Abstract
Macroecology strives to identify ecological patterns on broad spatial and temporal scales. One such pattern, Rapoport's rule, describes the tendency of species' latitudinal ranges to increase with increasing latitude. Several mechanisms have been proposed to explain this rule. Some invoke climate, either through glaciation driving differential extinction of northern species or through increased seasonal variability at higher latitudes causing higher thermal tolerances and subsequently larger ranges. Alternatively, continental tapering or higher interspecific competition at lower latitudes may be responsible. Assessing the incidence of Rapoport's rule through deep time can help to distinguish between competing explanations. Using fossil occurrence data from the Palaeobiology Database, we test these hypotheses by evaluating mammalian compliance with the rule throughout the Caenozoic of North America. Adherence to Rapoport's rule primarily coincides with periods of intense cooling and increased seasonality, suggesting that extinctions caused by changing climate may have played an important role in erecting the latitudinal gradients in range sizes seen today.
Keywords: Rapoport's rule, Caenozoic, macroecology, mammals, latitude, geographical range
1. Introduction
Species at higher latitudes tend to have larger latitudinal ranges. This pattern, called Rapoport's rule, was first formally identified to help explain another latitudinal trend in species distributions, that of increased species diversity at lower latitudes [1]. Since its conception, the generality of the rule has been called into question. Critics argue that Rapoport's rule is a local phenomenon [2], applicable to some regions and not others. Indeed, while Rapoport's rule is well evidenced for a wide variety of organisms in the temperate north [1,3,4], including arthropods, reptiles, mammals, birds, plants and pathogens [3–9], support for the rule is scarcer in tropical regions and the Southern Hemisphere [2,3,8].
Suggested explanations for Rapoport's rule include decreased land area closer to the equator [3,10], increased interspecific competition from greater species diversity at lower latitudes [3,5], differential rates of glacially driven extinction at higher latitudes [2,3,10], and increased climatic variability and thermal tolerance at higher latitudes [1,10,11]. These mechanisms are not mutually exclusive, and several may apply simultaneously in different ecological settings. However, we should observe Rapoport's rule: (i) in every epoch if the tapering continent [3,10] or high interspecific competition at low latitudes [3,5] primarily drives Rapoport's rule, as narrower North American southern land areas and latitudinal species diversity gradients have been present throughout the Caenozoic [12,13], though the latter strengthened closer to the present day [13] and do not appear to be ubiquitous for mammals through deep time [14]; (ii) in the Oligocene and/or Late Miocene onwards, if seasonality drives Rapoport's rule, as those epochs mark increasing seasonality [15,16] or (iii) in the Pleistocene alone, if differential extinction by Pleistocene glaciation drives Rapoport's rule.
We examine when Rapoport's rule holds for North American non-volant terrestrial mammals during the Caenozoic. Specifically, we test the presence or the absence of Rapoport's rule during the Palaeocene (66.0–56.0 Ma), Eocene (56.0–33.9 Ma), Oligocene (33.9–23.0 Ma), Miocene (23.0–5.3 Ma), Pliocene (5.3–2.6 Ma) and Pleistocene (2.6–0.01 Ma) epochs using approximately 35 000 individual fossil occurrences recorded in the Palaeobiology Database [17,18]. We also assess Rapoport's rule throughout the Caenozoic using North American land mammal age (NALMA) time bins [19]. By determining when Rapoport's rule occurs, we can evaluate potential causal mechanisms.
2. Material and methods
All Caenozoic mammalian occurrences were downloaded from the Palaeobiology Database on 12 March 2012 [17]. A total of 34 969 individual species occurrences were used in our analysis (Palaeocene, 3777; Eocene, 11 823; Oligocene, 2669; Miocene, 7568; Pliocene, 1890; Pleistocene, 7242). Each occurrence is based on the presence of one or more specimens identified to a given species per temporal unit [17]. As the Palaeobiology Database has epoch categorizations based on the Pliocene/Pleistocene boundary of 1.8 Ma, we instead sorted by midpoint age and reclassified occurrences according to a revised Pleistocene age of 2.6 Ma [18]. When a midpoint age was identical to a boundary date (e.g. 2.6 Ma) and the total age range was equally split between two epochs, the epoch assigned previously was given precedence.
Data were also sorted and analysed using NALMA time bins, with NALMA assignments made using minimum and maximum age estimates (noted in the Palaeobiology Database) if taxon-locality pairs lacked NALMA designations (NALMA age ranges are given in table 1). Species occurrences that spanned two NALMAs were assigned to both, whereas those spanning more than two were removed owing to lack of temporal resolution.
Table 1.
Results summarizing two-tailed linear regressions and Spearman's rank correlations between palaeolatitude midpoint and range for each NALMA of the Caenozoic.
| NALMA | max. age (Ma) | min. age (Ma) | n | linear regression |
Spearman's rank correlation |
|||
|---|---|---|---|---|---|---|---|---|
| slope | R2 | p-value | ρ | p-value | ||||
| Holocene | 0.012 | 0 | 9 | 0.58 | 0.21 | 0.22 | −0.09 | 0.81 |
| Rancholabrean | 0.3 | 0.012 | 117 | 1.02 | 0.44 | <0.0001* | 0.58 | <0.0001* |
| Irvingtonian | 1.8 | 0.3 | 100 | 0.38 | 0.06 | 0.01* | 0.05 | 0.64 |
| Blancan | 4.9 | 1.8 | 81 | 0.44 | 0.05 | 0.04* | 0.15 | 0.18 |
| Hemphillian | 10.3 | 4.9 | 50 | −0.20 | 0.02 | 0.28 | −0.18 | 0.22 |
| Clarendonian | 13.6 | 10.3 | 53 | −0.65 | 0.23 | <0.001* | −0.45 | 0.001* |
| Barstovian | 15.97 | 13.6 | 70 | −0.60 | 0.20 | <0.0001* | −0.27 | 0.02* |
| Hemingfordian | 20.43 | 15.97 | 41 | −1.29 | 0.58 | <0.0001* | −0.66 | <0.0001* |
| Arikareean | 30.8 | 20.43 | 58 | −1.12 | 0.33 | <0.0001* | −0.61 | <0.0001* |
| Whitneyan | 33.3 | 30.8 | 7 | 2.17 | 0.80 | <0.01* | 0.61 | 0.17 |
| Orellan | 33.9 | 33.3 | 35 | 0.93 | 0.16 | 0.02* | 0.63 | <0.0001* |
| Chadronian | 37.2 | 33.9 | 40 | −1.58 | 0.53 | <0.0001* | −0.41 | 0.01* |
| Duchesnean | 40.4 | 37.2 | 8 | −0.44 | 0.11 | 0.41 | 0.24 | 0.58 |
| Uintan | 46.2 | 40.4 | 30 | 1.04 | 0.33 | <0.001* | 0.53 | 0.002* |
| Bridgerian | 50.3 | 46.2 | 56 | −1.72 | 0.84 | <0.0001* | −0.57 | <0.0001* |
| Wasatchian | 55.8 | 50.3 | 126 | −0.30 | 0.02 | 0.09 | −0.83 | <0.0001* |
| Clarkforkian | 56.8 | 55.8 | 9 | −2.13 | 0.98 | <0.0001* | −0.58 | 0.11 |
| Tiffanian | 61.7 | 56.8 | 50 | −0.56 | 0.10 | 0.03* | −0.07 | 0.6111535 |
| Torrejonian | 63.3 | 61.7 | 26 | −0.18 | 0.02 | 0.48 | 0.08 | 0.68 |
| Puercan | 65.5 | 63.3 | 17 | −0.57 | 0.18 | 0.09 | −0.54 | 0.03* |
*Significant p-values (p < 0.05).
We determined the palaeolatitudinal range and midpoint of each species' maximum and minimum palaeolatitude in each time bin. Our method is comparable with Rohde's midpoint method [20], but differs in that each species is treated as a separate data point, similar to recent methods used to test Rapoport's rule (e.g. [9]). As the fossil record may fail to capture the total latitudinal range of all species, we also examined relationships between palaeolatitude mean and standard deviation. If multiple occurrences of a species appeared in a given locality for a given time bin, all but one of those occurrences were excluded. Best model, two-tailed linear regressions were performed using XLSTAT. Spearman's rank correlations were also performed to ensure assessment of nonlinear monotonic relationships.
Volant and marine mammals (i.e. members of the orders Chiroptera, Sirenia and Cetacea; the families Odobenidae, Otariidae and Phocidae; and the genus Enhydra) were excluded from this analysis. Species not appearing at 10 or more unique localities in an epoch or 5 or more unique localities in a NALMA were also excluded.
3. Results
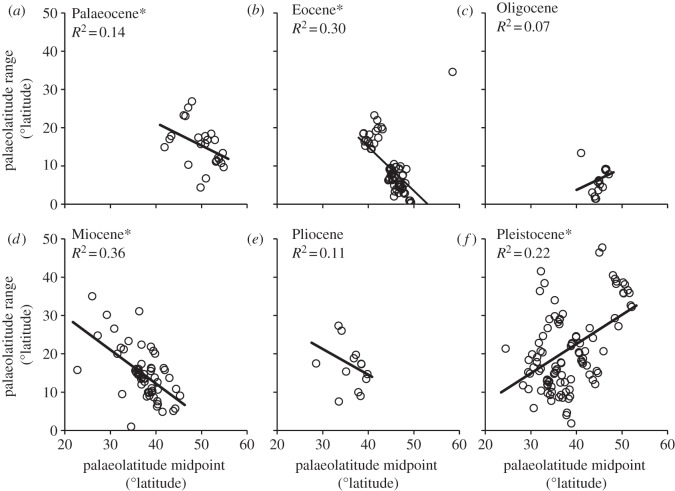
Rapoport's rule is not consistently observed in North American terrestrial mammals through time (figure 1a–f and table 1). Instead, the Pleistocene is the only epoch where linear regression of midpoint and range is both positive and significant (p < 0.05; figure 1; see the electronic supplementary material, table S1), though regressions of mean and standard deviation are positive and significant in both the Pleistocene and Oligocene (see the electronic supplementary material, table S2). Spearman's rank correlations demonstrate significant positive relationships during the Pleistocene and Oligocene for both midpoints/ranges and means/standard deviations (see the electronic supplementary material, tables S1 and S2).
Figure 1.
Palaeolatitude midpoint versus range for all species with 10 or more unique localities in each epoch (a–f), with trend lines and subsequent R2 values noted (asterisk (*) denotes statistically significant regressions, p < 0.05).
NALMA subdivisions provide increased temporal resolution and yield significant positive relationships between species’ midpoints and ranges during the Rancholabrean, Irvingtonian, Blancan, Whitneyan, Orellan and Uintan (table 1). Mean and standard deviation regressions are consistent during all NALMAs noted above except for the Blancan (see the electronic supplementary material, table S3). Spearman's rank correlations are positive and significant in the Rancholabrean, Orellan and Uintan for midpoint and range (table 1), and in the Rancholabrean, Orellan, Tiffanian and Torrejonian for mean and standard deviation (see the electronic supplementary material, table S3).
As Pleistocene fossil identification is often informed by what is currently living in the region [21], we tested Rapoport's rule separately for extinct and extant species in the Pleistocene epoch and the Rancholabrean and Irvingtonian NALMAs to ensure that observed fossil trends were not biased by modern distributions. Both extant and extinct species demonstrate Rapoport's rule in the Pleistocene and Rancholabrean (for both regression and Spearman's rank correlation of mean/standard deviation and midpoint/range), and only extinct species in the Irvingtonian (for regressions of mean/standard deviation and midpoint/range), suggesting that observed patterns throughout the Pleistocene are not merely reflecting patterns in the present (see the electronic supplementary material, tables S4 and S5).
4. Discussion
North American continental geography has not changed considerably during the Caenozoic. Specifically, narrower land areas in Central America have been apparent since the Jurassic [12]. Thus, failure to observe Rapoport's rule during all well-sampled time periods (table 1) suggests that reduced continent width at lower latitudes does not play a major mechanistic role, consistent with prior work [3]. Changes in interspecific competition are more difficult to assess through time. The fossil record evinces latitudinal gradients in diversity since the Palaeozoic, though mostly for marine fauna, with the gradient strengthening during the Caenozoic [13]. North American terrestrial mammals in the Palaeocene, however, do not increase in richness at the equator [14]. The temporal ubiquity of latitudinal diversity gradients must be better ascertained before firm conclusions regarding the interspecific competition hypothesis can be drawn, though we might still expect greater adherence to Rapoport's rule through deep time than observed.
Rapoport's rule may have been influenced by the onset of Pleistocene glaciations, as previously suggested [2,3,10]. Broadly speaking, the Caenozoic is a time of rapidly changing climate. Its sharpest global cooling event occurs at the Eocene–Oligocene transition 33.9 Ma [15,22], and full-scale Northern Hemisphere ice sheets are present only during the rapid cooling of the Late Pliocene and Pleistocene [22]. Of the six NALMA midpoint/range regressions that adhere to Rapoport's rule, two coincide with cooling at the Eocene–Oligocene transition and three with Plio–Pleistocene cooling and/or Pleistocene glaciations, possibly implicating rapid cooling—and the complex interactions of species and climate that result—as a causal mechanism for Rapoport's rule. As mean annual temperatures are colder and seasonal variation in temperature greater at higher latitudes, cooling effects would have been more pronounced in northern North America, and species with smaller ranges would have faced higher chance of extinction (perhaps having to disperse longer distances to find suitable habitat) [10,23]. As the Uintan corresponds to a time of climatic stability [22,24], further work is needed to clarify why Rapoport's rule occurred then (table 1).
High temperature seasonality may also have contributed to Rapoport's rule. Temperature seasonality dramatically increased at the Eocene/Oligocene boundary [15] and from the Late Miocene to the present [16,25], matching closely with cooling climates and the presence of Rapoport's rule through time. Higher seasonality at higher latitudes would result in species capable of tolerating a wider array of climatic conditions, enabling them to disperse farther north and south and across elevational barriers whose summer conditions at high elevations are no more severe than winters in the lowlands [1,10,11]. As Rapoport's rule is not observed in all time periods typified by increased seasonality, synergy between declining temperatures and increasing seasonality may have caused the larger latitudinal ranges of species at higher latitudes noted today.
Sampling efforts throughout the Caenozoic have varied considerably [26], and different geographical regions are better sampled during different time periods [27]. For example, terrestrial mammal fossil excavations in the Palaeocene and Eocene focus largely on the Western Interior of North America (e.g. Bighorn Basin, WY, USA), while post-Oligocene faunas are more broadly sampled. Additionally, biases arising from heterogeneity of taxonomic efforts throughout the Caenozoic may have influenced our analysis. Taxonomic revisions in well-studied time periods (e.g. primates of the Early Eocene) may decrease species' range size relative to less-studied time periods (e.g. the Oligocene) [28]. To mitigate biases resulting from differential taxonomic focus and collecting effort, we only considered species appearing at 5 or more and 10 or more unique localities under NALMA and epoch-scale bins, respectively. While we advise caution when interpreting analyses from less studied time bins, Rapoport's rule is clearly not ubiquitous throughout the Caenozoic.
Acknowledgements
We are grateful to the Palaeobiology Database, including J. Alroy, the major contributor of fossil data used in this paper. We would also like to thank J. Gillooly and anonymous reviewers for comments on earlier versions of this manuscript.
Data accessibility
This is Palaeobiology Database publication 186.
Funding statement
We are grateful to Vanderbilt University for financial support.
References
- 1.Stevens GC. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256 (doi:10.1086/284913) [Google Scholar]
- 2.Rohde K. 1996. Rapoport's rule is a local phenomenon and cannot explain latitudinal gradients in species diversity. Biodivers. Lett. 3, 10–13 (doi:10.2307/2999704) [Google Scholar]
- 3.Gaston KJ, Blackburn TM, Spicer JI. 1998. Rapoport's rule: time for an epitaph? Trends Ecol. Evol. 13, 70–74 (doi:10.1016/S0169-5347(97)01236-6) [DOI] [PubMed] [Google Scholar]
- 4.Letcher AJ, Harvey PH. 1994. Variation in geographical range size among mammals of the Palearctic. Am. Nat. 144, 30–42 (doi:10.1086/285659) [Google Scholar]
- 5.France R. 1992. The North American latitudinal gradient in species richness and geographical range of freshwater crayfish and amphipods. Am. Nat. 139, 342–354 (doi:10.1086/285330) [Google Scholar]
- 6.Hecnar SJ. 1999. Patterns of turtle species’ geographic range size and a test of Rapoport's rule. Ecography 22, 436–446 (doi:10.1111/j.1600-0587.1999.tb00580.x) [Google Scholar]
- 7.Price TD, Helbig AJ, Richman AD. 1997. Evolution of breeding distributions in the Old World leaf warblers (genus Phylloscopus). Evolution 51, 552–561 (doi:10.2307/2411127) [DOI] [PubMed] [Google Scholar]
- 8.Mourelle C, Ezcurra E. 1997. Rapoport's rule: a comparative analysis between South and North American columnar cacti. Am. Nat. 150, 131–142 (doi:10.1086/286060) [DOI] [PubMed] [Google Scholar]
- 9.Guernier V, Guégan J-F. 2009. May Rapoport's rule apply to human associated pathogens? Ecohealth 6, 509–521 (doi:10.1007/s10393-010-0290-5) [DOI] [PubMed] [Google Scholar]
- 10.Brown JH. 1995. Macroecology, pp. 112–116 Chicago, IL: University of Chicago Press [Google Scholar]
- 11.Fernández MH, Vrba ES. 2005. Rapoport effect and biomic specialization in African mammals: revisiting the climatic variability hypothesis. J. Biogeogr. 32, 903–918 (doi:10.1111/j.1365-2699.2004.01188.x) [Google Scholar]
- 12.Scotese CR. 2004. A continental drift flipbook. J. Geol. 112, 729–741 (doi:10.1086/424867) [Google Scholar]
- 13.Crame JA. 2001. Taxonomic diversity gradients through geological time. Divers. Distrib. 7, 175–189 (doi:10.1046/j.1472-4642.2001.00106.x) [Google Scholar]
- 14.Rose PJ, Fox DL, Marcot J, Badgley C. 2011. Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology 39, 163–166 (doi:10.1130/G31099.1) [Google Scholar]
- 15.Eldrett JS, Greenwood DR, Harding IC, Huber M. 2009. Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature 459, 969–973 (doi:10.1038/nature08069) [DOI] [PubMed] [Google Scholar]
- 16.Utescher T, Mosbrugger V, Ashraf AR. 2000. Terrestrial climate evolution in northwest Germany over the last 25 million years. Palaios 15, 430–449 (doi:10.1669/0883-1351(2000)015<0430:TCEING>2.0.CO;2) [Google Scholar]
- 17.Alroy J. 2012. Paleobiology database online archives. See http://paleodb.org [Google Scholar]
- 18.Walker JD, Geissman JW. 2009. GSA geologic time scale. GSA Today 19, 60 (doi:10.1130/1052-5173-19.4-5.60) [Google Scholar]
- 19.Woodburne MO. 1987. A prospectus of the North American Mammal Ages. In Cenozoic mammals of North America (ed. Woodburne MO.), pp. 285–290 Berkeley, CA: University of California Press [Google Scholar]
- 20.Rohde K, Heap M, Heap D. 1993. Rapoport's rule does not apply to marine teleosts and cannot explain latitudinal gradients in species richness. Am. Nat. 142, 1–16 (doi:10.1086/285526) [Google Scholar]
- 21.Bell CJ, Gauthier JA, Bever GS. 2010. Covert biases, circularity, and apomorphies: a critical look at the North American quaternary herpetofaunal stability hypothesis. Quat. Int. 217, 30–36 (doi:10.1016/j.quaint.2009.08.009) [Google Scholar]
- 22.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 23.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952 (doi:10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood DR, Wing SL. 1995. Eocene continental climates and latitudinal temperature gradients. Geology 23, 1044–1048 (doi:10.1130/0091-7613(1995)023<1044:ECCALT>2.3.CO;2) [Google Scholar]
- 25.Bernard A, Daux V, Lécuyer C, Brugal J-P, Genty D, Wainer K, Gardien V, Fourel F, Jaubert J. 2009. Pleistocene seasonal temperature variations recorded in the δ18O of Bison priscus teeth. Earth Planet. Sci. Lett. 283, 133–143 (doi:10.1016/j.epsl.2009.04.005) [Google Scholar]
- 26.Alroy J. 1996. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 285–311 (doi:10.1016/S0031-0182(96)00100-9) [Google Scholar]
- 27.Alroy J, Koch PL, Zachos JC. 2000. Global climate change and North American mammalian evolution. Paleobiology. 26, 259–288 (doi:10.1666/0094-8373(2000)26[259:GCCANA]2.0.CO;2) [Google Scholar]
- 28.Alroy J. 2002. How many named species are valid? Proc. Natl Acad. Sci. USA 99, 3706–3711 (doi:10.1073/pnas.062691099) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is Palaeobiology Database publication 186.