Abstract
By having phenotypically plastic traits, many organisms optimize their fitness in response to fluctuating threats. Freshwater snails with translucent shells, e.g. snails from the Radix genus, differ considerably in their mantle pigmentation patterns, with snails from the same water body ranging from being completely dark pigmented to having only a few dark patterns. These pigmentation differences have previously been suggested to be genetically fixed, but we propose that this polymorphism is owing to phenotypic plasticity in response to a fluctuating environment. Hence, we here aimed to assess whether common stressors, including ultraviolet radiation (UVR) and predation, induce a plastic response in mantle pigmentation patterns of Radix balthica. We show, in contrast to previous studies, that snails are plastic in their expression of mantle pigmentation in response to changes in UVR and predator threats, i.e. differences among populations are not genetically fixed. When exposed to cues from visually hunting fish, R. balthica increased the proportion of their dark pigmentation, suggesting a crypsis strategy. Snails increased their pigmentation even further in response to UVR, but this also led to a reduction in pattern complexity. Furthermore, when exposed to UVR and fish simultaneously, snails responded in the same way as in the UVR treatment, suggesting a trade-off between photoprotection and crypsis.
Keywords: inducible defence, phenotypic plasticity, pigmentation patterns, ultraviolet, predator threat
1. Introduction
In nature, organisms have to cope with environments that fluctuate both spatially and temporally and this can lead to situations where they are simultaneously exposed to multiple fluctuating stressors. Accordingly, many organisms have evolved different adaptations to deal with such changes and show phenotypic plasticity in behavioural, morphological or life-history traits in response to changing environments. However, theory predicts that these adaptive traits come with a cost (e.g. production and fitness costs), because they are not expressed in the absence of these threats [1]. By expressing phenotypic plasticity in different traits, organisms may optimize their fitness in response to simultaneously occurring abiotic and biotic threats, such as ultraviolet radiation (UVR) and predation [2]. High levels of UVR have severe consequences for organisms, including effects on growth, behaviour and DNA damage [3,4]. However, the ability to induce photoprotective pigmentation can counteract the negative effects of UVR [5–7]. Risk of predation is another environmental threat that may affect the level of pigmentation individuals express. For example, fishes, crustaceans and gastropods may change their pigmentation so that they resemble the substrate colour in a variable environment, a cryptic strategy that reduces predation risk by background matching [8–10].
Freshwater snails with translucent shells often differ in their mantle pigmentation patterns, e.g. snails from the genus Radix can range from being completely dark pigmented to phenotypes having only a few dark patterns [11]. This variation has previously been attributed to genetically fixed differences among species or populations [11]. It is well known that Radix balthica is polymorphic for a number of other traits, including shell and genital shapes [11], but also that they show phenotypic plasticity in behaviour [12], shell shape and life history in response to chemical cues from predators [1]. Mantle pigmentation may be another trait that is phenotypically plastic in Radix snails, as well as in other snails with translucent shells. However, there are to our knowledge no suggestions in the literature regarding a mechanism behind such differences. Hence, we aimed to assess whether R. balthica variation in mantle pigmentation is an adaptive, plastic response to two common stressors, UVR and predation. We hypothesized that: (i) UVR would increase pigmentation in the snail's mantle, and (ii) snails would also increase both the amount of pigmentation and the complexity of their patterns in the presence of fish.
2. Material and methods
Approximately 300 snails (R. balthica) were collected in a pond close to Lund, southern Sweden and brought to the laboratory to reproduce in three 8 l tanks. One week after the eggs had hatched, snails were collected and five snails were randomly assigned to each experimental container.
To study how predatory fish and UVR affect mantle pigmentation, we used 32 transparent plastic 2 l containers, placed on a dark brown bench and aerated. Each container was equipped with a refuge (a white ceramic tile (10 × 10 cm) raised 12 mm from the bottom using rubber legs). The four treatments, control, fish, UV and UV + fish, were replicated eight times. All snails were fed spinach leaves ad libitum. After eight weeks, we terminated the experiment, and mantle pigmentation was quantified in all snails (see below). The experiment was conducted at 20°C and in a 12 L : 12 D cycle.
(a). Predator treatment
The predator chemical cue was produced in a 70 l tank where we kept three crucian carp (Carassius carassius), which were fed six crushed snails (R. balthica) three times per week. We collected 0.1 l of tank water as the predator chemical cue and added this to the fish and UV + fish treatments twice a week. As a control, the same amount of tap water was added to the control and UV treatments.
(b). UV treatment
The UVR was produced by six fluorescent lamps (UV-A-340; Q-Panel, Cleveland, OH, USA) that were mounted 0.6 m above the experimental aquaria. The control and fish aquaria were covered with Plexiglas lids (Röhm GS 233, Φ 20 cm), which cut off radiation below approximately 370 nm, but allowed visual light (photosynthetically active radiation 400–700 nm) to go through. The UV and UV + fish aquaria were covered with UV-transparent Plexiglas lids (Röhm GS 2458, Φ 20 cm), letting through radiation down to a wavelength of 270 nm. There was no difference in transmittance between the two Plexiglas types, for more detailed information on spectral irradiance of fluorescent lamps and transmittance by the different Plexiglas lids, see [5].
(c). Pigment measurements
At the end of the experiment, snails were photographed from the dorsal view (Canon 350D with Sigma 150 mm f/2.8 APO Macro lens). Prior to photographing the camera was calibrated for white balance with a grey card (Lastolite Ezybalance 12%, 30 cm). In order to analyse mantle coloration as accurately as possible, the photos were stored as RAW files (.CR2 format) and prior to analysis they were converted to 8 bit binary pictures. To quantify mantle pigmentation, all pictures were adjusted for dark and white thresholds and mantle area. Threshold values between 0 and 93 were set as ‘dark’, whereas 93–255 were considered ‘light’ (with 0 being true black and 255 true white), because these values best corresponded with the pigmented and the unpigmented parts of the mantle. To quantify camouflage, we calculated the pigmentation complexity using the index of roundness [13]. The ratio of dark : light per mantle area and the pigmentation complexity were then analysed using ImageJ [14]. The effects of fish and UV on R. balthica mantle pigmentation were tested with nested two-way ANOVAs (aquaria are nested within the interaction term, UV × fish), followed by a Tukey's HSD test. All data met the assumptions of parametric tests. Data were deposited in the Dryad repository [15].
3. Results
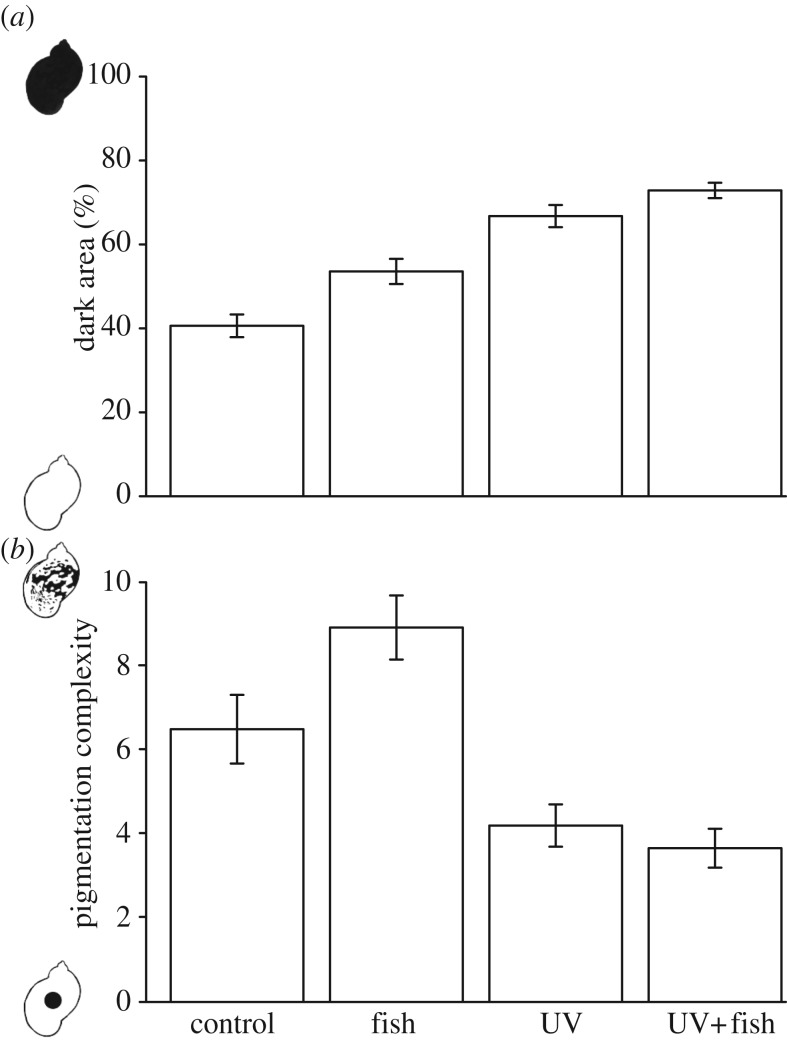
The coverage of dark pigmentation on the mantle increased in response to both fish (nested two-way ANOVA, F1,28 = 7.771, p = 0.009) and UV (F1,28 = 44.120, p < 0.001), but there was no significant interaction between UV and fish (F1,28 = 0.981, p = 0.33; figures 1 and 2a). The post hoc analysis showed that snails in the controls were significantly less pigmented compared with snails in the UV and UV + fish treatments (p < 0.001 and p < 0.001, respectively). Snails exposed to the control treatment were also marginally significantly less pigmented than snails in the fish treatment (p = 0.059). Snails exposed to fish were significantly less pigmented than snails in the UV + fish treatment (p = 0.003) and snails in the UV treatment (p = 0.05). However, there was no significant difference in dark pigmentation coverage between UV and UV + fish (p = 0.6).
Figure 1.

Pictures of freshwater snail R. balthica exposed to (from left) control, fish, UV or UV + fish. Individuals in the figure represent the mean pigment value for each treatment. Bar represents 0.1 cm. (Online version in colour.)
Figure 2.
(a) Percent of mantle area covered in dark pigment and (b) pigmentation complexity in freshwater snail R. balthica exposed to control, fish, UV or UV + fish. Values are means ± 1s.e.
The pigmentation complexity did not change in response to fish (nested two-way ANOVA, F1,28 = 0.404, p = 0.53), but there was a reduction in the complexity of the pigmentation when exposed to UV (nested two-way ANOVA, F1,28 = 6.497, p = 0.017, figures 1 and 2b). Also, there was no significant interaction between UV and fish (nested two-way ANOVA, F1,28 = 3.278, p = 0.081).
4. Discussion
The pigment measurements show that R. balthica is phenotypically plastic in the pigmentation of the mantle in response to predation risk and UVR. In response to fish, snails expressed a greater coverage of dark pigment, without affecting the complexity of their pigmentation. Exposure to UVR increased the amount of pigmentation even further, but at a cost of reduced pigmentation complexity. When exposed to both UVR and fish, snails induced the same amount of dark pigment and exhibited a comparable reduction in pigmentation complexity to the UVR treatment, indicating that snails are able to trade-off their camouflage for photoprotection. Also, as phenotypic plasticity underlies much of the considerable variation in mantle pigmentation observed in R. balthica, we can conclude that pigment pattern is not a reliable diagnostic feature for use in taxonomic determination [11].
Predation risk can vary both spatially and temporally, just as the severity of UVR may vary for organisms in the littoral zone owing to differences in water transparency (e.g. water colour, turbidity; [16]) and shading related to differences in canopy cover [17]. In addition, the dark photoprotective melanin compounds that protect organisms from harmful UVR are costly to produce [18]. Hence, it is highly probable that it is beneficial for the snails to be plastic in mantle pigmentation and that this plasticity may be adaptive. While photoprotective pigmentation can counteract the negative effects of UVR [6,7], it can also be used as camouflage [9,10]. An inducible morphological defence can increase a prey's chance of escaping an attack [19]; in this case, pigmentation linked to camouflage may decrease predation rate by reducing encounter rates with predators [10]. Furthermore, pigmentation can improve crypsis owing to, for example, background matching [8,9] or disruptive coloration in which prey try to break the outline of the body [20]. We showed that snails exposed to predatory fish increased the amount of pigmentation without affecting the pattern complexity. This suggests a disruptive coloration strategy, because it leads to more complex patterns even though the background was homogeneously brown. However, further studies are needed to test the intriguing hypothesis that snails with more complex pigmentation patterns are less vulnerable to predators.
Multiple threats impose a considerable amount of stress for the organism, especially when a response to one threat increases the vulnerability to another. Hence, animals have to trade-off responses to different threats or, alternatively, only respond to the most severe threat. For example, in environments where copepods are exposed to both predatory fish and UVR simultaneously, copepods ignore UVR and lose all their pigmentation to reduce predation [6]. In our study, both UVR and predators induced increased pigmentation in R. balthica. However, in response to UVR, snails induced pigmentation to an extent that resulted in a loss of pigmentation complexity. This indicates that R. balthica, in contrast to copepods, do not have to trade-off their pigmentation, but instead their cryptic patterns when exposed to both UVR and predators. Hence, while snails exposed to both UVR and fish still have their photoprotection, their camouflage might not be optimal.
In conclusion, we found that the level and complexity of mantle pigmentation in the freshwater snail R. balthica is owing to phenotypic plasticity in response to both UVR and predators. While exposure to predators increased the amount of dark pigmentation, UVR increased the dark pigmentation even further, but at the expense of pigmentation complexity. Hence, when exposed to both UVR and fish, snails have to trade-off camouflage against photoprotection, a trade-off that may increase predation risk.
Acknowledgements
We thank Ben Chapman for insightful comments on the manuscript and for improving the language.
Data accessibility
Data were deposited in the Dryad repository (doi:10.5061/dryad.9v9d3) [15].
Funding statement
This research was supported by a grant from the Swedish Research Council (to C.B.).
References
- 1.Brönmark C, Lakowitz T, Nilsson PA, Ahlgren J, Lennartsdotter C, Hollander J. 2012. Costs of inducible defence along a resource gradient. PLoS ONE 7, e30467 (doi:10.1371/journal.pone.0030467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson L-A. 2004. Plasticity in pigmentation induced by conflicting threats from predation and UV radiation. Ecology 85, 1005–1016 (doi:10.1890/02-0525) [Google Scholar]
- 3.Caldwell MM, Bjorn LO, Bornman JF, Flint SD, Kulandaivelu G, Teramura AH, Tevini M. 1998. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J. Photochem. Photobiol. B Biol. 46, 40–52 (doi:10.1016/s1011-1344(98)00184-5) [DOI] [PubMed] [Google Scholar]
- 4.Belden LK, Wildy EL, Blaustein AR. 2000. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. J. Zool. 251, 473–479 (doi:10.1111/j.1469-7998.2000.tb00803.x) [Google Scholar]
- 5.Hansson L-A, Hylander S, Sommaruga R. 2007. Escape from UV threats in zooplankton: a cocktail of behavior and protective pigmentation. Ecology 88, 1932–1939 (doi:10.1890/06-2038.1) [DOI] [PubMed] [Google Scholar]
- 6.Hansson L-A, Hylander S. 2009. Effects of ultraviolet radiation on pigmentation, photoenzymatic repair, behavior, and community ecology of zooplankton. Photochem. Photobiol. Sci. 8, 1266–1275 (doi:10.1039/b908825c) [DOI] [PubMed] [Google Scholar]
- 7.Williamson CE, Fischer JM, Bollens SM, Overholt EP, Breckenridge JK. 2011. Toward a more comprehensive theory of zooplankton diel vertical migration: integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnol. Oceanogr. 56, 1603–1623 (doi:10.4319/lo.2011.56.5.1603) [Google Scholar]
- 8.Johannesson K, Ekendahl A. 2002. Selective predation favouring cryptic individuals of marine snails (Littorina). Biol. J. Linn. Soc. 76, 137–144 (doi:10.1111/j.1095-8312.2002.tb01720.x) [Google Scholar]
- 9.Hargeby A, Stoltz J, Johansson J. 2005. Locally differentiated cryptic pigmentation in the freshwater isopod Asellus aquaticus. J. Evol. Biol. 18, 713–721 (doi:10.1111/j.1420-9101.2004.00837.x) [DOI] [PubMed] [Google Scholar]
- 10.Svanbäck R, Eklöv P. 2011. Catch me if you can: predation affects divergence in a polyphenic species. Evolution 65, 3515–3526 (doi:10.1111/j.1558-5646.2011.01398.x) [DOI] [PubMed] [Google Scholar]
- 11.Schniebs K, Gloer P, Vinarski MV, Hundsdoerfer AK. 2011. Intraspecific morphological and genetic variability in Radix balthica (Linnaeus 1758) (Gastropoda: Basommatophora: Lymnaeidae) with morphological comparison to other European Radix species. J. Conchol. 40, 657–678 [Google Scholar]
- 12.Ahlgren J, Brönmark C. 2012. Fleeing towards death—leech-induced behavioural defences increase freshwater snail susceptibility to predatory fish. Oikos 121, 1501–1506 (doi:10.1111/j.1600-0706.2012.20420.x). [Google Scholar]
- 13.Merilaita S. 1998. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. Lond. B 265, 1059–1064 (doi:10.1098/rspb.1998.0399). [Google Scholar]
- 14.Rasband WS. 1997–2012. ImageJ. Bethesda, MaD: US National Institutes of Health. See http://imagej.nih.gov/ij/. [Google Scholar]
- 15.Ahlgren J. 2013. Data from: camouflaged or tanned: plasticity in freshwater snail pigmentation. Dryad Digital Respository. (doi:10.5061/dryad.9v9d3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hargreaves BR. 2003. Water column optics and penetration of UVR. In UV effects in aquatic organisms and ecosystems (eds Helbling ER, Zagarese H.), pp. 59–105 Cambridge, UK: Royal Society of Chemistry [Google Scholar]
- 17.Frost PC, Mack A, Larson JH, Bridgham SD, Lamberti GA. 2006. Environmental controls of UV-B radiation in forested streams of northern Michigan. Photochem. Photobiol. 82, 781–786 (doi:10.1562/2005-07-22-RA-619) [DOI] [PubMed] [Google Scholar]
- 18.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- 19.Van Buskirk J, McCollum SA. 2000. Functional mechanisms of an inducible defence in tadpoles: morphology and behaviour influence mortality risk from predation. J. Evol. Biol. 13, 336–347 (doi:10.1046/j.1420-9101.2000.00173.x) [Google Scholar]
- 20.Stevens M, Merilaita S. 2009. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B 364, 481–488 (doi:10.1098/rstb.2008.0216). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were deposited in the Dryad repository (doi:10.5061/dryad.9v9d3) [15].