Abstract
The inaccessibility of open ocean habitat and the cryptic nature of small animals are fundamental problems when assessing the distribution of oceanic-stage sea turtles and other marine animals sharing similar life-history traits. Most methods that estimate patterns of abundance cannot be applied in situations that are extremely data limited. Here, we use a movement ecology framework to generate the first predicted distributions for the oceanic stage of the Kemp's ridley sea turtle (Lepidochelys kempii). Our simulations of particle dispersal within ocean circulation models reveal substantial annual variation in distribution and survival among simulated cohorts. Such techniques can help prioritize areas for conservation, and supply inputs for more realistic demographic models attempting to characterize population trends.
Keywords: ocean circulation model, distribution, movement ecology, sea turtle, Gulf of Mexico
1. Introduction
Knowing the distribution of a species and its variability through time is fundamental for successful species conservation and management [1]. The distribution of a species is commonly predicted by correlating occurrence records with environmental metrics to generate maps of habitat suitability or likelihood of occurrence [1,2]. Alternatively, distribution can be estimated from principles of movement ecology theory: the distribution of a species is mechanistically predicted by simulating the movement process of individuals within a realistic environmental model [3]. Compared with more frequently used tools for predicting distribution, for instance ecological niche models [1,2], the movement ecology approach may be particularly useful for species that occupy habitats which preclude sampling or that possess cryptic life-stages, for instance sea turtles [4,5]. In most sea turtle species, the young migrate from beaches into the ocean and are rarely encountered again until they return to coastal waters 2–15 years later [4–6]. However, predicting the distribution of oceanic-stage turtles is possible using particle-tracking software and the output of high-resolution ocean circulation models [7]. Nesting beaches, which serve as initiation points for simulations, are well defined for most species. Although swimming behaviour can affect sea turtle distributions [8], young turtles are relatively weak swimmers [6]; thus, as a first approximation, predictions of early oceanic-stage sea turtle distributions can be generated by simulating particle dispersal from nesting beaches based solely on ocean currents [4–12].
We use this approach to predict the distribution of the Kemp's ridley sea turtle (Lepidochelys kempii) across the Gulf of Mexico and to estimate their early survival. This critically endangered turtle nests almost exclusively in the western Gulf of Mexico, with concentrated nesting occurring in the vicinity of Ranch Nuevo in Tamaulipas, Mexico [13]. As such, the early life history of this species is probably constrained to the Gulf of Mexico [5]. The limited geographical range of these turtles combined with high-resolution ocean circulation models for this region provide a unique opportunity to examine how physical processes at the ocean surface influence the distribution of a life-stage in sea turtles that has been, thus far, impossible to estimate [4,5].
2. Material and methods
To simulate the movement of young (less than 2 years old) Kemp's ridleys, we extracted surface currents from the Gulf of Mexico Global Hybrid Coordinate Ocean Model (GOM HYCOM) [14]; GOM HYCOM output has a spatial resolution of 0.04° (approx. 4.5 km), daily snapshots of velocity and resolves mesoscale processes such as meandering currents, fronts, filaments and eddies [14]. Ichthyop (v. 2.21) particle-tracking software [15] calculated trajectories of virtual particles released within 0.04° × 0.04° cells centred 10 km offshore of nesting sites (table 1 and electronic supplementary material). Locations of hatchling releases were obtained from records for the 2009, 2010 and 2011 Kemp's ridley cohorts (Donna J. Shaver, Padre Island National Seashore, Division of Sea Turtle Science and Recovery; Patrick M. Burchfield and Luis J. Peña, Gladys Porter Zoo; Raul J. Gonzalez, Acuario de Veracruz, Mexico and Rosa C. Martinez Portugal, CONANP de Veracruz, Mexico 2012, personal communication). We used the mean relative abundance of hatchlings released at each location during the months of June, July and August to weight the proportion of particles that were released daily from each location for a particular month (table 1). Simulations were performed for the 2003–2010 cohorts, and particles were tracked for 515 days. ICHTHYOP implemented a Runge–Kutta fourth-order time-stepping method, whereby particle position was calculated every 30 min. Particles that encountered a coastline ‘bounced’ along the coast until currents changed to move them offshore [16]. To simulate the ‘frenzy-period’ of hatchling turtles, particles swam at 0.25 m s−1 in an offshore direction (±20°) during the first 48 h [5,16]; a maximum distance of 43.2 km in still water. The duration of the ‘frenzy-period’ is not described for Kemp's ridleys, this approximation is based on what is known in loggerheads (Caretta caretta) [16]. We determined distribution patterns for a cohort by summing the number of particles within each grid cell at daily intervals throughout the simulation. This highlighted locations that particles encountered, were retained, and, therefore, where Kemp's ridleys likely occur. Age structure across the Gulf was estimated by summing the ages of particles within a cell and dividing by the total number of particles counted within that cell as described above.
Table 1.
Parameters of simulated hatchling release.
| nesting region | latitudinal range | nesting sites | particles released (GOM HYCOM) | particles released (SABGOM) | percent of population (%) |
|---|---|---|---|---|---|
| TX, USA | 26.86–27.44° N | 9 | 88 | 2907 | 1.6 |
| Tamaulipas, Mexico | 22.50–23.77° N | 6 | 5026 | 166 052 | 94.1 |
| Veracruz, Mexico | 18.90–20.48° N | 5 | 228 | 7533 | 4.3 |
To assess the probability of eastward transport, we recorded the proportion of particles that crossed different longitudes (90° W, 85° W and 80° W). We also simulated mortality of particles based on water depths encountered during the first 185 days. Because shallower areas have greater predation risk [5], we specified increasing survival probabilities with increasing depth. At the end of each day, the likelihood of survival for particles in water less than 10 m = 98%, 10–50 m = 99%, 50–100 m = 99.5%, 100–200 m = 99.75% and greater than 200 m = 100% [16]. The actual likelihood of predation is not known; this simplification of mortality risk is meant for illustrative purposes and is therefore not represented in predicted distributions (figures 1 and 2).
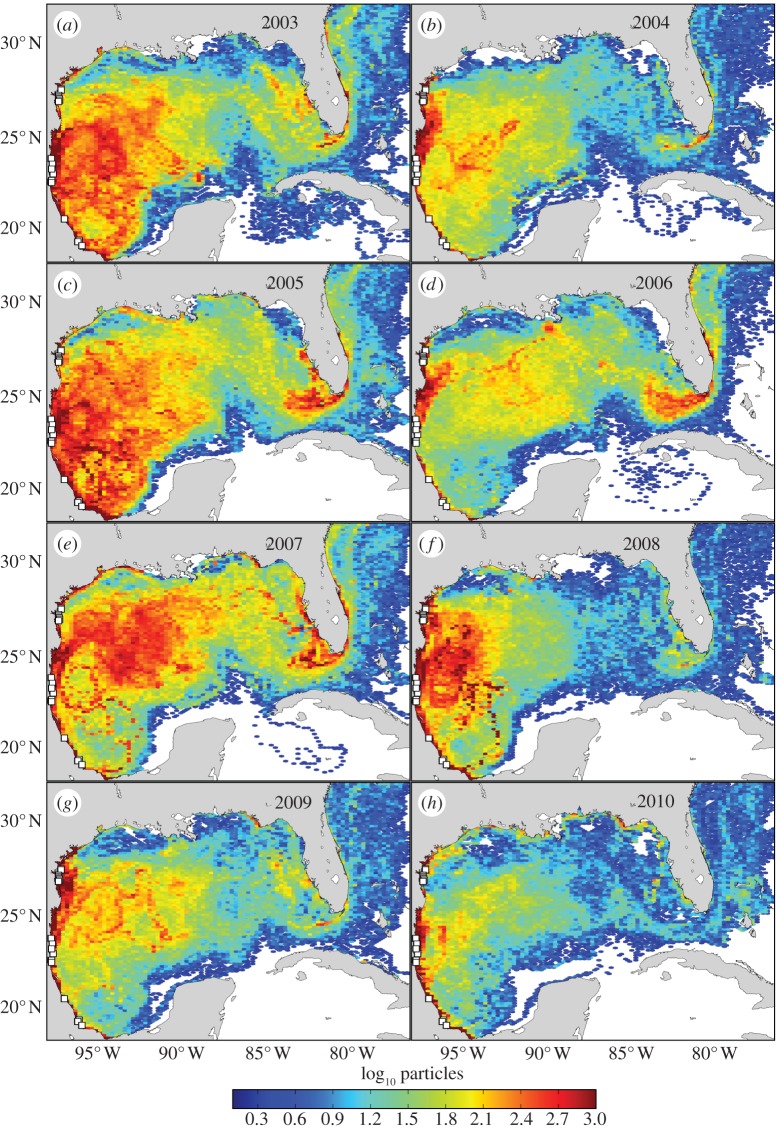
Figure 1.
Predicted abundance of oceanic-stage Kemp's ridley turtles in the Gulf of Mexico by cohort. White squares indicate particle release sites. Coloration is scaled logarithmically and indicates the number of particles within each grid cell throughout the 515-day tracking period for the (a) 2003, (b) 2004, (c) 2005, (d) 2006, (e) 2007, (f) 2008, (g) 2009 and (h) 2010 cohorts.
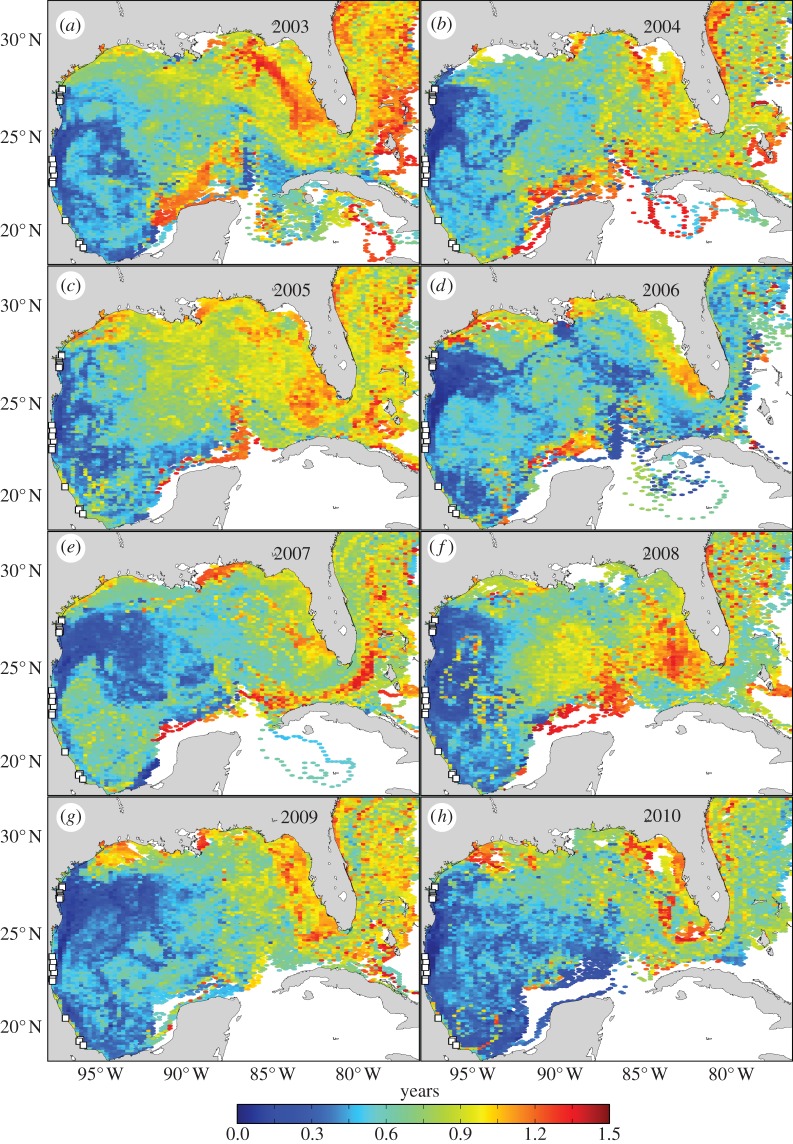
Figure 2.
Predicted age structure of oceanic-stage Kemp's ridley turtles in the Gulf of Mexico by cohort. White squares indicate particle release sites. Coloration indicates the mean age of particles within each grid cell throughout the 515-day tracking period for the (a) 2003, (b) 2004, (c) 2005, (d) 2006, (e) 2007, (f) 2008, (g) 2009 and (h) 2010 cohorts.
Transport predictions are more accurate with finer spatio-temporal resolution of ocean velocity fields [7] and with greater numbers of particles released [17]. We examined the sensitivity of our estimates of eastward transport and survival to these parameters using hindcast output from the South Atlantic Bight–Gulf of Mexico ocean circulation model (SABGOM) [18]. SABGOM has a spatial resolution of 5 km and hourly snapshots of ocean velocity fields from 1 June 2010 to 31 December 2010. We released approximately 33 times as many particles (in the same proportion) from nesting sites (table 1) and calculated their trajectories through 31 December 2010. Finally, we also modified the behaviour of particles encountering coastlines, rather than ‘bouncing’ (which might not depict actual near shore currents) particles in SABGOM simply ‘beached’.
3. Results
Simulations indicate that oceanic-stage Kemp's ridley turtles are likely to be distributed throughout the Gulf of Mexico and into the northwestern Atlantic (figure 1). Highest abundance was predicted in the western Gulf; more than half of all particles for each cohort remained west of 90° W for the entirety of the simulation (table 2). Coastal regions from southern Texas, USA to Tabasco, Mexico consistently had high abundance. Some cohorts also contributed substantially to the coastal waters of southwest Florida, USA and the Florida Keys (figure 1). Mean particle age generally increased from west to east across the Gulf of Mexico (figure 2). At times, however, rapid transport eastward and out of the Gulf occurred (figure 2d and table 2). Variation in predicted distributions resulted in differences in cohort survival spanning an order of magnitude (table 2). Survival and transport predictions appear robust with respect to the modelling parameters that we varied (table 2).
Table 2.
Percentage of particles transported eastward and simulated survival.
| simulation | east of 90° W (%) (eastern Gulf) | east of 85° W (%) (West Florida Shelf) | east of 80° W (%) (Atlantic Ocean) | survival (%) |
|---|---|---|---|---|
| GOM HYCOM 2003, 515 days | 36.7 | 29.1 | 21.2 | 14.0 |
| GOM HYCOM 2004, 515 days | 14.1 | 8.4 | 7.1 | 7.1 |
| GOM HYCOM 2005, 515 days | 43.9 | 32.8 | 20.0 | 17.5 |
| GOM HYCOM 2006, 515 days | 28.0 | 22.2 | 19.4 | 7.1 |
| GOM HYCOM 2007, 515 days | 44.4 | 35.7 | 28.4 | 20.1 |
| GOM HYCOM 2008, 515 days | 8.9 | 6.2 | 5.1 | 7.0 |
| GOM HYCOM 2009, 515 days | 17.6 | 12.3 | 10.1 | 8.0 |
| GOM HYCOM 2010, 515 days | 9.7 | 8.1 | 6.9 | 2.9 |
| GOM HYCOM June–December 2010 | 3.8 | 0.3 | 0.0 | 5.7 |
| SABGOM June–December 2010 | 4.6 | 0.6 | 0.2 | 6.6 |
4. Discussion
Given the limited data on oceanic-stage turtles, these predicted distributions fill a substantial gap regarding sea turtle ecology. These simulations identified locations of possible conservation importance and where field-based research efforts could be focused. The waters offshore of Tamaulipas, Mexico may serve as a nursery area for numerous Kemp's ridleys less than 1 year old (figures 1 and 2). Older turtles, nearing the age when recruitment to near shore waters occurs, are more likely to be distributed in the northern Gulf, eastern Gulf and western Atlantic (figure 2). Anthropogenic stressors to these regions, such as oil spills and incidental take in fisheries, may be particularly detrimental to the Kemp's ridley's population growth [14]. By contrast, young Kemp's ridleys are less likely to be found in the Caribbean Sea and on the Campeche Bank; potential threats in these areas might be less problematic to this life stage (figure 1).
Although our modelling assumptions necessarily simplify the behaviour of young turtles, the predictions are consistent with available in situ observations. Proposed hatchling dispersal trajectories based on surface drifters [5] were observed within our models (table 2). Likewise, sporadic reports of numerous small juveniles stranding along the Texas, USA coast [19] or caught in the waters of Alabama, USA [4] imply that the number of Kemp's ridleys entering these regions varies substantially, as predicted by our simulations (figure 1). Witherington et al. captured 38 Kemp's ridleys in the pelagic Saragassum community on the West Florida Shelf. These turtles had a mean straight carapace length of 233 mm (range: 175–276 mm) corresponding to approximately 1–2 years of age [6]. Our simulations suggest that turtles could reach these waters in less than a year—with the average age of particles around 1.2 years. Particles (or turtles) entering the West Florida Shelf would probably be retained by surface currents [16,20], which might facilitate turtles' ontogenetic shift to coastal foraging grounds in this region [20].
Our analyses imply that variation in the oceanic-stage distribution among cohorts (driven by variation in ocean circulation) strongly affects predicted survival and may, therefore, profoundly influence population trends of this species. Demographic models typically assume that natural mortality is constant from year to year for oceanic-stage Kemp's ridleys; variations in population trends are often attributed to anthropogenic factors [13]. Using spatially explicit and temporally variable estimates of survival as inputs for demographic models could help quantify the role of anthropogenic and environmental factors influencing population abundance. Further development of these models in tandem with empirical data on sea turtle ecology (e.g. swimming and diving behaviour, natural and anthropogenic mortality) hold considerable promise in aiding the conservation of this and other species that share similar life-history traits.
Acknowledgements
Padre Island National Seashore, Division of Sea Turtle Science and Recovery; Patrick Burchfield and Jaime Peña, Gladys Porter Zoo; Raul de J. Gonzalez Diaz Miro, Acuario de Veracruz and Rosa Ciria Martinez Portugal, CONAP de Veracruz provided hatchling production information.
Funding statement
K.L.M. was supported by the NOAA Oil Spill Supplemental Spend Plan. R.H. was supported by NASA grant no. 09-IDS09-0040, NOAA grant no. IOOS-2011-2002515 and GRI GISR grant no. 12-09/GoMRI-006.
References
- 1.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 2.Beaugrand G, Lenoir S, Ibanez F, Mante C. 2011. A new model to assess the probability of occurrence of a species based on presence-only data. Mar. Ecol. Prog. Ser. 424, 175–190 (doi:10.3354/meps08939) [Google Scholar]
- 3.Nathan R. 2008. An emerging movement ecology paradigm. Proc. Natl Acad. Sci. USA 105, 19 050–19 051 (doi:10.1073/pnas.0808918105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr A. 1980. Some problems of sea turtle ecology. Am. Zool. 280, 489–498 [Google Scholar]
- 5.Collard SB, Ogren LH. 1990. Dispersal scenarios for pelagic post-hatchling sea turtles. Bull. Mar. Sci. 47, 233–243 [Google Scholar]
- 6.Witherington B, Hirama S, Hardy R. 2012. Young sea turtles of the pelagic Sargassum-dominated drift community: habitat use, population density and threats. Mar. Ecol. Prog. Ser. 463, 1–22 (doi:10.3354/meps09970) [Google Scholar]
- 7.Putman NF, He R. 2013. Tracking the long-distance dispersal of marine organisms: sensitivity to ocean model resolution. J. R. Soc. Interface 10, 20120979 (doi:10.1098/rsif.2012.0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putman NF, Verley P, Shay T, Lohmann KJ. 2012. Simulating transoceanic migrations of young loggerhead sea turtles: merging magnetic navigation behavior with an ocean circulation model. J. Exp. Biol. 215, 1863–1870 (doi:10.1242/jeb.067587) [DOI] [PubMed] [Google Scholar]
- 9.Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G. 2010. Ontogenetic development of migration: Lagrangian drift trajectories suggest a new paradigm for sea turtles. J. R. Soc. Interface 7, 1319–1327 (doi:10.1098/rsif.2010.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamann M, Grech AB, Wolanski E, Lambrechts J. 2011. Modelling the fate of marine turtle hatchlings. Ecol. Model. 222, 1515–1521 (doi:10.1016/j.ecolmodel.2011.02.003) [Google Scholar]
- 11.Shillinger GL, Di Lorenzo E, Luo H, Bograd SJ, Hazen EL, Bailey H, Spotila JR. 2012. On the dispersal of leatherback turtle hatchlings from Mesoamerican nesting beaches. Proc. R. Soc. B 279, 2391–2395 (doi:10.1098/rspb.2011.2348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspar P, Benson SR, Dutton PH, Reveillere A, Jacob G, Meetoo C, Dehecq A, Fossette S. 2012. Oceanic dispersal of juvenile leatherback turtles: going beyond passive drift modeling. Mar. Ecol. Prog. Ser. 457, 265–284 [Google Scholar]
- 13.Crowder L, Heppell S. 2011. The decline and rise of a sea turtle: how Kemp's ridleys are recovering in the Gulf of Mexico. Solutions 2, 67–73 See http://thesolutionsjournal.anu.edu.au/node/859 [Google Scholar]
- 14.Chassignet EP, Hurlburt HE, Smedstad OM, Halliwell GR, Hogan PJ, Wallcraft AJ, Baraille R, Bleck R. 2007. The HYCOM (Hybrid Coordinate Ocean Model) data assimilative system. J. Mar. Syst. 65, 60–83 (doi:10.1016/j.jmarsys.2005.09.016) [Google Scholar]
- 15.Lett C, Verley P, Mullon C, Parada C, Brocier T, Penven P, Blanke B. 2007. A Lagrangian tool for modelling ichthyoplankton dynamics. Environ. Model. Softw. 23, 1210–1214 (doi:10.1016/j.envsoft.2008.02.005) [Google Scholar]
- 16.Putman NF, Scott R, Verley P, Marsh R, Hays GC. 2012. Natal site and offshore swimming influence fitness and long-distance ocean transport in young sea turtles. Mar. Biol. 159, 2117–2126 (doi:10.1007/s00227-012-1995-5) [Google Scholar]
- 17.Simons RD, Siegel DA, Brown KS. 2013. Model sensitivity and robustness in estimation of larval transport: a study of particle tracking parameters. J. Mar. Syst. 119–120, 19–29 (doi:10.1016/j.jmarsys.2013.03.004). [Google Scholar]
- 18.North EW, Adams EE, Schlag Z, Sherwood CR, He R, Hyun KH, Socolofsky SA. 2011. Simulating oil droplet dispersal from the Deepwater Horizon spill with a Lagrangian approach. Geophys. Monogr. Ser. 195, 217–226 (doi:10.1029/2011GM001102) [Google Scholar]
- 19.Zimmerman R. 1998. Characteristics and causes of Texas marine strandings. NOAA Technical Report NMFS 143, pp. 85, US Department of Commerce, Washington, DC, USA [Google Scholar]
- 20.Putman NF, Shay TJ, Lohmann KJ. 2010. Is the geographic distribution of nesting in the Kemp's ridley sea turtle shaped by the migratory needs of offspring? Integr. Comp. Biol. 50, 305–314 (doi:10.1093/icb/icq041) [DOI] [PubMed] [Google Scholar]