Abstract
The hero shrew's (Scutisorex somereni) massive interlocking lumbar vertebrae represent the most extreme modification of the vertebral column known in mammals. No intermediate form of this remarkable morphology is known, nor is there any convincing theory to explain its functional significance. We document a new species in the heretofore monotypic genus Scutisorex; the new species possesses cranial and vertebral features representing intermediate character states between S. somereni and other shrews. Phylogenetic analyses of DNA sequences support a sister relationship between the new species and S. somereni. While the function of the unusual spine in Scutisorex is unknown, it gives these small animals incredible vertebral strength. Based on field observations, we hypothesize that the unique vertebral column is an adaptation allowing these shrews to lever heavy or compressive objects to access concentrated food resources inaccessible to other animals.
Keywords: functional significance, hero shrew, new species, Scutisorex, vertebral column
1. Introduction
The hero shrew, Scutisorex somereni (Soricomorpha: Soricidae), was discovered in equatorial Africa in 1910 [1], but its most fascinating feature was not noted for another 7 years, when Allen [2] discovered a massive spine with interlocking bony tubercles that fortify the lumbar region and are the only bone-on-bone articulation known in mammals [3]. These interlocking processes derive from outpocketings of transverse and accessory processes [4], rendering the spine four times more robust (relative to body mass) than in any other vertebrate [5], and giving it five times the resilience to axial torsion of a typical mammalian backbone [3]. Allen [2] cited observations of adult men (approx. 70 kg) standing on Scutisorex (approx. 50–70 g) for several minutes, with the animals walking away, apparently unharmed, after their tormentor stepped off of them [2]. These animals' incredible strength resulted in the Mangbetu people of the Congo using them as talismans, believing that wearing part of the animal would provide invincibility and generating the common name of ‘hero shrew’ [6]. To evolutionary biologists, Scutisorex has been an enigma, with no known adaptive significance for the species' extremely unusual spine and incredible strength [3,4]. The only adaptive hypothesis offered thus far is that the robust spine and associated posture keeps the animal's body clear of wet ground in swampy habitats [6]. Alternatively, the interlocking vertebrae may represent a case of Gould & Lewontin's [7] Spandrels of San Marco hypothesis [3], according to which complex traits evolve as a consequence of adaptive factors unrelated to the trait itself.
Only one species is currently recognized within the genus Scutisorex. The form congicus [8] is considered a junior synonym of somereni [9,10]. Specimens across the known range of the genus (including historically identified congicus) exhibit the woolly pelage, rugose texture of the cranium and fortified lumbar region, consisting of 10–11 vertebrae with bony interlocking tubercles primarily on the lateral, but also on the dorsal and ventral aspects of each lumbar vertebra, which renders Scutisorex unique among mammals [11].
Here, we describe a second species of Scutisorex with a significantly different cranial morphology, a distinct pelage and fewer lumbar vertebrae, each with fewer processes.
2. Material and methods
We collected small mammals near the village of Baleko, Équateur Province, Democratic Republic of the Congo (DRC) (0.24127° S, 20.8833° E, 358 m.a.s.l.; see the electronic supplementary material), including one shrew with interdigitating lumbar vertebrae. We compared both morphological (crania and post-cranial skeleton) and molecular (nuclear and mitochondrial DNA sequences) characters between the specimen we collected and representatives of S. somereni from different localities within the known distribution of the genus.
3. Results
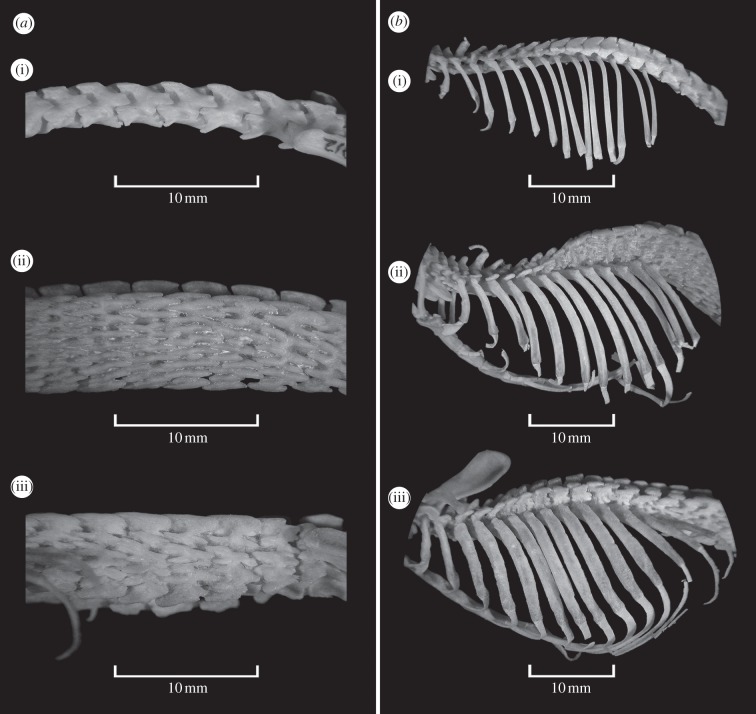
The specimen we collected differs from S. somereni by its smaller skull, modestly striated parietal region of the cranium (versus extreme rugosity), low lambdoidal ridge (versus very pronounced lambdoidal ridge; figure 1), fewer lumbar vertebrae, each with fewer lateral processes, more robust and flattened ribs (figure 2) and pelage consisting of shorter hairs.
Figure 1.
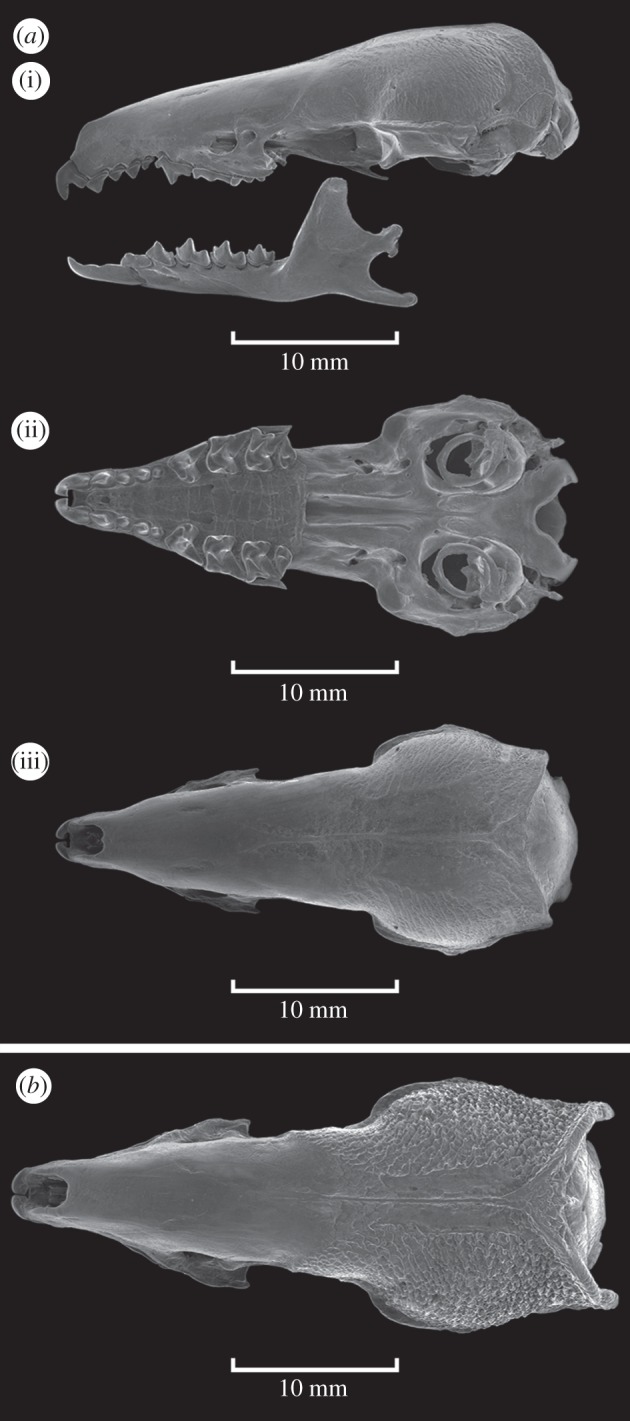
(a) Skull of Scutisorex thori (FMNH 219669). (i) Lateral view of cranium and mandible; (ii) ventral (occlusal) view of cranium; (iii) dorsal view of cranium. (b) Cranium of Scutisorex somereni (FMNH 43860) in dorsal view.
Figure 2.
Lateral views of (a) the lumbar region and (b) ribs of Crocidura olivieri ((i) FMNH 192912), Scutisorex somereni ((ii) FMNH 189277) and Scutisorex thori ((iii) FMNH 219669).
Bayesian phylogenetic analyses of two unlinked loci (see the electronic supplementary material) support a sister relationship between the new species and S. somereni (see the electronic supplementary material, figure S2). According to our molecular analyses, Scutisorex is either sister to other crocidurine shrews (mitochondrial: cytochrome b) or a member of a clade containing Sylvisorex, Suncus and Ruwenzorisorex (nuclear: von Willebrand factor), the latter a result similar to a previous inference [12]. From the mitochondrial topology, we estimated the divergence date between the two species of Scutisorex as approximately 4 Ma (95% highest posterior density: 1.8–8.9 Ma) and between Scutisorex and other crocidurines at approximately 14 Ma (7.6–30.1 Ma, see the electronic supplementary material, figure S2).
On the basis of the morphological differences and long history of isolation between the new specimen and S. somereni, we define a new species of shrew in the genus Scutisorex.
(a). New species
Scutisorex thori Stanley, Malekani and Gambalemoke.
(b). Holotype
FMNH 219669 (figures 1 and 2; electronic supplementary material, figures S1 and S3), an adult female collected on 9 June 2012 and prepared as a dried skin and cleaned skull and skeleton.
(c). Type locality
Baleko, Tshuapa District, Équateur Province, DRC (0.24127° S, 20.8833° E, 358 m.a.s.l.).
(d). Diagnosis
Scutisorex thori is a large shrew (47 g) with subtle crenulations on the parietal bones of the cranium, eight lumbar vertebrae with multiple interlocking tubercles and broad, flat ribs (figures 1 and 2; electronic supplementary material, figures S1 and S3; table 1).
Table 1.
Comparison of select cranial measurements (mm) for Scutisorex, given as mean, ±s.d. and range. Characters defined in the electronic supplementary material.
| character | S. thori n. sp. (n = 1) | S. somereni (n = 9) |
|---|---|---|
| condylo-incisive length | 30.34 | 33.33 ± 0.90 32.00 – 34.76 |
| length of upper toothrow | 13.00 | 14.76 ± 0.44 14.04 – 15.44 |
| least interorbital width | 6.85 | 7.06 ± 0.15 6.84 – 7.27 |
| bimaxillary width | 8.77 | 10.08 ± 0.32 9.60 – 10.44 |
| greatest width of the braincase | 13.69 | 14.52 ± 0.42 13.80 – 15.23 |
| height of the braincase | 9.31 | 9.98 ± 0.23 9.58 – 10.20 |
| width of third upper incisor | 0.88 | 1.18 ± 0.02 1.15 – 1.21 |
| width of upper canine | 0.92 | 1.21 ± 0.07 1.09 – 1.32 |
| length of third upper molar | 1.82 | 1.97 ± 0.12 1.83 – 2.18 |
| width of third upper molar | 0.82 | 0.89 ± 0.05 0.82 – 0.98 |
| maxillary plate | 1.53 | 2.08 ± 0.17 1.78 – 2.30 |
| length of mandible | 19.57 | 21.80 ± 0.73 20.85 – 22.90 |
| length of lower toothrow | 12.1 | 13.72 ± 0.35 13.15 – 14.18 |
(e). Description and comparisons
Scutisorex thori has a tail that is shorter than the head and body (72% of HB length), and a prominent ear conch. The vibrissae are thin and long (less than or equal to 25 mm) and ranged from black to almost translucent. The dorsal pelage (5–6 mm long) is grizzled with some hairs entirely pale, but others with brown tips. Longer black guard hairs (8–10 mm) are distributed throughout the dorsal pelage, but the longest are concentrated on the rump. The ventral pelage consists of pale grey short hairs (2–3 mm). The bicoloured tail (dark brown dorsum, pale venter) has a cream coloured tip and no long bristles. All feet have stout curved claws and short black hairs on the dorsal surface.
The skull is stout, but less so than that of S. somereni, which is longer and broader (table 1 and figure 1). The parietal bones have subtle crenulations, and distinct, but small (relative to S. somereni) lambdoidal ridges (figure 1). The third upper incisor and the upper canine are similarly sized, but the first premolar is smaller than either (figure 1).
Scutisorex thori differs from all mammals except S. somereni in having lumbar vertebrae reinforced with interlocking bony tubercles. Scutisorex thori differs from S. somereni as follows: the parietal bones of the skull are not extremely rugose; the lambdoidal ridges are less prominent and do not project posteriorly beyond the vertical plane of the occipital condyles (figure 1); fewer lumbar vertebrae present (8 versus 10–11 in S. somereni) that are wider with fewer lateral tubercles; the ribs are broader, flatter and more robust (figure 2). The fur is shorter (hairs of dorsal pelage 5–6 mm long versus 10 mm long in S. somereni) with a silky, rather than woolly texture (see the electronic supplementary material, figure S1). External measurements of the holotype (mm, except mass (g)) are total length, 251; tail length, 105; hindfoot length (nail included), 24; ear length, 14; and mass, 47 g. See table 1 for cranial measurements.
(f). Etymology
The species is named in honour of Thorvald ‘Thor’ Holmes, Jr of the Humboldt State University Vertebrate Museum, for his dedication to specimen-based mammal research and education. The epithet also invokes Thor, the god of strength in Norse mythology. We suggest the common name of Thor's hero shrew.
(g). Distribution
Scutisorex thori is only known from the area of Baleko near the Tshuapa River, Équateur Province, DRC.
(h). Habitat and ecology
The specimen was collected in seasonally flooded lowland forest near the Tshuapa River, Équateur Province, DRC. The animal was trapped as part of a survey effort that included 396 pitfall nights and 1000 trap nights over the course of 8 days. The animal was pregnant with one embryo (12 mm crown–rump length) in the right uterine horn. Although most S. somereni specimens are from localities in Uganda, Rwanda, Burundi and northeastern DRC [10,13], two specimens housed at the USNM (537692, 537693) were collected in the same province (Équateur) of DRC as S. thori, but north of the Congo River (see the electronic supplementary material, figure S4).
4. Discussion
Despite differences between the two species of Scutisorex, both share the fortified lumbar region of the vertebral column that renders the genus enigmatic. The presence of cartilaginous processes on the lumbar vertebrae during embryonic development in S. somereni demonstrates that the tubercles are not the result of secondary exostoses [4]. Biomechanical properties of the skeleton reveal the ribs and spine to be significantly more robust than those of other mammals, including fossorial species, but the limbs are not unusually sturdy [5]. The associated musculature is also modified [14], and observations of Scutisorex in captivity revealed that the animal is capable of turning 180° in tight spaces via sagittal flexion of the spine [14]. Extension, however, is more restricted and lateral bending is retarded by the interdigitated tubercles [5]. Thus, the spine and associated musculature are well suited for flexion during the application of heavy loads and can resist compressive and torsional forces during sagittal flexion [14]. An adaptive explanation for this unusual morphology has been elusive [14].
At a site near Tandala in Équateur Province, DRC, in 1979, L. W. Robbins was shown specific localities where local residents captured Scutisorex. All sites were in swampy palm forests where residents routinely collected beetle larvae by pulling the persistent bases of dead palm leaves away from standing tree trunks to recover the larvae residing between the leaf bases and tree trunk. The collectors indicated that Scutisorex were commonly encountered during this process and showed LWR runways around the base of these trees that they said were used by Scutisorex. Scutisorex can flex their fortified vertebral column considerably, especially sagitally [3], and the spine provides robust muscle attachments, especially for the epaxial longissimus muscles, which store elastic energy during flexion [14]. We hypothesize that these shrews position themselves between trunk and leaf bases, and use the spine and associated musculature to exert force between the trunk and the base of leaves, granting access to predictable and potentially concentrated sources of invertebrate larvae that are otherwise protected from predation. This same mechanism may be useful in levering heavy objects such as logs to gain access to aggregates of invertebrate prey such as earthworms (Oligochaeta). Churchfield et al. [15] found the diet of S. somereni to be predominately earthworms (Oligochaeta), but to also include Coleoptera, Formicidae, and lepidopteran and dipteran larvae. All of these are known to concentrate under items that afford protection from predation such as rocks and logs [16]. Access to this high quality, predictable energy source may have provided an evolutionary advantage, allowing the evolution of the reinforced torso. The greater number of lumbar vertebrae in S. somereni compared with S. thori may provide a longer lever arm during spinal flexion in the former; future behavioural studies may indicate different foraging behaviours.
No inferences of the evolutionary steps necessary to generate the unique Scutisorex morphology have been made, because Scutisorex has been thought of as a monotypic lineage most closely related to ‘normal’ shrews [4]. This unique spinal morphology, without intermediate examples, has been cited as an instance of ‘the fuel of punctuated evolutionary events’ [14]. However, the fewer lumbar vertebrae with fewer tubercles and less crenulated parietal bones found in the new species, along with the long evolutionary time estimated in our mtDNA gene tree, suggest that the evolution of the Scutisorex morphology was incremental, with the cumulative addition of vertebrae and tubercles and increasing crenulation of the cranium.
Acknowledgements
We thank Rebecca Banasiak, Anna Goldman and Andrea Niedzielski, who provided assistance with specimen preparation and figures. DNA sequencing was conducted in the Pritzker Molecular Laboratory, Field Museum. We are grateful to M. Anderson, R. Bieler, J. P. Brown, S. Carroll, T. Bakambana Luemba, T. Lumbsch, L. Kalemba Ndimba, C. Oliveros, O. Rieppel, M. A. Rogers, E. Sargis, B. Strack, J. Stanley and D. Willard for logistical support and advice. The National Science Foundation (DEB-1145251) provided funding. Three anonymous reviewers provided helpful comments on an earlier draft of this manuscript.
References
- 1.Thomas O. 1910. A new genus of fruit-bats and two new shrews from Africa. Ann. Mag. Nat. Hist. 6, 111–114 (doi:10.1080/00222931008692827) [Google Scholar]
- 2.Allen J. 1917. The skeletal characters of Scutisorex. Bull. Am. Mus. Nat. Hist. 37, 769–784 [Google Scholar]
- 3.Cullinane DM, Bertram JEA. 2000. The mechanical behaviour of a novel mammalian vertebral joint. J. Anat. 197, 627–634 (doi:10.1046/j.1469-7580.2000.19740627.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed A, Klima M. 1978. Zur Entwicklung und Funktion der Lendenwirbelsäule bei der Panzerspitzmaus Scutisorex somereni (Thomas, 1910). Z. Saugetierkd. 43, 1–17 [Google Scholar]
- 5.Cullinane DM, Aleper D, Bertram JEA. 1998. The functional and biomechanical modifications of the spine of Scutisorex somereni, the hero shrew: skeletal scaling relationships. J. Zool. 244, 447–452 (doi:10.1111/j.1469-7998.1998.tb00049.x) [Google Scholar]
- 6.Kingdon J. 1974. East African mammals, vol. IIA Chicago, IL: University of Chicago Press [Google Scholar]
- 7.Gould S, Lewontin R. 1979. The spandrels of San Marco and the Panglossian Paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581–598 (doi:10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 8.Thomas O. 1915. List of mammals (exclusive of Ungulata) collected on the upper Congo by Dr Christy of the Congo Museum, Tervueren. Ann. Mag. Nat. Hist. 8, 465–481 (doi:10.1080/00222931508693740) [Google Scholar]
- 9.Hollister N. 1916. Shrews collected by the Congo expedition of the American Museum. Bull. Am. Mus. Nat. Hist. 35, 663–680 [Google Scholar]
- 10.Hutterer R. 2005. Order Soricomorpha. In Mammal species of the world: a taxonomic and geographic reference (eds Wilson DE, Reeder DM.), pp. 220–311 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 11.Nowak R. 2001. Walker's mammals of the world, 6th edn. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 12.Quérouil S, Hutterer R, Barrière P, Colyn M, Kerbis Peterhans JC, Verheyen E. 2001. Phylogeny and evolution of African shrews (Mammalia: Soricidae) inferred from 16s rRNA sequences. Mol. Phylogenet. Evol. 20, 185–195 (doi:10.1006/mpev.2001.0974) [DOI] [PubMed] [Google Scholar]
- 13.Heim de Balsac H, Meester J. 1972. Order Insectivora. In The mammals of Africa (eds Meester J, Setzer H.), pp. 1–29 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 14.Cullinane DM, Aleper D. 1998. The functional and biomechanical modifications of the spine of Scutisorex somereni, the hero shrew: spinal musculature. J. Zool. 244, 453–458 (doi:10.1111/j.1469-7998.1998.tb00050.x) [Google Scholar]
- 15.Churchfield S, Dieterlen F, Hutterer R, Dudu A. 2007. Feeding ecology of the armored shrew, from the northeastern Democratic Republic of Congo. J. Zool. 273, 40–45 (doi:10.1111/j.1469-7998.2007.00297.x) [Google Scholar]
- 16.Borror OJ, De Long DM, Triplehorn CA. 1981. An introduction to the study of insects. Philadelphia, PA: Saunders College Publishing [Google Scholar]