
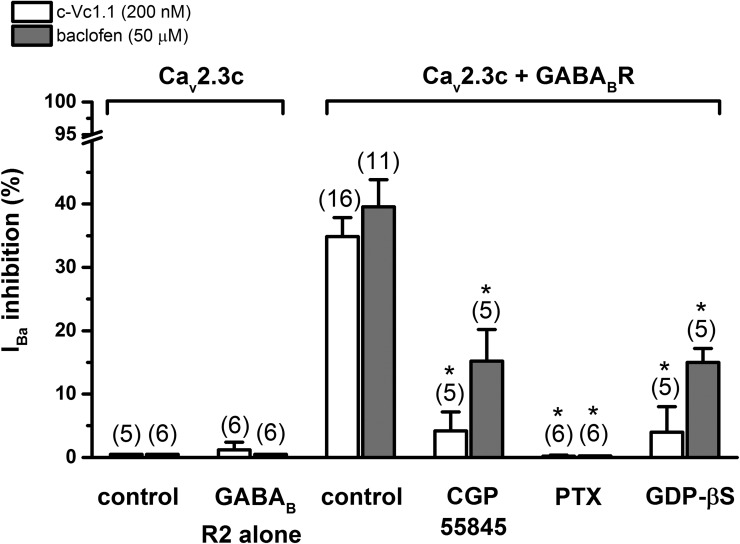
We evaluated the VI pathway leading to Cav2.3 channel modulation by determining the fraction of IBa that could be inhibited under various experimental conditions (Fig. 5). In HEK cells stably expressing Cav2.3c channels (Cav2.3 cells) or Cav2.3 cells coexpressing GABABR R2 subunits, neither 200 nM c-Vc1.1 nor 50 µM baclofen inhibits IBa, indicating that a fully functional GABABR heterodimer is needed for proper signaling. In Cav2.3/GABABR cells, the selective GABABR antagonist CGP55845 (1 µM) did not change IBa amplitude or kinetics but strongly antagonized IBa inhibition by c-Vc1.1 and reduced the effect of baclofen by ∼60% compared with control. This confirmed that GABABR needed to be activated for c-Vc1.1 and baclofen inhibitory effects to occur. When the hydrolysis-resistant GDP analogue GDP-β-S (500 µM) was added to the intracellular recording solution, Vc1.1 and baclofen inhibitory effects were almost identically reduced. Overnight treatment with 1 µg/ml PTX abolished c-Vc1.1 and baclofen inhibitory pathway(s) in Cav2.3/GABABR cells, suggesting that the effects were mediated by Gi and/or Go proteins.
Figure 5.
c-Vc1.1 and baclofen inhibit stably expressed Cav2.3c calcium channels via G protein–coupled GABABRs. Neither compound inhibits IBa in the absence of GABABRs (see also Fig. 2 C) or the absence of GABABR R1 subunits. 1 µM CGP55845 or 500 µM GDP-β-S significantly reduces c-Vc1.1 and baclofen responses, respectively. A 24-h pretreatment with 1 µg/ml PTX abolishes the effect of c-Vc1.1 or baclofen. Data are mean ± SEM (one-way ANOVA; *, P < 0.001 vs. controls; Cav2.3/GABABR cells with c-Vc1.1 or baclofen, respectively).
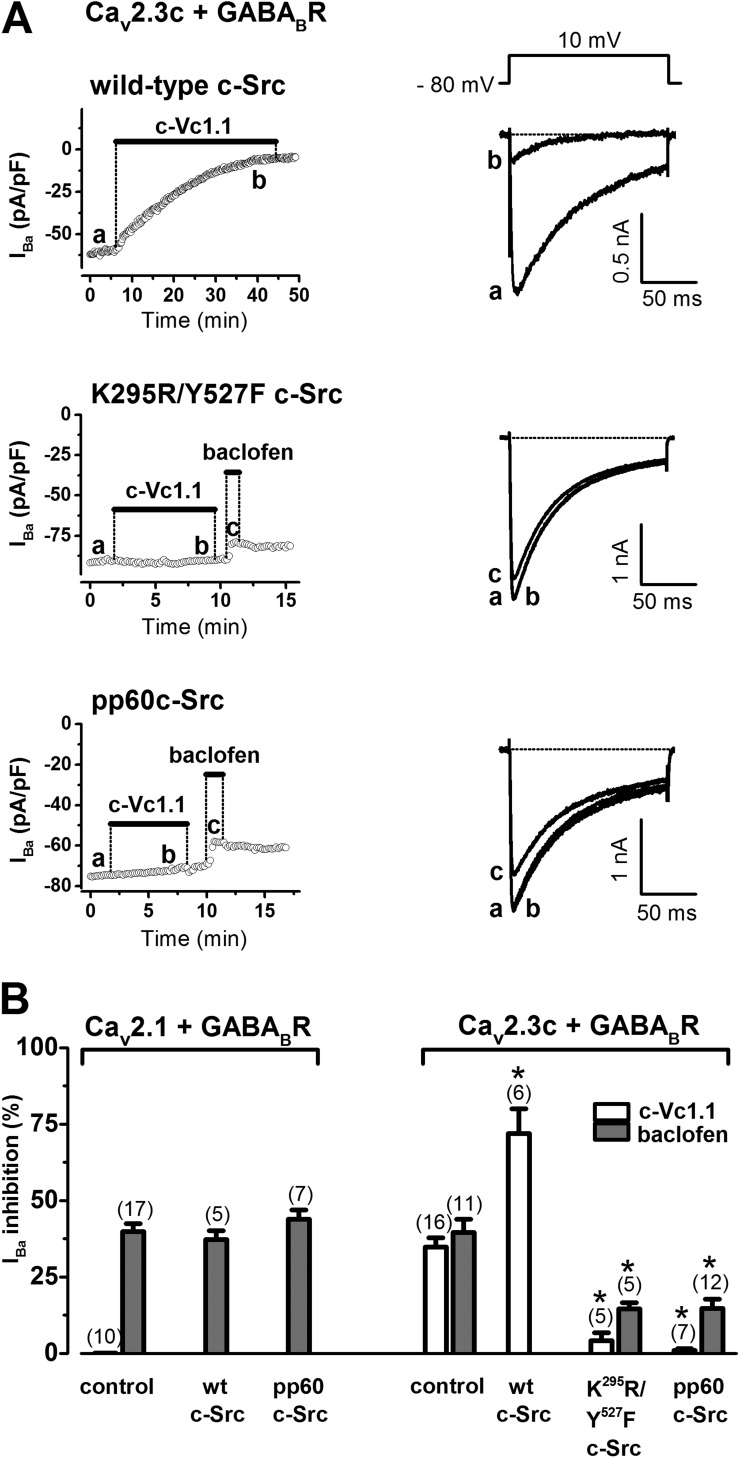
We previously showed that Vc1.1 inhibition of N-type (Cav2.2) calcium channel currents can be blocked by a phosphorylated synthetic pp60c-Src peptide (Callaghan et al., 2008). This is probably a result of pp60c-Src binding to the SH2 domain of native c-Src protein in rat DRG neurons. Therefore, we examined in more detail the role of c-Src in the GABABR-mediated Cav2.1 or Cav2.3 channel inhibition by baclofen and c-Vc1.1 in the HEK expression system (Fig. 6). We changed endogenous HEK cell c-Src protein levels (Luttrell et al., 1999) by including cDNAs of wild-type or mutant c-Src in our expression system. In Cav2.1/GABABR cells, wild-type c-Src protein overexpression or inclusion of the pp60c-Src peptide (50 µM) in the intracellular solution did not affect baclofen inhibition of IBa. However, in Cav2.3/GABABR cells, wild-type c-Src protein overexpression dramatically increased the fraction of IBa inhibited by c-Vc1.1 compared with control.
Figure 6.
The role of c-Src proteins in GABABR-mediated inhibition by c-Vc1.1 in HEK cells stably expressing human Cav2.3c channels and GABABRs. (A) Time course of IBa through Cav2.3c channels in the absence (control) and presence of 200 nM c-Vc1.1 or 50 µM baclofen. Bars indicate c-Vc1.1 or baclofen application. Overexpression of wild-type c-Src protein increases the IBa fraction inhibited by c-Vc1.1 (top). Overexpression of the double mutant c-Src (K295R/Y527F) (middle) or pretreatment with the phosphorylated pp60c-Src peptide (50 µM) (bottom) abolishes the effect of c-Vc1.1 and reduces baclofen inhibition of IBa, respectively. Representative current traces (right) are shown at the times indicated by lowercase letters (see Fig.1 B for control). IBa was evoked by 150 ms, 0.1-Hz depolarizations to 10 mV, from an HP of −80 mV (voltage inset). Peak current amplitudes were plotted as a function of time. Horizontal dotted lines indicate zero-current levels. (B) Average data (±SEM) of IBa inhibition by c-Vc1.1 or baclofen after pp60c-Src peptide (50 µM) pretreatment or wild-type c-Src coexpression in Cav2.1/GABABR or Cav2.3/GABABR cells, respectively, or double mutant c-Src (K295R/Y527F) coexpression in Cav2.3/GABABR cells (*, P < 0.001 vs. controls; one-way ANOVA). The number of experiments is in parentheses.
To further evaluate the effect of c-Src on Cav2.3/GABABR cells, we overexpressed the K295R/Y527F c-Src double mutant, which is kinase inactive and functions as a dominant-negative inhibitor of wild-type c-Src (Gao et al., 1997). This construct reduced the effect of baclofen compared with control and abolished c-Vc1.1’s inhibitory effect. The effect of K295R/Y527F c-Src was recapitulated with the pp60c-Src peptide, suggesting that c-Src kinase activity is needed for VI inhibition of IBa by c-Vc1.1 and baclofen (Fig. 6, A and B).
Tyrosines 1761 and 1765 are needed in the C terminus for c-Src phosphorylation of Cav2.3 channels
Alternative splicing of Cav2.1, Cav2.2, and Cav2.3 genes creates channels with distinct kinetic, pharmacological, and modulatory properties (Bourinet et al., 1999; Bell et al., 2004; Fang et al., 2007; Gray et al., 2007). It has been reported that GPCR-mediated inhibition of the nociceptor-specific Cav2.2[e37a] channel occurred via VD and VI pathways. In HEK cells coexpressing GABABRs and Cav2.2[e37a] channels, the baclofen-induced VI component required a tyrosine (Y) residue in e37a to be phosphorylated (Raingo et al., 2007).
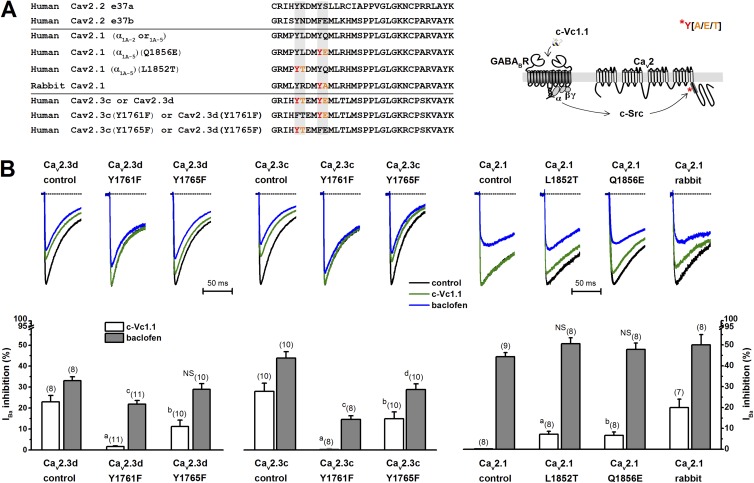
Alignment of the Cav2.2 channel e37a and e37b regions with the corresponding e37 regions in human Cav2.3c, Cav2.3d, and Cav2.1 (α1A-2 or α1A-5), and rabbit Cav2.1 channels, indicated a degree of structural conservation and the presence of tyrosine kinase consensus sites (Fig. 7 A). We hypothesized that Y residues within e37 at the proximal C terminus in Cav2.3 channels could serve as substrates for phosphorylation by c-Src. Using a publicly available catalog of phosphorylation motifs (Amanchy et al., 2007), we identified putative c-Src kinase phosphorylation sites in human Cav2.3 and rabbit Cav2.1, but not in human Cav2.1 channels. In both human Cav2.3c and Cav2.3d splice variants, the Y1761 and Y1675 (as numbered in GenBank accession no. L29385) are followed by a threonine (T) or glutamic acid (E), respectively, as are potential substrates for Src kinases. Remarkably, in rabbit Cav2.1, the second Y residue followed by alanine (A) also represents a Src motif described in the literature. In contrast, in the human Cav2.1 splice variants α1A-2 or α1A-5, the consensus Y1851 and Y1855 residues (as numbered in RefSeq accession no. NM_001174080) lack the neighboring amino acids that are needed to generate known Src kinase substrates for phosphorylation (Amanchy et al., 2007).
Figure 7.
Kinase-dependent phosphorylation of the intracellular proximal C terminus is needed for VI Cav2.3 channel inhibition by c-Vc1.1. (A) Amino acid sequence alignments of e37 regions in splice variants of Cav2.2, Cav2.1, and Cav2.3 and mutant Cav2.1 and Cav2.3 genes. Y (red), followed by A, E, or T (orange), is shown as a possible substrate for c-Src phosphorylation. (Right) Proposed model for c-Vc1.1–induced intracellular signaling leading to Cav2 channel inhibition. Asterisk indicates phosphorylation. (B) Average IBa inhibition in the presence of 200 nM c-Vc1.1 or 50 µM baclofen in HEK 293T cells transiently cotransfected with wild-type human Cav2.3d (α1E-d) and Cav2.3c (α1E-c); Cav2.1 (α1A-5); rabbit Cav2.1 channel subunits; human e37 mutants Cav2.3d (Y1761F), Cav2.3d (Y1765F), Cav2.3c (Y1761F), Cav2.3c (Y1765F), Cav2.1 (α1A-5) (L1852T), or Cav2.1 (α1A-5) (Q1852E); auxiliary human Cav channel subunits α2bδ-1 and β3; and human GABABR subunits. Representative normalized IBa traces (current insets) of wild-type or mutant channels are shown in the absence (control) or presence of 200 nM c-Vc1.1 or 50 µM baclofen (only 65 ms of the 150-ms current traces are plotted). IBa was evoked by depolarizations to +10 mV (Cav2.3c) or 15 mV (Cav2.1), applied at 0.1 Hz from an HP of −80 mV. Dotted lines indicate zero-current levels. Bar graphs show mean ± SEM; n, number of experiments in parentheses. Labels a, b, and c denote statistically significant differences between various mutants and control; NS, not significantly different from wild-type modulation. See Table 3 for statistical evaluation of the data.
We conducted a structure–function study in the e37 region to identify the amino acid residues responsible for the different sensitivity to c-Vc1.1. We also tested their contribution to c-Src–mediated inhibition in HEK cells transiently coexpressing GABABRs and transiently expressing Cav2.3c or Cav2.3d splice variants, or human or rabbit Cav2.1 channels. In patch-clamp experiments, 200 nM c-Vc1.1 inhibited human Cav2.3c and Cav2.3d channels but did not affect human Cav2.1 (α1A-5) (Fig. 7 B and Table 3). This confirmed previous results in HEK cells stably expressing Cav2.1 (α1A-2) or Cav2.3c channels in the presence of GABABRs (Figs. 1 and 2). In all experiments, 50 µM baclofen inhibited IBa. As predicted, c-Vc1.1 also inhibited rabbit Cav2.1 channels, likely because of the presence of a putative c-Src phosphorylation site in the C terminus (Fig. 7 A).
Table 3.
Summary of IBa inhibition by baclofen and c-Vc1.1 in HEK 293T cells transiently coexpressing GABABRs and Cav2.1 or Cav2.3 channels
| Cav2 channel | IBa inhibition (%) | |
| c-Vc1.1 (200 nM) | Baclofen (50 µM) | |
| Human Cav2.3d | 23 ± 2.9 (8) | 33.1 ± 1.8 (8) |
| Human Cav2.3d (Y1761F) | 1.7 ± 0.3 (11)a | 21.9 ± 1.8 (11)c |
| Human Cav2.3d (Y1765F) | 11.3 ± 3.0 (10)b | NS28.9 ± 2.7 (10) |
| Human Cav2.3c | 28.0 ± 4.0 (10) | 44.0 ± 3.0 (10) |
| Human Cav2.3c (Y1761F) | 0.37 ± 0.3 (8)a | 14.6 ± 1.8 (8)c |
| Human Cav2.3c (Y1765F) | 15.0 ± 3.2 (10)b | 28.9 ± 2.7 (10)d |
| Human Cav2.1 | 0 (8) | 44.4 ± 1.9 (9) |
| Human Cav2.1 (L1852T) | 7.2 ± 1.4 (8)a | NS50.6 ± 2.8 (8) |
| Human Cav2.1 (Q1856E) | 6.6 ± 1.7 (8)b | NS47.9 ± 3.0 (8) |
| Rabbit Cav2.1 | #20.0 ± 4.0 (7) | #50.0 ± 5.0 (8) |
Values represent mean ± SEM; n, number of experiments in parentheses. NS, not significantly different from wild-type modulation. One-way ANOVA with Bonferroni post-hoc testing was used to test for statistically significant differences except when comparing the effect of c-Vc1.1 on wild-type or mutant Cav2.1 (one-way ANOVA on ranks with Tukey test). Data marked with a hash symbol were not included in the statistical analysis. Note that the percentage of inhibition with transiently expressed human Cav2.1 or Cav2.3c channels and transiently coexpressed GABABRs (above) is similar (within the statistical margin of error) to that obtained with stably expressed human Cav2.1 or Cav2.3c channels in the presence of transiently coexpressed GABABRs (see Table 1).
P < 0.001 versus Cav2.3d with c-Vc1.1; P < 0.001 versus Cav2.3c with c-Vc1.1; and P = 0.002 versus human Cav2.1 with c-Vc1.1.
P = 0.003 versus Cav2.3d with c-Vc1.1; P = 0.008 versus Cav2.3c with c-Vc1.1; and P = 0.002 versus human Cav2.1 with c-Vc1.1.
P = 0.003 versus Cav2.3d with baclofen and P < 0.001 versus Cav2.3c with baclofen.
P < 0.001 versus Cav2.3c with baclofen.
Mutational analyses of the e37 region in the proximal C termini of Cav2.3d or Cav2.3c demonstrated that the Y1761F mutation completely abolished c-Vc1.1 inhibition of IBa, and the Y1765F mutation significantly reduced the c-Vc1.1 inhibition (Fig. 7 B and Table 3). These results suggest that tyrosines 1761 and 1765 are critical for mediating the effects of Vc1.1. Interestingly, these mutants, except the Cav2.3d (Y1765F), also reduced baclofen inhibition of IBa, which indicates that these Y residues are also involved in baclofen signaling. We explored how mutation affects the VI component of inhibition, in the absence and presence of a depolarizing prepulse, via baclofen inhibition of IBa through Cav2.3d (Y1765F) channels. Experiments were performed with either 0.5 or 10 mM EGTA in the intracellular recording solution to (a) identify any effects of intracellular divalent cations on IBa facilitation (Zühlke et al., 1999), and (b) rule out modulation by phospholipids (Delmas et al., 2005) (Fig. S1). The results showed that the effect of baclofen was solely mediated by a VI pathway, independent of a classical Gβγ binding.
We also generated human Cav2.1 (α1A-5) (L1852T) and Cav2.1 (α1A-5) (Q1856E) channel mutants. Remarkably, the introduced putative c-Src phosphorylation sites conveyed sensitivity to c-Vc1.1 in these channels. Baclofen modulation was not affected by the Cav2.1 (L1852T) or Cav2.1 (Q1856E) channel mutants (Fig. 7 B and Table 3). Collectively, these data suggest that specific c-Src phosphorylation sites in the C terminus are needed for α-conotoxin c-Vc1.1 inhibition of Cav2.3 and Cav2.1 channels. However, it remains possible that other residues are also involved in mediating baclofen’s inhibition of Cav2.3 channels.
DISCUSSION
In this study, we efficiently reconstituted human Cav2.1 and Cav2.3 channel modulation via human G protein–coupled GABABRs. Baclofen, a GABABR agonist, inhibited IBa through both channels; however, α-conotoxin Vc1.1 only inhibited Cav2.3 channels. The effect of Vc1.1 on Cav2.3 channels was completely VI and depended on the presence of specific c-Src phosphorylation sites in the C terminus of the human α1E (Cav2.3). These results define Cav2.3 channels as new targets for analgesic α-conotoxins.
Cav2 channels and chronic pain
It is well established that Cav2.2 channel inhibition by antagonists or via GPCRs produces analgesia in animals and humans (Altier and Zamponi, 2004). GABABR-mediated inhibition of Cav2.1 or Cav2.2 channels in various neurons is well documented (Cox and Dunlap, 1992; Mintz and Bean, 1993; Lambert and Wilson, 1996) and has been shown to involve VD and VI second messenger pathways (Dolphin and Scott, 1986; Diversé-Pierluissi et al., 1997). We showed that a subset of α-conotoxins, including Vc1.1, also selectively inhibit Cav2.2 channels by acting as G protein–coupled GABABR agonists (Callaghan et al., 2008; Callaghan and Adams, 2010; Clark et al., 2010; Daly et al., 2011). This mechanism may help relieve nerve injury–induced neuropathic pain (Klimis et al., 2011).
Studies involving pharmacological and genetic approaches have also established Cav2.3 channels as potential targets for drugs that treat chronic pain (Saegusa et al., 2000; Qian et al., 2013). The anti-nociceptive role of Cav2.3 channels was demonstrated in rat dorsal horn neurons (Matthews et al., 2007), and their inhibition was associated with high efficiency opioid therapy without tolerance (Yokoyama et al., 2004). Cav2.3 channels are ubiquitously expressed in the central and peripheral nervous systems, but their physiological roles and modulation is not well understood. They typically conduct a small proportion of whole-cell Ca2+ current and are difficult to isolate in neurons (Schneider et al., 2013).
Baclofen and α-conotoxin Vc1.1 differentially inhibit Cav2.1 and Cav2.3 channels
Our results demonstrate that baclofen or GABA inhibits IBa to a similar extent in Cav2.1/GABABR and Cav2.3/GABABR cells (Figs. 1 and 2). Throughout this study, we used cells with similar electrophysiological characteristics and applied supra-maximal doses of baclofen (50 µM) or c-Vc1.1 (200 nM) to make sure receptors were fully activated and rule out the possibility that differences were caused by cell variability.
In Cav2.1 channel–expressing cells, baclofen inhibition was VD and could be relieved by a strong depolarizing prepulse, reflecting transient dissociation of G protein βγ subunits from the channel. The 20-ms prepulses and 5-ms interpulses were considered suitable for VD IBa relief in Cav2.1 channels (Currie and Fox, 1997). Our results on the voltage dependence of inhibition are consistent with previous studies demonstrating baclofen inhibition of Cav2.1 channels in adrenal chromaffin cells and cerebellar Purkinje neurons (Mintz and Bean, 1993; Currie and Fox, 1997). We did not analyze the time course of IBa activation in the presence of baclofen or c-Vc1.1 in Cav2.1/GABABR or Cav2.3/GABABR cells. However, in Cav2.1/GABABR cells, the time course of IBa activation considerably slowed in the presence of baclofen when compared with control. This is a hallmark of VD Gβγ binding to the α1A (Cav2.1) subunit (Fig. 3 B). Interestingly, baclofen only caused a small and statistically insignificant shift of the I-V and G-V relationships in Cav2.1/GABABR cells (Figs. 3 A and 4), which was independent of the intracellular EGTA concentration. Bourinet et al. (1996) also reported a similar slight positive shift of the µ opioid receptor–activated Cav2.1 channel I-V relationship, suggesting possible differences between Cav2.1 and Cav2.2 channel–modulating membrane-delimited pathways. In Cav2.3/GABABR cells, the time course of IBa activation in the presence of baclofen or c-Vc1.1 seemed unaffected. In these cells, c-Vc1.1 did not affect the I-V relationship, but baclofen caused a hyperpolarizing I-V shift, which suggests that there may be an additional signaling mechanism. Our results show that neither baclofen nor Vc1.1 elicits VD inhibition of Cav2.3 channels via GABABRs. However, it has been shown that the rat brain α1Elong splice variant could be inhibited in a VD manner via D2 dopamine receptors (Page et al., 1998). Our alignment of the human α1E-c or α1E-d and rat α1Elong splice variants (not depicted) indicate that the N-terminal sequence responsible for VD inhibition of rat α1Elong variant is present in human α1E-c and α1E-d. Therefore, future experiments should determine whether or not human Cav2.3 channels can be inhibited via D2 receptors in a VD manner. VD modulation may depend on the type of GPCR and specific signal transduction mechanism elicited by the GPCR-specific ligand.
The VI pathway leading to Cav2.3 channel inhibition by baclofen or c-Vc1.1 could be disrupted by GDP-β-S, a GDP analogue that keeps Gα permanently associated with Gβγ. In all cases, PTX treatment abolished baclofen and c-Vc1.1 inhibition of IBa, indicating that GABABRs couple with G proteins of the Gi/Go superfamily in cells expressing Cav2.3 channels (Fig. 5). Analysis of the VI pathway in Cav2.3/GABABR cells indicated that signaling mechanisms that contribute to Cav2.3 channel inhibition, downstream of G protein subunits, involve c-Src kinase activation. For example, wild-type c-Src overexpression increased IBa inhibition, whereas the dominant-negative double mutant c-Src or the pp60c-Src peptide abolished c-Vc1.1 inhibition of IBa (Fig. 6). This suggested that Cav2.3 channels are a potential c-Src substrate. It has been demonstrated that certain protein tyrosine kinases can be direct effectors of G proteins (Bence et al., 1997), and GABA inhibition of Cav2.2 channels involves direct Gαo activation of Src kinase (Diversé-Pierluissi et al., 1997). However, further studies are needed to elucidate whether baclofen or Vc1.1 inhibition of Cav2.3 channels involves direct Gi/Go activation of c-Src.
In Cav2.1 and Cav2.2 channels, intracellular N and C termini and cytoplasmic loops connecting domains I–IV have been shown to interact with other proteins and are targeted by second messenger pathways, including phosphorylation by kinases (Zamponi and Currie, 2013). Many of these interaction or modulatory sites can also be identified in Cav2.3 channels (Schneider et al., 2013). Furthermore, alternative splicing, recognized as a mechanism for creating functional diversity in VGCCs (Gray et al., 2007), results in a series of Cav2.3 splice variants (Williams et al., 1994) with similar biophysical properties (Pereverzev et al., 2002). Of these, Cav2.3c represents the major neuronal type variant, which is dominantly expressed in the adult central nervous system (Schneider et al., 2013), whereas Cav2.3d, the variant cloned from human fetal brain (Schneider et al., 1994), shows minor in vivo expression in the adult brain (Pereverzev et al., 2002). Interestingly, the endocrine splice variant Cav2.3e was also identified in nociceptive trigeminal ganglion and DRG neurons together with Cav2.3a (Fang et al., 2007, 2010). Importantly, the e37 region containing the putative c-Src phosphorylation sites can be identified in all Cav2.3 splice variants.
There is evidence of multiple Src interaction sites in various VGCCs. For example, Src interacts with both the II–III linker and C-terminal tail regions of the L-type Ca2+ channel α1c subunit (Dubuis et al., 2006). c-Src kinases also appear to be pre-associated with N-type VGCCs, efficiently modulating their function (Schiff et al., 2000). In addition, c-Src kinases have been implicated in the GABABR-mediated inhibition of Cav2.2 channels by baclofen (Raingo et al., 2007) and Vc1.1 (Callaghan et al., 2008).
To date, GABABR-mediated modulation of Cav2.3 channels has not been reconstituted in expression systems, and c-Src phosphorylation of Cav2.3 channels has not been demonstrated. Conserved Y residues within the e37 region can be identified across all Cav2 family members and appear to be key substrates for phosphorylation by various tyrosine kinases. Our analysis of the e37 regions in Cav2.1 and Cav2.3 channels predicted Src kinase motifs in human Cav2.3 channels. These motifs were absent in human Cav2.1 (Fig. 7). Although phosphorylation site prediction can be error prone, it is a useful tool to determine whether e37 regions contain sequence contexts typical of c-Src motifs described previously in the literature (Amanchy et al., 2007). It is generally accepted that the amino acid sequence motif around the tyrosine residue and three-dimensional structure of the substrate proteins contribute to phosphorylation site specificity (Pawson et al., 2001). Our structure–function studies confirmed that Y[A/E/T] sequences are the likely c-Src phosphorylation substrates and represent key switches for the molecular mechanisms involved. The lack of any effect of Vc1.1 on human Cav2.1 channels agrees with the absence of a c-Src motif in the e37 region and marginal VI component in cells expressing these channels. Interestingly, c-Src motif Y[K] can also be identified in the Cav2.2[e37a] channel, whereas such a motif is absent in Cav2.2[e37b]. Therefore, further studies are required to dissect the effects of Vc1.1 on Cav2.2 channel splice variants. Our results also suggest that the effects of baclofen on Cav2.3 channels are, at least partially, controlled by c-Src phosphorylation. Given that PTX completely abolishes the inhibition by baclofen, an additional PTX-sensitive pathway is probably also involved. Future studies should be aimed at directly correlating Cav2.3 channel phosphorylation and dephosphorylation with inhibition and (the absence of) recovery, respectively. Clearly, further experiments are also needed to confirm that c-Src kinase directly phosphorylates Cav2.3 channels in vivo.
Therapeutic implications of Cav2.3 channel inhibition
Few studies have examined Cav2.3 (R-type) channel modulation in neurons, where a combination of specific inhibitors is needed to completely block various VGCCs while preserving the R-type calcium channel. In thalamocortical neurons, R-type current modulation by baclofen has been demonstrated and could be antagonized by CGP55845 (Guyon and Leresche, 1995). In rat DRG neurons, we also observed R-type current inhibition by baclofen in the presence of specific L-, N- and P/Q-type channel blockers (not depicted). However, further studies are needed to demonstrate what contribution the R-type current component makes to the whole-cell calcium conductance inhibited by Vc1.1 in nociceptive neurons.
GABABR activation produces anti-nociceptive effects in animal models of acute or chronic pain (Pan et al., 2008; Bowery, 2010). Baclofen is mainly injected into the spine to manage spasticity and neuropathic pain and as an adjuvant analgesic for relieving cancer pain (Zuniga et al., 2000; Yomiya et al., 2009). Its oral dose must be carefully regulated because of possible side effects. Vc1.1 does not compete with baclofen for binding to receptors, but it targets the interface between the GABABR ectodomains (see Adams and Berecki, 2013). Vc1.1 was tested in human clinical trials, but its development was discontinued because of its lack of potency at human α9α10 nicotinic acetylcholine receptor, which was proposed to be the molecular target (McIntosh et al., 2009). However, with the emergence of new α-conotoxin–based pharmacological tools that act on neuronal VGCCs via the GABABR, its development is likely to resume. It remains to be established if analgesic α-conotoxins can be used as specific Cav2.2[e37a] and Cav2.3 channel inhibitors for the treatment of chronic pain.
In conclusion, we identified a previously unrecognized mechanism of α-conotoxin Vc1.1 and baclofen inhibition of Cav2.3 channels that involves GABABRs. We systematically examined the intracellular pathways and elucidated the molecular details that determine c-Src phosphorylation of the Cav2.1 and Cav2.3 channel C termini. Although the physiological significance of kinase-mediated Cav2.3 channel inhibition is unclear, it may have long-term influence over Ca2+-dependent intracellular signaling, exocytosis, and/or gene transcription in neurons.
Supplementary Material
Acknowledgments
We thank D. Lipscombe for the rat β3 clone, J.W. Lynch for the eGFP clone, F. Meunier for the rabbit Cav2.1 clone, T. Schneider for the human Cav2.3d clone, J. Striessnig for the human Cav2.1 clone, J. Ulrich for the wild-type and mutant mouse c-Src clones, and G.W. Zamponi for the rat α2δ-1 clone.
This work was supported by National Health and Medical Research Council grants 1034642 (to G. Berecki and R.J. Clark) and 569927 (to D.J. Adams). R.J. Clark is an Australian Research Council (ARC) Future Fellow, and D.J. Adams is an ARC Australian Professorial Fellow.
The authors declare no competing financial interests.
Sharona E. Gordon served as editor.
Footnotes
Abbreviations used in this paper:
- DRG
- dorsal root ganglion
- GABA
- γ-aminobutyric acid
- GABAB
- GABA type B
- GABABR
- GABAB receptor
- GDP-β-S
- guanosine 5′-[β-thio]diphosphate trilithium salt
- GPCR
- G protein–coupled receptor
- HEK
- human embryonic kidney
- HP
- holding potential
- IBa
- Ba2+ current
- PTX
- pertussis toxin
- VD
- voltage dependent
- VGCC
- voltage-gated calcium channel
- VI
- voltage independent
References
- Adams D.J., Berecki G. 2013. Mechanisms of conotoxin inhibition of N-type (Cav2.2) calcium channels. Biochim. Biophys. Acta. 1828:1619–1628 10.1016/j.bbamem.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Altier C., Zamponi G.W. 2004. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol. Sci. 25:465–470 10.1016/j.tips.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Altier C., Dale C.S., Kisilevsky A.E., Chapman K., Castiglioni A.J., Matthews E.A., Evans R.M., Dickenson A.H., Lipscombe D., Vergnolle N., Zamponi G.W. 2007. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 27:6363–6373 10.1523/JNEUROSCI.0307-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanchy R., Periaswamy B., Mathivanan S., Reddy R., Tattikota S.G., Pandey A. 2007. A curated compendium of phosphorylation motifs. Nat. Biotechnol. 25:285–286 10.1038/nbt0307-285 [DOI] [PubMed] [Google Scholar]
- Bannister R.A., Melliti K., Adams B.A. 2004. Differential modulation of CaV2.3 Ca2+ channels by Gαq/11-coupled muscarinic receptors. Mol. Pharmacol. 65:381–388 10.1124/mol.65.2.381 [DOI] [PubMed] [Google Scholar]
- Bean B.P. 1989. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 340:153–156 10.1038/340153a0 [DOI] [PubMed] [Google Scholar]
- Bell T.J., Thaler C., Castiglioni A.J., Helton T.D., Lipscombe D. 2004. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 41:127–138 10.1016/S0896-6273(03)00801-8 [DOI] [PubMed] [Google Scholar]
- Bence K., Ma W., Kozasa T., Huang X.Y. 1997. Direct stimulation of Bruton’s tyrosine kinase by Gq-protein α-subunit. Nature. 389:296–299 10.1038/38520 [DOI] [PubMed] [Google Scholar]
- Bourinet E., Soong T.W., Stea A., Snutch T.P. 1996. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc. Natl. Acad. Sci. USA. 93:1486–1491 10.1073/pnas.93.4.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E., Soong T.W., Sutton K., Slaymaker S., Mathews E., Monteil A., Zamponi G.W., Nargeot J., Snutch T.P. 1999. Splicing of α1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat. Neurosci. 2:407–415 10.1038/8070 [DOI] [PubMed] [Google Scholar]
- Bowery N.G. 2010. Historical perspective and emergence of the GABAB receptor. Adv. Pharmacol. 58:1–18 10.1016/S1054-3589(10)58001-3 [DOI] [PubMed] [Google Scholar]
- Callaghan B., Adams D.J. 2010. Analgesic α-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels (Austin). 4:1–4 [DOI] [PubMed] [Google Scholar]
- Callaghan B., Haythornthwaite A., Berecki G., Clark R.J., Craik D.J., Adams D.J. 2008. Analgesic α-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J. Neurosci. 28:10943–10951 10.1523/JNEUROSCI.3594-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B., Bekkers J.M. 1999. GABAB, opioid and α2 receptor inhibition of calcium channels in acutely-dissociated locus coeruleus neurones. Br. J. Pharmacol. 127:1533–1538 10.1038/sj.bjp.0702693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A., Kenakin T. 2002. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54:323–374 10.1124/pr.54.2.323 [DOI] [PubMed] [Google Scholar]
- Clark R.J., Fischer H., Nevin S.T., Adams D.J., Craik D.J. 2006. The synthesis, structural characterization, and receptor specificity of the α-conotoxin Vc1.1. J. Biol. Chem. 281:23254–23263 10.1074/jbc.M604550200 [DOI] [PubMed] [Google Scholar]
- Clark R.J., Jensen J., Nevin S.T., Callaghan B.P., Adams D.J., Craik D.J. 2010. The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angew. Chem. Int. Ed. Engl. 49:6545–6548 10.1002/anie.201000620 [DOI] [PubMed] [Google Scholar]
- Cox D.H., Dunlap K. 1992. Pharmacological discrimination of N-type from L-type calcium current and its selective modulation by transmitters. J. Neurosci. 12:906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny H., de Faoite A., Huynh T.G., Yasuda T., Berecki G., Adams D.J. 2012. γ-Aminobutyric acid type B (GABAB) receptor expression is needed for inhibition of N-type (Cav2.2) calcium channels by analgesic α-conotoxins. J. Biol. Chem. 287:23948–23957 10.1074/jbc.M112.342998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie K.P., Fox A.P. 1997. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J. Neurosci. 17:4570–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G., Haedo R.J., Warren V.A., Ratliff K.S., Bugianesi R.M., Rush A., Williams M.E., Herrington J., Smith M.M., McManus O.B., Swensen A.M. 2008. A high-throughput assay for evaluating state dependence and subtype selectivity of Cav2 calcium channel inhibitors. Assay Drug Dev. Technol. 6:195–212 10.1089/adt.2008.136 [DOI] [PubMed] [Google Scholar]
- Daly N.L., Callaghan B., Clark R.J., Nevin S.T., Adams D.J., Craik D.J. 2011. Structure and activity of α-conotoxin PeIA at nicotinic acetylcholine receptor subtypes and GABAB receptor-coupled N-type calcium channels. J. Biol. Chem. 286:10233–10237 10.1074/jbc.M110.196170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P., Coste B., Gamper N., Shapiro M.S. 2005. Phosphoinositide lipid second messengers: New paradigms for calcium channel modulation. Neuron. 47:179–182 10.1016/j.neuron.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Diversé-Pierluissi M., Remmers A.E., Neubig R.R., Dunlap K. 1997. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc. Natl. Acad. Sci. USA. 94:5417–5421 10.1073/pnas.94.10.5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A.C., Scott R.H. 1986. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (−)-baclofen. Br. J. Pharmacol. 88:213–220 10.1111/j.1476-5381.1986.tb09489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuis E., Rockliffe N., Hussain M., Boyett M., Wray D., Gawler D. 2006. Evidence for multiple Src binding sites on the α1c L-type Ca2+ channel and their roles in activity regulation. Cardiovasc. Res. 69:391–401 10.1016/j.cardiores.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Fang Z., Park C.K., Li H.Y., Kim H.Y., Park S.H., Jung S.J., Kim J.S., Monteil A., Oh S.B., Miller R.J. 2007. Molecular basis of Cav2.3 calcium channels in rat nociceptive neurons. J. Biol. Chem. 282:4757–4764 10.1074/jbc.M605248200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Hwang J.H., Kim J.S., Jung S.J., Oh S.B. 2010. R-type calcium channel isoform in rat dorsal root ganglion neurons. Korean J. Physiol. Pharmacol. 14:45–49 10.4196/kjpp.2010.14.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre I., Moczydlowski E., Schild L. 1995. Specificity for block by saxitoxin and divalent cations at a residue which determines sensitivity of sodium channel subtypes to guanidinium toxins. J. Gen. Physiol. 106:203–229 10.1085/jgp.106.2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Zoller K.E., Ginsberg M.H., Brugge J.S., Shattil S.J. 1997. Regulation of the pp72syk protein tyrosine kinase by platelet integrin αIIbβ3. EMBO J. 16:6414–6425 10.1093/emboj/16.21.6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S., Kasyanov A.M., Pietrobon D., Voronin L.L., Cherubini E. 2001. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J. Neurosci. 21:8715–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A.C., Raingo J., Lipscombe D. 2007. Neuronal calcium channels: Splicing for optimal performance. Cell Calcium. 42:409–417 10.1016/j.ceca.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A., Leresche N. 1995. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. J. Physiol. 485:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M., Schallhorn A., Wurm F.M. 1996. Transfecting mammalian cells: Optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24:596–601 10.1093/nar/24.4.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T. 1989. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflugers Arch. 414:145–149 10.1007/BF00580956 [DOI] [PubMed] [Google Scholar]
- Klimis H., Adams D.J., Callaghan B., Nevin S., Alewood P.F., Vaughan C.W., Mozar C.A., Christie M.J. 2011. A novel mechanism of inhibition of high-voltage activated calcium channels by α-conotoxins contributes to relief of nerve injury-induced neuropathic pain. Pain. 152:259–266 10.1016/j.pain.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Lambert N.A., Wilson W.A. 1996. High-threshold Ca2+ currents in rat hippocampal interneurones and their selective inhibition by activation of GABAB receptors. J. Physiol. 492:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R.W. 1989. α-Adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 340:639–642 10.1038/340639a0 [DOI] [PubMed] [Google Scholar]
- Luebke J.I., Dunlap K., Turner T.J. 1993. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 11:895–902 10.1016/0896-6273(93)90119-C [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Ferguson S.S., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F., Kawakatsu H., Owada K., Luttrell D.K., et al. 1999. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 283:655–661 10.1126/science.283.5402.655 [DOI] [PubMed] [Google Scholar]
- Matthews E.A., Bee L.A., Stephens G.J., Dickenson A.H. 2007. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur. J. Neurosci. 25:3561–3569 10.1111/j.1460-9568.2007.05605.x [DOI] [PubMed] [Google Scholar]
- McIntosh J.M., Absalom N., Chebib M., Elgoyhen A.B., Vincler M. 2009. α9 nicotinic acetylcholine receptors and the treatment of pain. Biochem. Pharmacol. 78:693–702 10.1016/j.bcp.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pereverzev A., Grabsch H., Hescheler J., Schneider T. 1997. Receptor-mediated modulation of recombinant neuronal class E calcium channels. FEBS Lett. 408:261–270 10.1016/S0014-5793(97)00437-7 [DOI] [PubMed] [Google Scholar]
- Mintz I.M., Bean B.P. 1993. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 10:889–898 10.1016/0896-6273(93)90204-5 [DOI] [PubMed] [Google Scholar]
- Ottolia M., Platano D., Qin N., Noceti F., Birnbaumer M., Toro L., Birnbaumer L., Stefani E., Olcese R. 1998. Functional coupling between human E-type Ca2+ channels and µ opioid receptors expressed in Xenopus oocytes. FEBS Lett. 427:96–102 10.1016/S0014-5793(98)00401-3 [DOI] [PubMed] [Google Scholar]
- Page K.M., Cantí C., Stephens G.J., Berrow N.S., Dolphin A.C. 1998. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J. Neurosci. 18:4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.L., Wu Z.Z., Zhou H.Y., Chen S.R., Zhang H.M., Li D.P. 2008. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 117:141–161 10.1016/j.pharmthera.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Gish G.D., Nash P. 2001. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 11:504–511 10.1016/S0962-8924(01)02154-7 [DOI] [PubMed] [Google Scholar]
- Pereverzev A., Leroy J., Krieger A., Malécot C.O., Hescheler J., Pfitzer G., Klöckner U., Schneider T. 2002. Alternate splicing in the cytosolic II-III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels: Electrophysiological characterization of isoforms. Mol. Cell. Neurosci. 21:352–365 10.1006/mcne.2002.1179 [DOI] [PubMed] [Google Scholar]
- Pexton T., Moeller-Bertram T., Schilling J.M., Wallace M.S. 2011. Targeting voltage-gated calcium channels for the treatment of neuropathic pain: a review of drug development. Expert Opin. Investig. Drugs. 20:1277–1284 10.1517/13543784.2011.600686 [DOI] [PubMed] [Google Scholar]
- Qian A., Song D., Li Y., Liu X., Tang D., Yao W., Yuan Y. 2013. Role of voltage gated Ca2+ channels in rat visceral hypersensitivity change induced by 2,4,6-trinitrobenzene sulfonic acid. Mol. Pain. 9:15 10.1186/1744-8069-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingo J., Castiglioni A.J., Lipscombe D. 2007. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 10:285–292 10.1038/nn1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H., Kurihara T., Zong S., Minowa O., Kazuno A., Han W., Matsuda Y., Yamanaka H., Osanai M., Noda T., Tanabe T. 2000. Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc. Natl. Acad. Sci. USA. 97:6132–6137 10.1073/pnas.100124197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M.L., Siderovski D.P., Jordan J.D., Brothers G., Snow B., De Vries L., Ortiz D.F., Diversé-Pierluissi M. 2000. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 408:723–727 10.1038/35047093 [DOI] [PubMed] [Google Scholar]
- Schneider T., Wei X., Olcese R., Costantin J.L., Neely A., Palade P., Perez-Reyes E., Qin N., Zhou J., Crawford G.D., et al. 1994. Molecular analysis and functional expression of the human type E neuronal Ca2+ channel alpha 1 subunit. Receptors Channels. 2:255–270 [PubMed] [Google Scholar]
- Schneider T., Dibué M., Hescheler J. 2013. How “Pharmacoresistant” is Cav2.3, the major component of voltage-gated R-type Ca2+ channels. Pharmaceuticals (Basel). 6:759–776 10.3390/ph6060759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekter L.R., Taussig R., Gillard S.E., Miller R.J. 1997. Regulation of human neuronal calcium channels by G protein βγ subunits expressed in human embryonic kidney 293 cells. Mol. Pharmacol. 52:282–291 [DOI] [PubMed] [Google Scholar]
- Simen A.A., Miller R.J. 2000. Involvement of regions in domain I in the opioid receptor sensitivity of α1B Ca2+ channels. Mol. Pharmacol. 57:1064–1074 [PubMed] [Google Scholar]
- Stephens G.J., Cantí C., Page K.M., Dolphin A.C. 1998. Role of domain I of neuronal Ca2+ channel α1 subunits in G protein modulation. J. Physiol. 509:163–169 10.1111/j.1469-7793.1998.163bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. 1993. Different types of calcium channels mediate central synaptic transmission. Nature. 366:156–158 10.1038/366156a0 [DOI] [PubMed] [Google Scholar]
- Terashima T., Xu Q., Yamaguchi S., Yaksh T.L. 2013. Intrathecal P/Q- and R-type calcium channel blockade of spinal substance P release and c-Fos expression. Neuropharmacology. 75:1–8 10.1016/j.neuropharm.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.E., Marubio L.M., Deal C.R., Hans M., Brust P.F., Philipson L.H., Miller R.J., Johnson E.C., Harpold M.M., Ellis S.B. 1994. Structure and functional characterization of neuronal α1E calcium channel subtypes. J. Biol. Chem. 269:22347–22357 [PubMed] [Google Scholar]
- Wu L.G., Borst J.G., Sakmann B. 1998. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc. Natl. Acad. Sci. USA. 95:4720–4725 10.1073/pnas.95.8.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Kurihara T., Saegusa H., Zong S., Makita K., Tanabe T. 2004. Blocking the R-type (Cav2.3) Ca2+ channel enhanced morphine analgesia and reduced morphine tolerance. Eur. J. Neurosci. 20:3516–3519 10.1111/j.1460-9568.2004.03810.x [DOI] [PubMed] [Google Scholar]
- Yomiya K., Matsuo N., Tomiyasu S., Yoshimoto T., Tamaki T., Suzuki T., Matoba M. 2009. Baclofen as an adjuvant analgesic for cancer pain. Am. J. Hosp. Palliat. Care. 26:112–118 10.1177/1049909108327968 [DOI] [PubMed] [Google Scholar]
- Zamponi G.W., Currie K.P. 2013. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta. 1828:1629–1643 10.1016/j.bbamem.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke R.D., Pitt G.S., Deisseroth K., Tsien R.W., Reuter H. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162 10.1038/20200 [DOI] [PubMed] [Google Scholar]
- Zuniga R.E., Schlicht C.R., Abram S.E. 2000. Intrathecal baclofen is analgesic in patients with chronic pain. Anesthesiology. 92:876–880 10.1097/00000542-200003000-00037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.