Abstract
Locomotion persists across all manner of internal and external perturbations. The objective of this study was to identify locomotor compensation strategies in rodent models of peripheral nerve injury. We found that hip-to-toe limb length and limb angle was preferentially preserved over individual joint angles after permanent denervation of rat ankle extensor muscles. These findings promote further enquiry into the significance of limb-level function for neuromechanical control of legged locomotion.
Keywords: rodent, biomechanics, locomotion, nerve injury
1. Introduction
Simple biomechanical models robustly predict complex locomotor behaviours. Walking and running dynamics across vertebrate and invertebrate taxa may be calculated from low degree of freedom templates based on whole limb kinematics [1–3]. Recordings of dorsal spinocerebellar tract neurons indicate that the central nervous system (CNS) integrates afferent feedback into neural representations of limb posture [4–7], suggesting that whole limb kinematics are physiologically relevant parameters for locomotor control.
Whole limb kinematics is composed of redundant combinations of constituent joint kinematics. The classical motor control problem of redundant degrees of freedom may actually be a solution to the problem of maintaining consistent behaviour [8]. Humans exploit joint-level redundancies in the short term to stabilize cycle-to-cycle limb function [9–13]. After peripheral nerve injury in cats, limb behaviour is preserved long-term through coordinated changes across all joints of the injured limb [14]. These short- and long-term compensation strategies suggest that whole limb kinematics is more critical to locomotor control than joint kinematics.
We tested the general hypothesis that an injury compensation strategy exists by which post-injury joint angle combinations are selected to achieve consistency, or minimal change, in whole limb kinematics. We hypothesized that hip-to-toe limb length and limb angle after peripheral nerve injury in rats would correlate highly with pre-injury measurements, whereas post-injury joint kinematics would assume new trajectories. We further hypothesized that the magnitude of change in joint kinematics would reflect the degree of injury, whereas whole limb kinematics remains invariant.
2. Material and methods
Peripheral nerve injuries were induced under aseptic conditions and isoflurane gas anaesthesia, via incisions in the posterior popliteal fossa of the left hindlimb, exposing branches of the tibial nerve leading to the four major ankle extensor muscles: medial and lateral gastrocnemius, soleus and plantaris muscles. Two unilateral interventions included: a ‘severe’ injury that permanently paralysed all four major ankle plantarflexor muscles, and a ‘moderate’ injury, sparing medial gastrocnemius. After nerve branch transection, we verified denervation of target muscles by electrically stimulating the proximal tibial nerve.
Twenty-three adult male Sprague-Dawley rats (Rattus norvegicus) were pair-housed and provided food and water ad libitum. Some rats of the severe (n = 9) and moderate (n = 10) injury groups were tested prior to surgery; however, these trials were treated as independent and pooled into the control group (n = 15), providing more conservative statistical results than a repeated measures design.
Each rat was acclimated to treadmill locomotion prior to data collection and surgery. Within the treadmill (Columbia Instruments), animals were oriented perpendicular to the beam of a custom, high-speed X-ray video system (200 Hz) [15]. A single trial consisted of 10 s of X-ray exposure during steady walking at 0.4 ms–1. One daily session yielded 10–12 trials per rat, amounting to approximately 300 strides each; over 17 000 total strides were recorded.
X-ray video data were corrected for distortion and enhanced for contrast (NI Vision, National Instruments) [15]. Hindlimb features were manually digitized frame-by-frame then low-pass filtered (7 Hz) using open source software [16]. Six anatomical landmarks (fourth distal phalanx, fourth metatarsal head, lateral malleolus of fibula, lateral femoral epicondyle, greater trochanter and caudal margin of ischium) defined four included joint angles (metatarsophalangeal (MTP), ankle, knee and hip joints; figure 1a). The limb vector was defined from fourth distal phalanx to greater trochanter; whole limb kinematics is the angle and length of this vector. Each stride was time-normalized to 100% of the gait cycle. We calculated mean kinematics trajectories for each animal, and then averaged across animals for grand mean trajectories of each experimental condition.
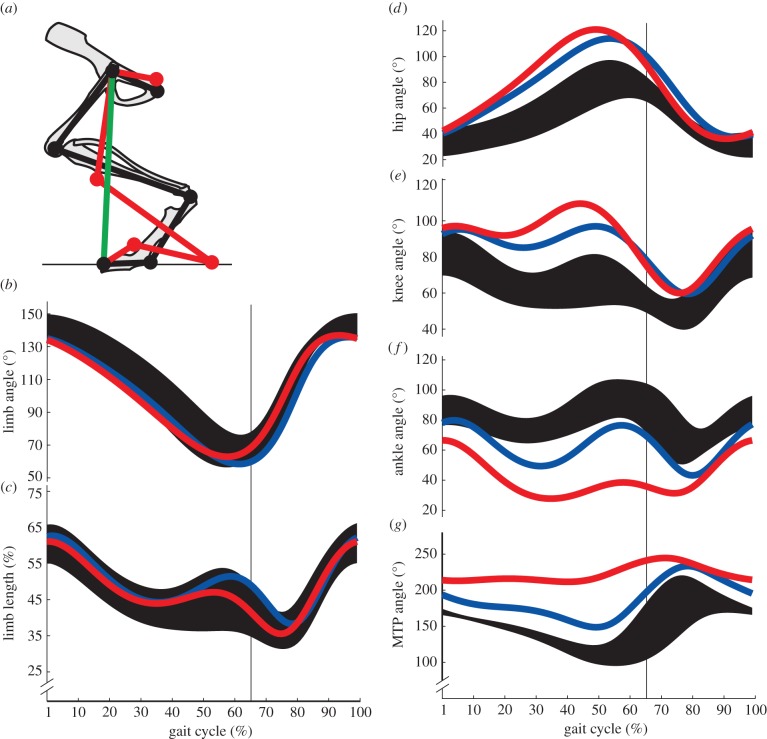
Figure 1.
(a) Representative data depicting sagittal plane kinematics model of the rat hindlimb. Limb segments of control (black) and severe PNI injury (red) rats are depicted during mid-stance sharing a common limb vector (green). (b) Mean limb angle, (c) limb length, (d) hip, (e) knee, (f) ankle and (g) MTP joint angle trajectories plotted over a normalized gait cycle. Control data (black, n = 15) are represented by ±1 s.d. about the mean and compared with mean trajectories after moderate (blue, n = 10) and severe (red, n = 9) triceps surae injuries. Vertical bars represent stance-to-swing transition.
We calculated coefficients of determination to represent goodness-of-fit between kinematics trajectories of control and injury groups over the gait cycle. Our formula (equations (2.1)–(2.3)) relates how much of the variance accounted for (VAF) in post-injury kinematics is explained by the control, uninjured patterns [17]
| 2.1 |
| 2.2 |
| 2.3 |
where 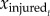 is the mean value of one kinematics variable at normalized time t across all trials of each individual injured rat,
is the mean value of one kinematics variable at normalized time t across all trials of each individual injured rat, 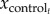 is the mean value at time t across all control rat trajectories and
is the mean value at time t across all control rat trajectories and 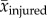 is the mean value over the entire gait cycle. Note that negative VAF values occur if SSE is greater than SST, which is possible if control data are a sufficiently poor model of post-injury data. We performed t-tests of the hypothesis that the majority of the variance in limb and joint parameters across individual rats for each injury condition would be explained by mean kinematics of the control rats (i.e. mean VAF > 0.5). All calculations and statistical analyses were performed in MATLAB.
is the mean value over the entire gait cycle. Note that negative VAF values occur if SSE is greater than SST, which is possible if control data are a sufficiently poor model of post-injury data. We performed t-tests of the hypothesis that the majority of the variance in limb and joint parameters across individual rats for each injury condition would be explained by mean kinematics of the control rats (i.e. mean VAF > 0.5). All calculations and statistical analyses were performed in MATLAB.
3. Results and discussion
Whole limb kinematics was conserved after peripheral nerve injury (see electronic supplementary material, video). Mean limb angle (figure 1b) and limb length (figure 1c) trajectories for both severe (red) and moderate (blue) injury groups were within 1 s.d. of mean control trajectories (black). By contrast, denervation of ankle extensor muscles significantly altered mean joint kinematics, which typically fell outside 1 s.d. of mean control trajectories. Changes were consistent across experimental groups: the ankle joint (figure 1f) was more yielding, or flexed, post-injury, whereas hip (figure 1d) and knee (figure 1e) joints were more extended compared with controls. The MTP joint became flexed after injury, though it is typically hyperextended in controls (figure 1g). The magnitude of changes depended on the extent of injury; denervation of all four muscles produced greater deviations from baseline joint kinematics than when medial gastrocnemius was spared.
VAF of post-injury joint kinematics (table 1) confirms these findings. Correlation between control and post-injury whole limb kinematics concluded that the trajectories matched, as mean VAF of limb angle and length were all significantly greater than 0.5. No joint-level comparison in either injury group had an average VAF value greater than 0.5.
Table 1.
Average VAF in post-injury kinematics. t-tests indicated whether control kinematics explained significantly greater than 50% of the variability of post-injury data.
| severe PNI | moderate PNI | |
|---|---|---|
| limb angle | VAF = 0.82 ± 0.09, (p < 0.001)* | VAF = 0.91 ± 0.02, (p < 0.001)* |
| limb length | VAF = 0.69 ± 0.08, (p < 0.001)* | VAF = 0.61 ± 0.10, (p < 0.005)* |
| hip angle | VAF = 0.35 ± 0.18, (p = 0.981) | VAF = 0.57 ± 0.17, (p = 0.160) |
| knee angle | VAF = −2.38 ± 0.83, (p = 1.000) | VAF = −1.49 ± 1.04, (p = 1.000) |
| ankle angle | VAF = −7.20 ± 2.22, (p = 1.000) | VAF = −2.10 ± 1.78, (p = 0.999) |
| MTP angle | VAF = −26.04 ± 19.14, (p = 0.998) | VAF = −0.32 ± 0.85, (p = 0.993) |
*p < 0.05.
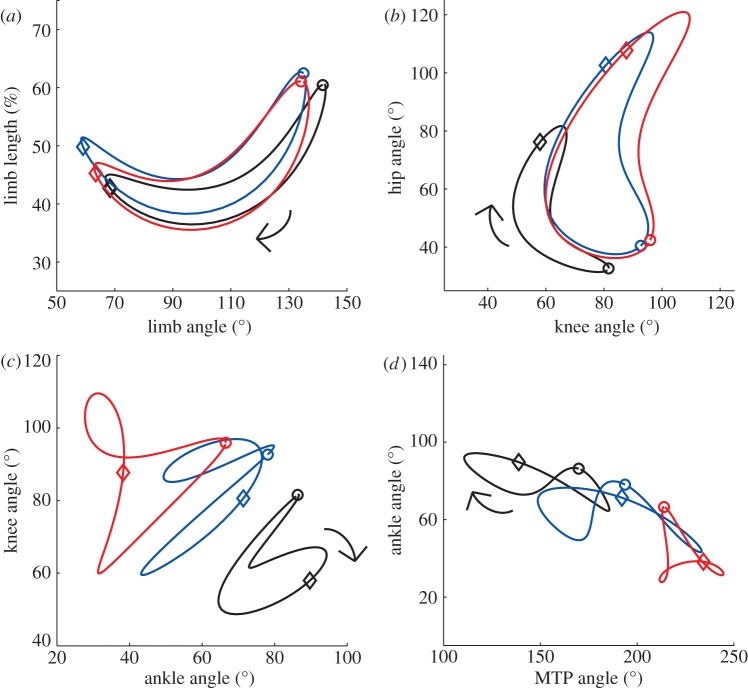
Conservation of whole limb kinematics is achieved despite disruption of interjoint coordination. Figure 2 represents coordination patterns within whole limb and joint kinematics. Plotting two limb kinematic parameters against each other (figure 2a) demonstrated that the uninjured limb coordination relationship had been largely conserved. Hip–knee joint angle relationships (figure 2b) for both injury groups retained roughly the same shape, but very different positions in the state space. Knee–ankle (figure 2c) and ankle–MTP (figure 2d) joint angle relationships were more radically different. These plots support the notion that limb-level function had been conserved post-injury, while joint-level function had been dramatically altered.
Figure 2.
(a) Coordination between limb length and angle. Angle–angle plots between contiguous joint pairs: (b) hip–knee, (c) knee–ankle and (d) ankle–MTP. These results use the same conventions as figure 1. Stance phase begins with paw contact (circles); swing phase with paw lift-off (diamonds). Arrows represent the direction of time.
Surgical denervation of triceps surae muscles places a major constraint on ankle joint function during locomotion and significantly alters joint kinematics of the injured hindlimb. Both experimental groups exhibited increased flexion at the ankle during early stance phase. This was compensated by just enough increased extension at the hip and knee joints to maintain uninjured whole limb kinematics during post-injury locomotion. This general strategy persisted across variation in injury severity, time post-surgery and interjoint coordination patterns. Our findings imply that maintenance of pre-injury whole limb kinematics, but not joint kinematics, is a goal of injury compensation during legged locomotion. Within this strategy, the intact major ankle plantarflexor of moderately injured rats attenuated the disruption of knee, ankle and MTP joint angles compared with the severe injury group. Hip joint angles were more similar for both injury groups, indicative of its importance to leg angle.
These results, along with recent neurophysiological experiments, imply a critical role for whole limb kinematics in locomotor control. The CNS is thought to integrate limb postural information within the spinal cord into sensory representations of whole limb behaviour [4–7]. The CNS may also issue motor commands that correspond to these neural representations of whole limb kinematics, which may be decoded at the spinal level to coordinate motor redundant joints. This line of reasoning agrees with findings that the execution of descending motor commands involves activating combinations of synergistic motor units [18]. These findings posit that sensorimotor control of the limbs involves low-dimensional, task-level neural representations of function.
Any given limb posture may be achieved through an infinite combination of joint angle configurations. In human hopping, joint kinematics change with each hop, but coordinate to make whole limb kinematics invariant over many cycles [9,11]. If limb function preservation by interjoint coordination is a fundamental principle of locomotor control, then temporary deviations should be treated in the same manner as chronic physiological deficits. Cats with peripheral nerve injuries maintained pre-injury whole limb kinematics as did rats, despite relatively extended limb postures [14]. Our results represent a third mammalian order observed to exhibit this general mechanism of locomotor compensation. Although further studies are warranted, this trait may be conserved for legged locomotion among mammals.
Rats are commonly used as animal models of peripheral nerve injury, but their performance is typically quantified by discrete endpoint measures that can fail to discriminate subtle gait impairments [15]. Some investigators have begun to assess joint or whole limb kinematics after peripheral nerve injury, but none have studied both together [19,20]. Limb kinetics are an important characteristic of locomotion; significant changes to joint moment and power profiles of the injured hindlimb have been observed in rats with lesions equivalent to our ‘severe’ case [21], and agree with changes in joint kinematics that we have observed. Our study distinguishes itself in that we overcome skin movement errors in joint kinematics using our X-ray-based kinematics method, as well as our unique focus on the interrelation between joint and whole limb kinematics. Human patients with stroke-induced hemiparesis will typically exhibit gait asymmetries that correlate significantly with ankle spasticity and decrements in propulsive forces of the paretic limb [22,23]. Owing to the nature of human subjects research, however, it is difficult to observe human limping without complicating factors such as co-morbidities and intersubject variability. Also, studies of human gait pathology typically focus on individual joints or more general stride parameters such as step length rather than whole limb kinematics as we studied here. We began our line of research in a precise and well-defined rat injury model to identify the fundamental traits of injury compensation that may be common to other forms of legged locomotion.
Neurophysiological mechanisms could be involved in stabilizing whole limb kinematics after injury. For example, the ankle extensor muscle, gastrocnemius, in cats exerts inhibitory feedback on knee extensor muscles, resulting in reinforced coordination between knee and ankle joints [24]. This could explain how the moderate injury group matches controls, but would not explain the performance of the severe injury group as both heads of the gastrocnemius were denervated. Both surgeries spared smaller extrinsic toe flexor muscles that cross the ankle and could account for some, but probably not much, residual degree of ankle extension. The post-injury joint kinematics may also be a product of passive biomechanical mechanisms, owing to the passive tension generated by muscles crossing multiple joints as well as from interaction torques generated between limb segments. In a locomoting decerebrate cat, however, whole limb kinematics was not preserved after the same neuromuscular injury suggesting that biomechanics alone are not sufficient for locomotor compensation and that some descending input from the brain is required [25].
Anatomical redundancies of the neuromuscular system ensure that failure of one part does not impede function of the whole. Conservation of whole limb kinematics appears to be an implicit goal of legged locomotion, persisting despite permanent disruption of individual joint kinematics. Animals manipulating motor redundancies at one anatomical level to satisfy goals at another level deserve further investigation, as this may represent a universal compensation mechanism that improves behavioural and locomotor plasticity.
Acknowledgements
We thank Juliet Jacobsen, Ramya Parthasarathy and Josie van Loozen for data collection and analysis assistance. We also thank Tyson Hedrick (UNC-Chapel Hill) for his digitizing software (DLT), and members of the Comparative Neuromechanics Laboratory for technical assistance.
We performed all experimental procedures in accordance with a protocol approved by the Georgia Institute of Technology IACUC.
Funding statement
This work was supported by NIH grant no. AR054760 to Y.H.C.
References
- 1.Cavagna GA, Heglund NC, Taylor CR. 1977. Mechanical work in terrestrial locomotion: two basic mechanisms in minimizing energy expenditure. Am. J. Physiol. 233, VAF43–VAF61 [DOI] [PubMed] [Google Scholar]
- 2.Blickhan R. 1989. The spring-mass model for running and hopping. J. Biomech. 22, 1217–1227 (doi:10.1016/0021-9290(89)90224-8) [DOI] [PubMed] [Google Scholar]
- 3.McMahon TA, Cheng GC. 1990. The mechanics of running: how does stiffness couple with speed? J. Biomech. 23, 65–78 (doi:10.1016/0021-9290(90)90042-2) [DOI] [PubMed] [Google Scholar]
- 4.Bosco G, Poppele RE. 2000. Reference frames for spinal proprioception: kinematics based or kinetics based?. J. Neurophysiol. 83, 2946–2955 [DOI] [PubMed] [Google Scholar]
- 5.Bosco G, Poppele RE, Eian J. 2000. Reference frames for spinal proprioception: limb endpoint based or jointlevel based? J. Neurophysiol. 83, 2931–2945 [DOI] [PubMed] [Google Scholar]
- 6.Poppele RE, Bosco G, Rankin AM. 2002. Independent representations of limb axis length and angle in spinocerebellar response components. J. Neurophysiol. 87, 409–422 [DOI] [PubMed] [Google Scholar]
- 7.Bosco G, Eian J, Poppele RE. 2006. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp. Brain Res. 175, 83–96 (doi:10.1007/s00221-006-0530-7) [DOI] [PubMed] [Google Scholar]
- 8.Bernstein NA. 1967. The co-ordination and regulation of movements. New York, NY: Pergamon Press [Google Scholar]
- 9.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. 2007. Modular control of limb movements during human locomotion. J. Neurosci. 27, 11 149–11 161 (doi:10.1523/JNEUROSCI.2644-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang YH, Roiz RA, Auyang AG. 2008. Intralimb compensation strategy depends on the nature of joint perturbation in human hopping. J. Biomech. 41, 1832–1839 (doi:10.1016/j.jbiomech.2008.04.006) [DOI] [PubMed] [Google Scholar]
- 11.Auyang AG, Yen JT, Chang YH. 2009. Neuromechanical stabilization of leg length and angle through interjoint compensation during human hopping. Exp. Brain Res. 192, 253–264 (doi:10.1007/s00221-008-1582-7) [DOI] [PubMed] [Google Scholar]
- 12.Yen JT, Auyang AG, Chang YH. 2009. Joint-level kinetic redundancy is exploited to control limb-level forces during human hopping. Exp. Brain Res. 196, 439–451 (doi:10.1007/s00221-009-1868-4) [DOI] [PubMed] [Google Scholar]
- 13.Yen JT, Chang YH. 2010. Rate-dependent control strategies stabilize limb forces during human locomotion. J. R. Soc. Interface 7, 801–810 (doi:10.1098/rsif.2009.0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YH, Auyang AG, Scholz JP, Nichols TR. 2009. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J. Exp. Biol. 212, 3511–3521 (doi:10.1242/jeb.033886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman JM, Chang YH. 2010. High-speed X-ray video demonstrates significant skin movement errors with standard optical kinematics during rat locomotion. J. Neurosci. Methods 186, 18–24 (doi:10.1016/j.jneumeth.2009.10.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 (doi:10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 17.Vogt WP. 1999. Dictionary of statistics and methodology. Thousand Oaks, CA: Sage Publications [Google Scholar]
- 18.Ting L, McKay JL. 2007. Neuromechanics of muscle synergies for posture and movement. Curr. Opin. Neurobiol. 17, 622–628 (doi:10.1016/j.conb.2008.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varejao ASP, Cabrita AM, Meek MF, Bulas-Cruz J, Filipe VM, Gabriel RC, Ferreira AJ, Geuna S, Winter DA. 2003. Ankle kinematics to evaluate functional recovery in crushed rat sciatic nerve. Muscle Nerve 27, 706–714 (doi:10.1002/mus.10374) [DOI] [PubMed] [Google Scholar]
- 20.Sabatier MJ, To BN, Nicolini J, English AW. 2011. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J. Exp. Biol. 214, 1007–1016 (doi:10.1242/jeb.051508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett SW, Lanovaz JL, Muir GD. 2012. The biomechanics of locomotor compensation after peripheral nerve lesion in the rat. Behav. Brain Res. 229, 391–400 (doi:10.1016/j.bbr.2012.01.040) [DOI] [PubMed] [Google Scholar]
- 22.Hsu AL, Tang PF, Jan MH. 2003. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch. Phys. Med. Rehabil. 84, 1185–1193 (doi:10.1016/S0003-9993(03)00030-3) [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. 2007. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 88, 43–49 (doi:10.1016/j.apmr.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 24.Ross KT, Nichols TR. 2009. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J. Neurophysiol. 101, 184–197 (doi:10.1152/jn.90338.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahl VA, Nichols TR. 2011. Short-term effects of muscular denervation and fasciotomy on global limb variables during locomotion in the decerebrate cat. Cells Tissues Organs 193, 325–335 (doi:10.1159/000323679) [DOI] [PMC free article] [PubMed] [Google Scholar]