Abstract
Telomere length and dynamics are increasingly scrutinized as ultimate determinants of performance, including age-dependent mortality and fecundity. Few studies have investigated longevity in relation to telomere length (TL) in the wild and none has analysed longevity in relation to TL soon after hatching, despite the fact that telomere shortening may mostly occur early in life. We show that TL in nestling barn swallows (Hirundo rustica) in the wild does not predict longevity. However, TL positively covaries with body size, suggesting that individuals with large TL can afford to grow larger without paying the cost of reduced TL, and/or that benign rearing conditions ensure both large body size and low rates of telomere shortening. Overall, our study hints at a role of TL in developmental processes, but also indicates a need for further analyses to assess the expectation that TL in young individuals predicts longevity in the wild.
Keywords: body size, longevity, nestlings, sex, telomere length
1. Introduction
Individuals differ greatly in longevity and fecundity. Because natural selection acts on the genetic component of such variation by promoting genotypes that maximize lifetime reproductive success, understanding the ultimate mechanisms that underpin variation in life-history traits is pivotal to evolutionary studies. A prominent source of variation in lifetime reproductive success is caused by the difference among individuals in the rate at which deterioration of performance with age (i.e. ‘senescence’) occurs, generating variation in longevity and/or age-specific fecundity [1].
A general mechanism that affects senescence is the dynamics of telomeres [2], which are highly conserved DNA repeat sequences located at the termini of linear chromosomes [3]. Telomeres accomplish multiple functions, including protection of chromosomes from loss of encoding parts caused by the ‘end replication’ problem, that is, failure of DNA polymerase to fully replicate linear DNA at its 3′ end [4]. Telomere shortening can be countered by telomerase enzymatic action, which tends to elongate telomeres [5]. With the loss of telomerase activity, telomere erosion prevails over restoration, most often leading to telomere shortening with age [6]. Once a threshold telomere length (TL) has been attained, cells enter replicative senescence and may incur apoptosis, reducing the regenerative capacity of the corresponding tissues [2].
Telomere shortening can thus impair bodily functions and reduce life expectancy. Initial TL shows significant heritability [7,8], and the rate of telomere shortening during life varies considerably among individuals, also as a consequence of variation in exposure to oxidative stress [9]. Among-individual variation in TL and dynamics has been suggested as an explanation of variation in performance (e.g. breeding success; [10]) and residual longevity among age-matched individuals [2]. Adult birds with short telomeres are more likely to disappear from their breeding colony, suggesting reduced viability [11–13]. Adult Seychelles warblers (Acrocephalus sechellensis) with large TL and a small rate of telomere shortening have enhanced survival [14]. Notably, most studies have analysed viability in relation to TL of adults. However, telomere dynamics may be most rapid among young [15], and studies analysing covariation of longevity with TL in early life-stages are needed to fully understand selection on TL. Only one such study has been carried out: in captive zebra finches (Taeniopygia guttata), TL measured 25 days post-hatching positively predicted longevity [15]. Importantly, conditions in the wild differ from those in captivity, and whether such a relationship holds under non-artificial conditions still remains to be assessed.
Here, for the first time in any wild population, we analyse whether TL soon after hatching predicts longevity, using the barn swallow (Hirundo rustica) as a short-lived model organism. Most studies of the phenotypic correlates of telomere dynamics in birds have focused on viability, whereas fewer have dealt with morphological traits, generally showing a negative association between TL and body size [6]. We thus also analysed covariation between TL and body size, and length of the wing and tail feathers, which are important traits with respect to natural and sexual selection in barn swallows [16], at sexual maturity.
2. Material and methods
Barn swallows are small, long-distance migratory passerines that most often breed colonially in rural buildings [16]. We marked nestlings and sampled blood between day 10 and 13 post-hatching over the 1997–2008 breeding seasons. Less than 5% of the offspring were recruited locally as 1-year-old breeders [17]. Keel length (reflecting body size), and wing and tail length were measured in the year of recruitment. The number of recruits per year ranged between 1 and 14. Only one pair of siblings was included. Excluding years with fewer than three recruits or controlling for year effects in the analyses did not alter the results qualitatively. Barn swallows in our population have very high breeding philopatry. In addition, we captured the vast majority of the breeding individuals every year. Thus, individuals that were not captured in the colony where they bred in the previous year could be considered dead [17], allowing us to measure longevity. We used accelerated failure time (AFT) and Cox regression models to investigate variation in longevity in relation to TL (see also [17] for coding of longevity). Right censoring occurred for only 8.3% of the observations.
(a). Telomere length analysis
Full methodological details are given in the electronic supplementary material. Total DNA was extracted from red blood cells. Three micrograms of DNA were digested and resolved on an agarose gel by pulsed field gel electrophoresis. Gels were UV cross-linked, denaturated, neutralized and transferred onto nylon membranes. Filters were hybridized in rapid hybridization buffer in the presence of a digoxigenin-labelled telomeric probe (TTAGGG)4. Telomere restriction fragments were detected by autoradiography and densitometric analysis. Mean TL was calculated following the study of Horn et al. [6] as TL = ∑(ODi)/∑(ODi/MWi), where ODi is the optical density at position i and MWi is molecular weight (see electronic supplementary material) at position i. Following the study of Bauch et al. [10], the upper TL limit was set at 30 kb, whereas the lower limit was set at 2 kb. Repeating the analyses using 50, 40 or 20 kb as upper limits to calculate TL50, TL40 and TL20 (collectively TLx) led to similar results (see below).
3. Results
Mean TLx values at the four TL thresholds were positively correlated (r ≥ 0.984, n = 60, p < 0.001), and the same was the case within each sex (r ≥ 0.983, n = 47 males, n = 13 females, p < 0.001).
TL30 did not differ between sexes (t58 = 0.85, p = 0.397, mean for males: 7.44 (0.23) kb; females: 7.90 (0.57)), and similar results were obtained for TL50, TL40 and TL20 (unsigned t58 < 1.00, p > 0.32). TLx values did not vary among years (ANOVA; p > 0.05; other details not shown).
TL30 did not significantly predict longevity after sexual maturity (AFT; 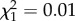 , p = 0.943; figure 1) while controlling for the effect of sex (
, p = 0.943; figure 1) while controlling for the effect of sex (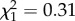 , p = 0.576), after excluding the non-significant sex-by-TL30 interaction from the model (
, p = 0.576), after excluding the non-significant sex-by-TL30 interaction from the model (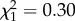 , p = 0.587). Similarly, models of longevity with TL20, TL40 and TL50 as predictors showed no effect on longevity (
, p = 0.587). Similarly, models of longevity with TL20, TL40 and TL50 as predictors showed no effect on longevity (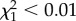 , p > 0.95). The non-significant effect of TLx on longevity remained qualitatively unchanged when we controlled for hatching date or colony of origin (effect of TLx:
, p > 0.95). The non-significant effect of TLx on longevity remained qualitatively unchanged when we controlled for hatching date or colony of origin (effect of TLx: 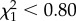 , p > 0.3). AFT models excluding years with fewer than three recruits confirmed no significant effects of TLx (
, p > 0.3). AFT models excluding years with fewer than three recruits confirmed no significant effects of TLx (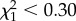 , p > 0.60; n = 51) while controlling for sex effects. Cox regression models stratifying data by hatching year confirmed the lack of significant effects of TLx (details not shown).
, p > 0.60; n = 51) while controlling for sex effects. Cox regression models stratifying data by hatching year confirmed the lack of significant effects of TLx (details not shown).
Figure 1.
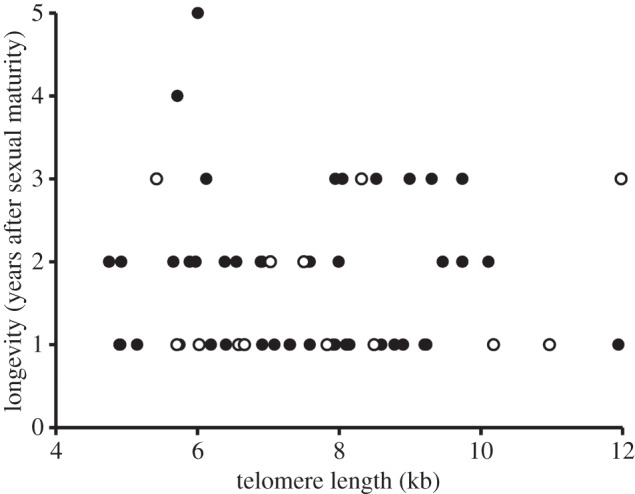
Longevity after maturity, expressed as year of disappearance from the colony, in relation to TL30 as nestlings. Closed circles: males; open circles: females.
Keel length covaried differentially with TL30 in either sex (table 1). The relationship was significantly positive among males (t52.5 = 4.04, n = 46, p < 0.001) but not females (t50.8 = −1.58, n = 13, p = 0.121; figure 2). These effects were confirmed in analyses with the other TLx as predictors (table 1). However, wing and tail length did not covary with TLx (table 1).
Table 1.
Linear mixed models of adult morphological traits in relation to TL30 in nestlings, with hatching year as a random factor. Main effects were estimated excluding interaction effects when non-significant. Sample size is 46 for males (45 for tail length) and 13 for females.
| F | d.f. | p | |
|---|---|---|---|
| keel length | |||
| sex | 7.65 | 1,53.4 | 0.008 |
| TL30 | 0.91 | 1,53.5 | 0.352 |
| a,bsex × TL30 | 12.67 | 1,54.5 | 0.001 |
| wing length | |||
| sex | 3.63 | 1,56 | 0.062 |
| TL30 | 0.08 | 1,56 | 0.774 |
| sex × TL30 | 0.05 | 1,55 | 0.818 |
| tail length | |||
| sex | 40.77 | 1,55 | <0.001 |
| TL30 | 0.55 | 1,55 | 0.462 |
| sex × TL30 | 0.64 | 1,54 | 0.429 |
aCoefficient (s.e.) for males 25.4 (6.28) and females −14.7 (9.31).
bAnalyses with TL50, TL40 or TL20 as predictors confirmed the significant (p < 0.005) sex-by-TL interaction effect on keel length.
Figure 2.
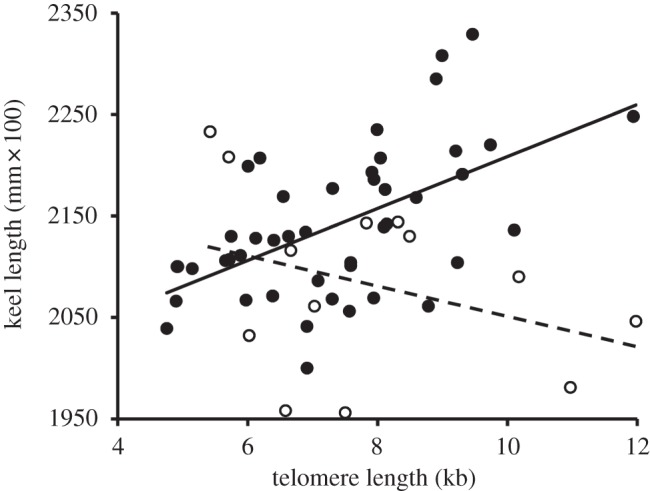
Relationship between adult body size and TL30. Linear regression lines for males (continuous line, closed circles) and females (dashed line, open circles) are shown.
4. Discussion
We found no evidence that TL soon after hatching predicts longevity of barn swallows. This finding was based on a large sample of recruits. However, because the sample of females was small, we emphasize that the results for females as well as the lack of sex-by-TL effect on longevity should be considered with caution.
The only previous study where longevity was analysed in relation to TL of young was carried out in captivity and showed a strong statistically significant positive effect [15]. The striking difference between the two studies may depend on extrinsic mortality factors (e.g. predation; [18]) in the wild having overwhelming effects on those of TL by causing mortality in barn swallows. Alternatively, the selection regime in captivity may amplify any relationship between TL and longevity that would be more difficult to detect in the wild. In addition, TL may predict viability before maturity. High natal dispersal propensity in our system prevents such an analysis. The latter interpretation, however, is not corroborated by the study of zebra finches [15] itself, as that study provided no evidence of a particularly strong effect of TL on survival before maturity. Finally, a different pattern of association between longevity and TL might exist among individuals that dispersed, although the mechanisms underlying such a differential relationship with respect to natal dispersal remain obscure. Hence, the present study does not support the idea that TL predicts longevity. Since longevity is a major determinant of lifetime reproductive success [17], our study provides no evidence for selection on TL, at least in males, for which we had a large sample. However, it should also be considered that telomere restriction fragment analysis by Southern blot or real-time PCR might have masked the relationship between TL and longevity [19], although that was not the case in other studies [6,15].
We found no evidence of sex-related variation in TL, in agreement with previous studies of nestlings [15,20,21]. Because hatching failure and nestling mortality rates in barn swallows are low [17], it seems unlikely that the lack of a sex difference resulted from TL-related differential mortality before blood sampling in either sex, with males and females differing in TL at the zygote stage.
We found that male nestlings with large TL achieved larger body size. The association between TL and body size could result from TL being causally linked to somatic growth, suggesting that growing to large body size entails no net cost in terms of telomere shortening. This is at odds with comparative evidence for mammals, which indicates that body size negatively covaries with telomerase activity [22]. Alternatively, the association may result from positive covariation of both variables with rearing conditions. Favourable pre-fledging conditions, including the effects of parental care, can both enhance somatic growth and reduce telomere shortening, for example, as a consequence of buffering of pro-oxidant production by an antioxidant-rich diet [9]. The relationship between body size and TL might thus represent a side effect of benign rearing conditions enhancing growth and protecting telomeres from oxidative damage. The fact that this relationship held only in males may depend on males being more sensitive to rearing conditions.
In conclusion, we have investigated, for the first time in the wild, the relation between TL in early life and longevity but failed to disclose an association. By contrast, we found a positive association between body size and TL, which may arise either as a consequence of large TL allowing an effect on growth or as a side effect of benign nest conditions. Clearly, more empirical research is needed to assess the generality of the links between TL and longevity in the wild.
References
- 1.Kirkwood TBL, Austad SN. 2000. Why do we age? Nature 408, 233–238 (doi:10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 2.Monaghan P, Haussmann M. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53 (doi:10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 3.Xin H, Liu D, Songyang Z. 2008. The telosome/shelterin complex and its functions. Genome Biol. 9, 232 (doi:10.1186/gb-2008-9-9-232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolzan AD, Bianchi MS. 2006. Telomeres, interstitial telomeric repeat sequences, and chromosomal aberrations. Mutat. Res. 612, 189–214 (doi:10.1016/j.mrrev.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 5.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (doi:10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 6.Horn T, Robertson BC, Gemmell NJ. 2010. The use of telomere length in ecology and evolutionary biology. Heredity 105, 497–506 (doi:10.1038/hdy.2010.113) [DOI] [PubMed] [Google Scholar]
- 7.Baird DM. 2006. Telomeres. Exp. Gerontol. 41, 1223–1227 (doi:10.1016/j.exger.2006.09.009) [DOI] [PubMed] [Google Scholar]
- 8.Horn T, Robertson BC, Will M, Eason DK, Elliott GP, Gemmell NJ. 2011. Inheritance of telomere length in a bird. PLoS ONE 6, e17199 (doi:10.1371/journal.pone.0017199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomarker of oxidative stress? Free Radic. Biol. Med. 44, 235–246 (doi:10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 10.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflect phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 20122540 (doi:10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haussman MF, Winkler DW, Vleck CM. 2005. Longer telomeres associated with higher survival in birds. Biol. Lett. 1, 212–214 (doi:10.1098/rsbl.2005.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683 (doi:10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids . Proc. R. Soc. B 276, 3157–3165 (doi:10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 22, 249–259 (doi:10.1111/mec.12110) [DOI] [PubMed] [Google Scholar]
- 15.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748 (doi:10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner A. 2006. The barn swallow. London, UK: T and AD Poyser [Google Scholar]
- 17.Saino N, Romano M, Ambrosini R, Rubolini D, Boncoraglio G, Caprioli M, Romano A. 2012. Longevity and lifetime reproductive success of barn swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 81, 1004–1012 (doi:10.1111/j.1365-2656.2012.01989.x) [DOI] [PubMed] [Google Scholar]
- 18.Wasser DE, Sherman PW. 2010. Avian longevities and their interpretation under evolutionary theories of senescence. J. Zool. 280, 103–155 (doi:10.1111/j.1469-7998.2009.00671.x) [Google Scholar]
- 19.Foote CG, Vleck D, Vleck CM. 2013. Extent and variability of interstitial telomeric sequences and their effect on estimates of telomere length. Mol. Ecol. Res. 13, 417–428 (doi:10.1111/1755-0998.12079) [DOI] [PubMed] [Google Scholar]
- 20.Foote CG, Gault EA, Nasir L, Monaghan P. 2011. Telomere dynamics in relation to early growth conditions in the wild in the lesser black-backed gull. J. Zool. 283, 203–209 (doi:10.1111/j.1469-7998.2010.00774.x) [Google Scholar]
- 21.Barrett ELB, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell. 10, 913–921 (doi:10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 22.Gorbunova V, Seluanov A. 2009. Coevolution of telomerase activity and body mass in mammals. Mech. Ageing Dev. 130, 3–9 (doi:10.1016/j.mad.2008.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]


