Abstract
While ageing is commonly associated with exponential increase in mortality with age, mortality rates paradoxically decelerate late in life resulting in distinct mortality plateaus. Late-life mortality plateaus have been discovered in a broad variety of taxa, including humans, but their origin is hotly debated. One hypothesis argues that deceleration occurs because the individual probability of death stops increasing at very old ages, predicting the evolution of earlier onset of mortality plateaus under increased rate of extrinsic mortality. By contrast, heterogeneity theory suggests that mortality deceleration arises from individual differences in intrinsic lifelong robustness and predicts that variation in robustness between populations will result in differences in mortality deceleration. We used experimental evolution to directly test these predictions by independently manipulating extrinsic mortality rate (high or low) and mortality source (random death or condition-dependent) to create replicate populations of nematodes, Caenorhabditis remanei that differ in the strength of selection in late-life and in the level of lifelong robustness. Late-life mortality deceleration evolved in response to differences in mortality source when mortality rate was held constant, while there was no consistent response to differences in mortality rate. These results provide direct experimental support for the heterogeneity theory of late-life mortality deceleration.
Keywords: ageing, heterogeneity, mortality plateau, nematodes, robustness, stress
1. Introduction
The process of ageing has long been described as an exponential increase in age-specific mortality rates with age [1]. However, in many taxa, ranging from invertebrates to vertebrates to humans, such increase during early- to mid-life decelerates or even ceases in very late ages, forming a distinct phase often called late-life mortality plateaus [1–4]. From the early days of this discovery, when the deceleration of mortality rates in late ages was considered unlikely, the evolution of late-life mortality deceleration remains a source of contention among biologists and biodemographers [5–12]. Currently, there are two major sets of theories explaining the phenomenon of late-life mortality deceleration: heterogeneity theory suggests that the deceleration arises from variation in properties of individuals in a population [5], while Hamiltonian theory postulates that the diminished strength of natural selection shapes the deceleration of mortality in late-life [11].
Heterogeneity theory relies on the fact that individuals in a population differ in their ‘robustness’, a lifelong property that can be viewed as a sum of genetic and environmental factors that determine an individual's likelihood of survival—less-robust individuals are more likely to die [5]. Mortality deceleration arises because selection removes less-robust individuals when they are young, leaving more robust individuals who survive selection to define the mortality rate of the population in late ages [3,4,10,12]. At least some versions of the heterogeneity theory predict that selection for physiological robustness, such as stress resistance, will cause the properties of late-life mortality deceleration, such as rate or timing-of-onset, to differ [7]. This prediction has been previously tested by comparing the mortality in populations of Drosophila melanogaster evolving under starvation resistance with control populations, but different analyses of the same dataset caused different authors to reach opposing conclusions [6,7].
Hamilton's formal analysis of senescence suggests that the strength of natural selection acting on age-dependent fitness components continuously declines starting from the age at first reproduction [13]. After the organisms stop reproducing, natural selection declines to zero and can no longer distinguish differences in fitness at different ages. Therefore, age-specific mortality decelerates in parallel with the strength of natural selection [9]. Because reproductive span is dependent on somatic lifespan [13], the onset of the mortality deceleration should evolve in accordance with the rate of extrinsic mortality, such that higher rate of extrinsic mortality results in earlier onset of deceleration in mortality [11]. The effect of different sources of mortality (e.g. random versus condition-dependent) is beyond the scope of this theory as populations should evolve similar rates of deceleration in late-life mortality when the rate of extrinsic mortality, and therefore the strength of selection in late-life, is held constant [9].
In this study, we set out to test these two contemporary theories of late-life mortality deceleration by using nematodes Caenorhabditis remanei that evolved under different rate and source of extrinsic mortality [14]. We used experimental evolution to test the predictions by independently manipulating extrinsic mortality rate (high or low) and mortality source (random or condition-dependent), and creating replicate populations that differ in the strength of selection in late-life as well as in the level of lifelong robustness [14]. Heterogeneity theory predicts that extrinsic mortality source should affect the evolution of mortality deceleration, whereas Hamiltonian theory predicts that mortality deceleration should be determined by extrinsic mortality rate.
2. Material and methods
We employed experimental lines that evolved from the wild-type strain SP8 of C. remanei. This strain harbours a substantial amount of standing genetic variation for life-history traits [14]. We subjected four replicate populations to four life-history regimes (n = 16 in total) by varying the rate (high (H) and low (L)) and source (random (R) and condition-dependent (C-d)) of extrinsic mortality in a 2 × 2 factorial design (from here on abbreviated as ‘HR’, ‘LR’, ‘HC-d’ and ‘LC-d’, respectively; see [14] for details). We used heat-shock to induce condition-dependent mortality because: (i) it has been shown that heat-shock increases the proportion of robust individuals and changes heterogeneity distribution in Caenorhabditis elegans [15]; and (ii) resistance to heat-shock is correlated with longevity in Caenorhabditis nematodes [16]. High mortality imposed by heat-shock indeed resulted in the evolution of long-lived nematodes [14]. After 12 generations of experimental evolution (see the electronic supplementary material), we estimated age-specific mortality rates of both sexes in the resultant populations, which we report here.
Mortality data were analysed both within each population (n = 30 (in one case 20) per sex, see the electronic supplementary material, tables S1 and S3) and by pooling data from populations within each regime (n = 120 (in one case 110) per sex, see figure 1 and table 1; electronic supplementary material, table S2 and S3). We fitted the data to the Gompertz family of nested models (Gompertz, Gompertz-Makeham, Logistic and Logistic-Makeham) using maximum-likelihood approach implemented in WinModest software [17]. The best-fit model was decided by log-likelihood ratio (LLR) test [17]. The best fit for all main treatment groups was provided by the Logistic model (see the electronic supplementary material, table S2), μx = aebx/[1 + (as/b)(ebx − 1)], where μx is the age-specific mortality at age x, a is the baseline mortality, b is the exponential increase in mortality with age (rate-of-senescence) and s is the rate-of-deceleration in mortality at late ages. To test whether individual parameters (a, b and s) differed between the experimental regimes, we (i) used a general linear model to compare parameters derived from each population (see figure 2 and the electronic supplementary material, table S1); and (ii) compared the log likelihoods (LLs) of models, where the parameter estimates were constrained to be the same (null hypothesis), with unconstrained models, where all parameter values were allowed to vary (alternative hypothesis) [17]. The significance was estimated by using the value of –2 (LLnull hypothesis − LLalternative hypothesis) with χ2 distribution and degrees of freedom equal to the number of constraint parameters (here, 1) [17] (table 1).
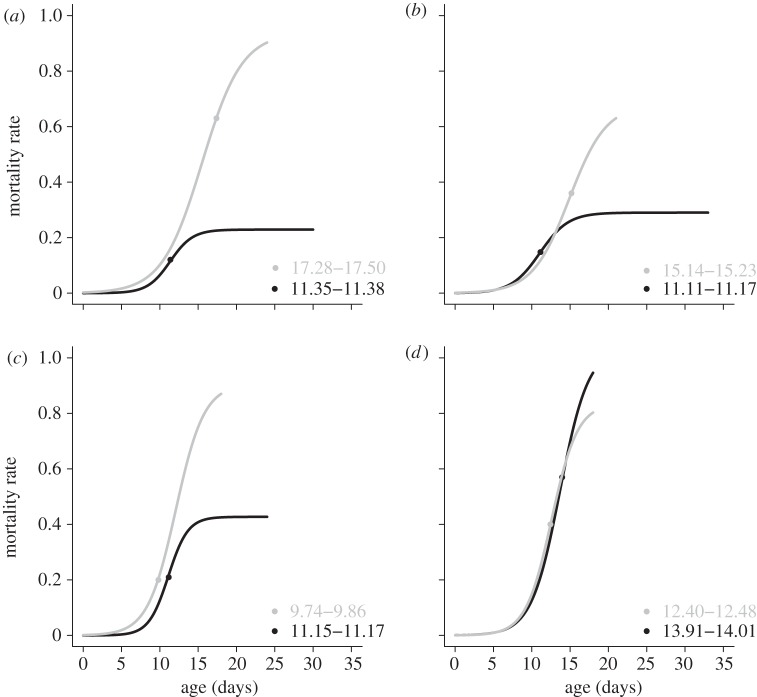
Figure 1.
Mortality rates (curves) and the inflection points that define the start of mortality deceleration (points on respective curves; numbers at lower right are respective 95% CIs) of populations of C. remanei evolved under high rate ((a) males, (b) females) and low rate ((c) males, (d) females) of extrinsic mortality. Condition-dependent mortality, black; random mortality, grey.
Table 1.
Asterisks denote parameters with significant difference in pairwise comparisons in LLR test. (95% CIs are given in parentheses. ***p < 0.0005; **p < 0.001; *p < 0.05.)
| selection regime | sex | a | b | s |
|---|---|---|---|---|
| high mortality rate | ||||
| condition-dependent | males | 0.0001 (0.00001–0.00096)* | 0.68167 (0.48084–0.96638)* | 2.97665 (1.76266–5.02675)*** |
| random | males | 0.00251 (0.00101–0.00620)* | 0.38196 (0.30027–0.48588)* | 0.40718 (0.16748–0.98992)*** |
| condition-dependent | females | 0.00007 (0.00001–0.00054)* | 0.78466 (0.59588–1.03324)** | 1.83663 (1.14506–2.94589)* |
| random | females | 0.0013 (0.00040–0.00422)* | 0.54146 (0.41597–0.70482)** | 0.59788 (0.26426–1.35266)* |
| low mortality rate | ||||
| condition-dependent | males | 0.00074 (0.00017–0.00332) | 0.53959 (0.38819–0.75003) | 1.85975 (1.04385–3.31337)* |
| random | males | 0.0014 (0.00043–0.00458) | 0.41526 (0.31076–0.55491) | 0.61059 (0.25187–1.48024)* |
| condition-dependent | females | 0.00065 (0.00016–0.00261) | 0.53957 (0.41124–0.70794) | 0.51997 (0.20662–1.30852) |
| random | females | 0.00049 (0.00011–0.00214) | 0.59108 (0.45082–0.77499) | 0.70720 (0.33348–1.49974) |
Figure 2.
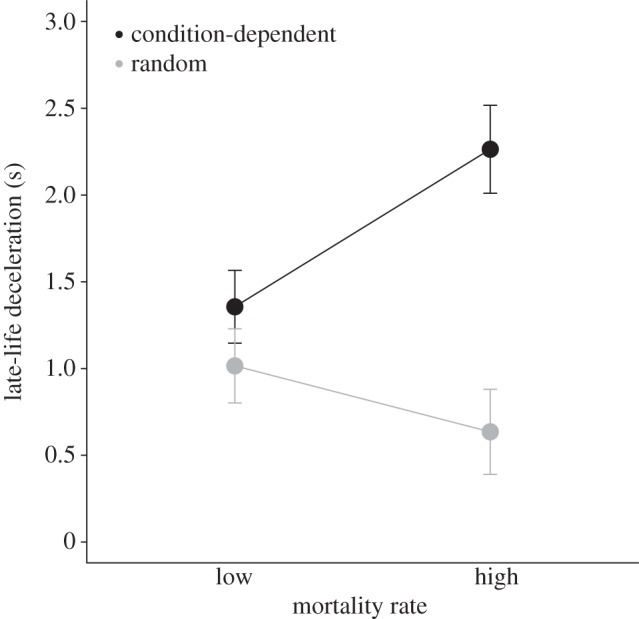
The effect of mortality rate and mortality source on the evolution of late-life deceleration parameter (s). Because there were no significant effects of sex (see electronic supplementary material, table S1), data are pooled across sexes. Values are the least-squares means ± s.e. from the general linear model.
The onset of mortality deceleration in late ages was estimated by the inflection points of mortality curves (i.e. when the exponential increase in age-specific mortality stopped; see the electronic supplementary material for details). A general linear model was used to test the effects of selection on the inflection points in both sexes (table 2).
Table 2.
The full factorial general linear model of the effects of extrinsic mortality rate, mortality source and sex on the evolution of the onset of late-life mortality deceleration.
| effects | d.f. | F-ratio | p-value |
|---|---|---|---|
| mortality rate | 1,12 | 0.1076 | 0.7485 |
| mortality source | 1,12 | 8.4432 | 0.0132 |
| sex | 1,12 | 0.7283 | 0.4102 |
| mortality rate × sex | 1,12 | 3.4727 | 0.0870 |
| mortality source × sex | 1,12 | 8.9735 | 0.0112 |
| mortality rate × mortality source | 1,12 | 0.7653 | 0.3988 |
| mortality rate × mortality source × sex | 1,12 | 0.1468 | 0.7083 |
3. Results
To test for the role of mortality source and mortality rate in shaping the late-life mortality deceleration, we first focused on pairwise comparisons of the deceleration in mortality (s) between experimental regimes with (i) the same rate but different sources (heterogeneity theory), or (ii) the same source but different rates of extrinsic mortality (Hamiltonian theory). In the first set of comparisons, the deceleration was more rapid in condition-dependent mortality regimes than in random mortality regimes in both sexes when the mortality rate was high, and in males when the mortality rate was low (table 1). On the contrary, in the second set of comparisons, all pairs except for one (HC-d versus LC-d females) showed the same deceleration in mortality. Besides, at high mortality rate, condition-dependence resulted in lower baseline mortality (a) in both sexes, but faster rate-of-senescence (b) in males only (table 1). The results were the same when using general linear mixed model on parameters derived from population-based analyses (see the electronic supplementary material, tables S1 and S3). There was a significant effect of mortality source on s (p = 0.0013), but not of mortality rate, and there were no interactions with sex. The effect of source on s was most apparent under high mortality regimes, resulting in source × rate interaction (p = 0.0218; figure 2, see the electronic supplementary material, table S1).
Condition-dependent mortality also resulted in earlier onset of mortality deceleration in both sexes (p = 0.0132), while there was no effect of extrinsic mortality rate on inflection points (figure 1 and table 2).
4. Discussion
Our results support the theory that late-life mortality deceleration arises from compositional heterogeneity of robustness between individuals in a population. Populations selected for increased robustness under heat-shock showed an earlier onset of mortality deceleration, as well as a more rapid deceleration of mortality in late ages than populations exposed to random death, despite experiencing the same rate of extrinsic mortality. On the contrary, there was little evidence that extrinsic mortality rate affected the evolution of late-life mortality.
Robustness determines the likelihood of survival of an individual, and consequently the mortality function of groups of individuals sharing a similar level of robustness [5]. Robustness is assumed to be positively related to the organisms’ general physiological condition and associated traits, such as stress resistance [6]. As a result of having a better physiological condition, more robust individuals should have lower intrinsic mortality. Indeed, a recent study indicates that genes involved in lifespan regulation also contribute to stress resistance and robustness [18]. In our experiment, condition-dependent mortality selected for individuals that are better at resisting increased ambient temperature [19], a trait that has been shown to be associated with whole-organism performance and longevity [16]. Therefore, the proportion of robust individuals should increase over time in the course of evolution under condition-dependent mortality.
While the two theories tested here are not mutually exclusive and both can contribute to the occurrence of late-life mortality deceleration, heterogeneity theory provides the best description of our data. The source of extrinsic mortality has profound effects on the evolution of ageing in general, and the evolution of late-life mortality deceleration in particular. Condition-dependent mortality resulted in the evolution of an earlier onset and a more rapid deceleration of mortality in late ages. However, while this experiment introduces an extra layer of complexity in comparison to earlier studies that employed only random mortality, more work is required to understand the generality of these patterns. Organisms experience multiple sources of mortality in nature (predation, disease and abiotic stress), many of which are likely to be condition-dependent, while others may not. The interaction between these multifarious mortality sources is likely to shape intrinsic mortality rates. We hope that our study will help focus much-needed research effort on the role of extrinsic mortality source in the evolution of ageing.
Funding statement
The study was supported by Swedish Research Council and ERC Starting-Grant-2010 (A.A.M.), Department of Animal Ecology (H-y.C.) and Wenner-Gren Foundations (F.Z.).
References
- 1.Charlesworth B, Partridge L. 1997. Ageing: levelling of the grim reaper. Curr. Biol. 7, R440–R442 (doi:10.1016/S0960-9822(06)00213-2) [DOI] [PubMed] [Google Scholar]
- 2.Vaupel JW, et al. 1998. Biodemographic trajectories of longevity. Science 280, 855–860 (doi:10.1126/science.280.5365.855) [DOI] [PubMed] [Google Scholar]
- 3.Carey JR, Liedo P, Orozco D, Vaupel JW. 1992. Slowing of mortality rates at older ages in large medfly cohorts. Science 258, 457–461 (doi:10.1126/science.1411540) [DOI] [PubMed] [Google Scholar]
- 4.Curtsinger JW, Fukui HH, Townsend DR, Vaupel JW. 1992. Demography of genotypes: failure of the limited life-span paradigm in Drosophila melanogaster. Science 258, 461–463 (doi:10.1126/science.1411541) [DOI] [PubMed] [Google Scholar]
- 5.Vaupel JW, Manton KG, Stallard E. 1979. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454 (doi:10.2307/2061224) [PubMed] [Google Scholar]
- 6.Drapeau MD, Gass EK, Simison MD, Mueller LD, Rose MR. 2000. Testing the heterogeneity theory of late-life mortality plateaus by using cohorts of Drosophila melanogaster. Exp. Gerontol. 35, 71–84 (doi:10.1016/S0531-5565(99)00082-0) [DOI] [PubMed] [Google Scholar]
- 7.Steinsaltz D. 2005. Re-evaluating a test of the heterogeneity explanation for mortality plateaus. Exp. Gerontol. 40, 101–113 (doi:10.1016/j.exger.2004.11.010) [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Rea SL, Yashin AI, Johnson TE. 2006. Visualizing hidden heterogeneity in isogenic populations of C. elegans. Exp. Gerontol. 41, 261–270 (doi:10.1016/j.exger.2006.01.003) [DOI] [PubMed] [Google Scholar]
- 9.Rauser CL, Mueller LD, Travisano M, Rose MR. 2009. Evolution of aging and late life. In Experimental evolution (eds Garland T, Rose MR.), pp. 551–584 Berkeley, CA: University of California Press [Google Scholar]
- 10.Vaupel JW. 2010. Biodemography of human ageing. Nature 464, 536–542 (doi:10.1038/nature08984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller LD, Rauser CL, Rose MR. 2011. Does aging stop? New York, NY: Oxford University Press [Google Scholar]
- 12.Brooks A, Lithgow GJ, Johnson TE. 1994. Mortality rates in a genetically heterogeneous population of Caenorhabditis elegans. Science 263, 668–671 (doi:10.1126/science.8303273) [DOI] [PubMed] [Google Scholar]
- 13.Hamilton WD. 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45 (doi:10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 14.Chen H-Y, Maklakov AA. 2012. Longer life span evolves under high rates of condition-dependent mortality. Curr. Biol. 22, 2140–2143 (doi:10.1016/j.cub.2011.12.009) [DOI] [PubMed] [Google Scholar]
- 15.Yashin AI, Cypser JW, Johnson TE, Michalski AI, Boyko SI, Novoseltsev VN. 2002. Heat shock changes the heterogeneity distribution in populations of Caenorhabditis elegans: does it tell us anything about the biological mechanism of stress response? J. Gerontol. A. Biol. Sci. Med. Sci. 57, B83–B92 (doi:10.1093/gerona/57.3.B83) [DOI] [PubMed] [Google Scholar]
- 16.Amrit FRG, Boehnisch CML, May RC. 2010. Phenotypic covariance of longevity, immunity and stress resistance in the Caenorhabditis nematodes. PLoS ONE 5, e9978 (doi:10.1371/journal.pone.0009978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pletcher S. 1999. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 12, 430–439 (doi:10.1046/j.1420-9101.1999.00058.x) [Google Scholar]
- 18.Yashin AI, Wu D, Arbeev KG, Stallard E, Land KC, Ukraintseva SV. 2012. How genes influence life span: the biodemography of human survival. Rejuvenation Res. 15, 374–380 (doi:10.1089/rej.2011.1290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Maklakov A. 2013. The worm that lived: evolution of rapid aging under high extrinsic mortality revisited. Worm 2, e23704 (doi:10.4161/worm.23704) [DOI] [PMC free article] [PubMed] [Google Scholar]