Abstract
An outstanding feature of the orchid family is that approximately 30–40% of the species have non-rewarding flowers and deploy various modes of deception to attract pollinators, whereas the remaining species engage in pollination mutualisms based on provision of floral rewards. Here, we explore the direction, frequency and reversibility of transitions between deceptive and rewarding pollination systems in the radiation of the large African genus Disa, and test whether these transitions had consequences for diversification. By optimizing nectar production data for 111 species on a well-resolved phylogeny, we confirmed that floral deception was the ancestral condition and that nectar production evolved at least nine times and was lost at least once. Transitions to nectar production first occurred ca 17 million years ago but did not significantly affect either speciation or extinction rates. Nectar evolved independently of a spur, which was lost and gained multiple times. These results show that nectar production can be a highly labile trait and highlight the need for further studies of the genetic architecture of nectar production and the selective factors underlying transitions between deception and mutualism.
Keywords: diversification rates, floral deception, mimicry, nectary, phylogenetic signal, pollination
1. Introduction
Floral deception is one of the great enigmas of the orchid family. First observed in the eighteenth century by the European naturalist Christian Sprengel [1], and firmly disbelieved by Darwin [2], who considered insect pollinators too smart to fall for ‘so gigantic an imposture’, floral deception is now known to occur in at least 30–40% of the ca 27 000 species in the orchid family [3]. Non-rewarding orchids achieve pollination by deploying signals that their pollinators associate with food, sexual partners or oviposition sites [3,4]. However, they tend to receive fewer pollinator visits and have lower rates of pollination than their rewarding relatives [5,6]. Some even suggested that floral deception is a sub-optimal condition in orchids and persists only because the mutational steps required for reward production are improbable [7].
Studies that simulated mutations for nectar production in non-rewarding orchids show that overall pollination success increases following addition of artificial nectar, but that pollinators also visit more flowers per plant, which, in most cases, increases self-pollination and hence the likelihood of inbreeding depression and pollen discounting [8–11]. From models based on these empirical results, Johnson et al. [9] suggested that selection in orchids will generally favour nectar production when pollinators are scarce and deception when pollinators are common. Because of its benefits for outcrossing, some have argued that deception may not only be maintained by selection, but also enhance rates of speciation, and thus partly explain the extraordinary species richness of the family [12].
Studies of European and Australian terrestrial orchids, belonging to the subtribe Orchidinae and tribe Diuridae, respectively, suggest that floral deception was ancestral and that nectar production evolved more than once in each of these lineages [13–16]. These transitions are apparent mostly at or above the genus level, with the exceptions of Dactylorhiza and Orchis in Europe and Diuris in Australia, in each of which at least one transition between reward states occurred [15–17]. Our understanding of the fine-scale patterns of nectar evolution in orchids has been limited, however, because the combination of reliable data on floral rewards and well-sampled species-level phylogenies is seldom available.
The large African orchid genus Disa represents a classic case of floral diversification, with numerous examples of both deceptive and mutualistic pollination systems and both spurred and un-spurred flowers. Johnson et al. [18] and Smithson [14] inferred three independent origins of nectar production in the genus, but grossly underestimated the number of transitions as they had data on reward production for fewer than 30 species. Here, we present the first in-depth analysis of reward transitions in Disa, based on field studies of a large sample (111 species or 62%) of the 183 species in the genus. We asked (i) what the frequency and direction of transitions were between floral deception and nectar production, (ii) whether nectar production was associated with the evolution of spurs, and (iii) whether reward status affected rates of speciation or extinction.
2. Material and methods
Phylogenetic relationships in Disa were inferred by Bytebier et al. [19]. Nectar production and occurrence of a spur (derived from the median sepal in Disa) were coded as binary characters. Nectar production was established by examination of flowers with a hand lens, confirmation of sugar content in fluids with a refractometer, and, in many cases, high-pressure liquid chromatography to identify sugars (details to be reported elsewhere). Only taxa whose phylogenetic relationships were established and for which reliable information on nectar production was available (see the electronic supplementary material) were included, which amounted to 111 species in our analyses. This corresponds to 62% of recognized Disa taxa and represents all sections of Disa, with the exception of the monotypic section Ovalifoliae [20].
We reconstructed ancestral character states by parsimony and maximum likelihood (ML) using Mesquite [21]. ML revealed slow rates of trait evolution (see electronic supplementary material), conditions under which ML suffers from insufficient information for correct parameter estimation, and under which parsimony performs more accurate ancestral state reconstruction [22–25]. We therefore report only parsimony results here, but note that they are strongly supported by ML results. Maximum-likelihood analyses were performed using chronograms, as tests for trait phylogenetic signal indicated that chronograms were more appropriate to capture patterns of trait evolution (see the electronic supplementary material).
We used Pagel's correlation test [26] to examine whether existence of spurs is a prerequisite for the evolution of nectar production, and the BiSSE module in Mesquite [27] to examine whether rates of speciation, extinction and diversification differed between rewarding and deceptive lineages (for details see the electronic supplementary material).
3. Results
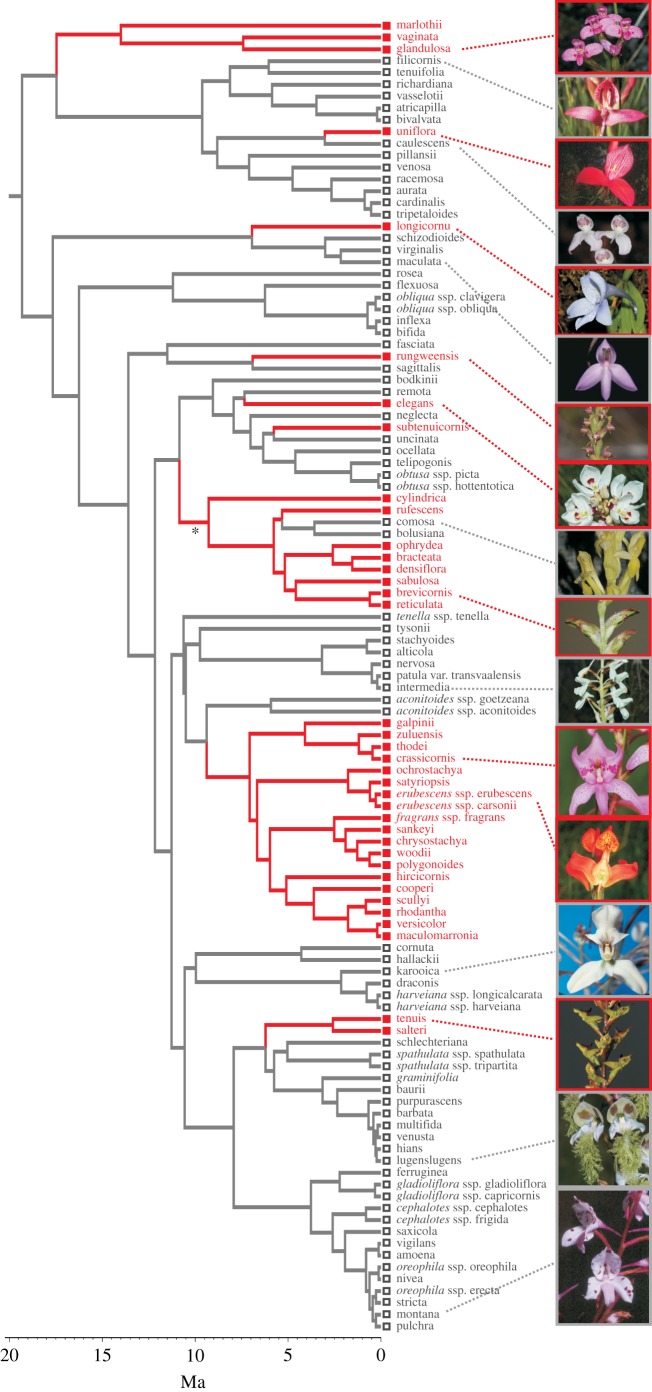
The ancestral Disa was unequivocally reconstructed as non-rewarding (1000/1000 chronograms). Nectar production was reconstructed as having evolved nine times (between 17 and 3 Ma) from non-rewarding ancestors (median; minimum = 8, maximum = 11 times) and lost once (median; minimum = 0, maximum = 2; figure 1). These conclusions are strongly supported by unequivocal reconstructions of eight of the nine transitions (figure 1). The root of Disa was unequivocally reconstructed as having spurred flowers (see electronic supplementary material, figure S1). Spurs were reconstructed as having been lost five times (median; minimum = 4, maximum = 6), and regained twice (median, minimum = 2, maximum = 4) (see electronic supplementary material). The presence of spurs and nectar production did not evolve in a correlated fashion (D4 = 2.52, p = 0.269).
Figure 1.
Evolution of nectar production in Disa, showing the maximum clade credibility tree, obtained from 1000 chronograms, over which trait evolution was optimized. Non-rewarding taxa, open symbols; rewarding taxa, filled symbols. Parsimony support for state changes (number of trees in which a transition is reconstructed /number of trees that contain that node) was unequivocal (1000/1000), with the exception of one transition indicated by an asterisk (i.e. 763/994). (Online version in colour.)
The null hypotheses of equal speciation, extinction and diversification rates, respectively, in rewarding and deceptive lineages could not be rejected (speciation rate (λ): average D1 = 3.91 × 10−5, p > 0.95; extinction rate (μ): average D1 = −1.58 × 10−4, p > 0.95; diversification rate: average D2 = 3.96 × 10−5, p > 0.95, n = 1000 chronograms per rate).
4. Discussion
Our finding that nectar production evolved at least nine times from non-rewarding ancestors in Disa, with at least one reversal to floral deception (figure 1), dispels older arguments that the evolution of nectar production in orchids is mutation-limited and generally improbable [7]. Although there is detectable phylogenetic signal in floral reward systems in Disa (electronic supplementary material), the overall picture is that transitions between deceptive and rewarding pollination systems are not strongly constrained. Instead, transitions between reward states are likely to reflect adaptive processes, whereby rewards are selected when they enhance fitness under certain ecological circumstances (such as pollen limitation arising from infrequent pollinator visits), while under other circumstances deception enhances fitness and thereby constitutes an alternative ‘evolutionarily stable strategy’ [3,9,28]. Nectar is not the only, or even necessarily the best, escape from pollen limitation traps. Food, mate or oviposition-site mimicry in deceptive orchids often enables them to achieve levels of fecundity comparable with those of rewarding species while simultaneously maximizing cross-pollination due to pollinators’ behavioural responses to the lack of rewards [3,29]. Batesian food deception evolved at least six times and sexual mimicry once in sections of Disa dominated by non-rewarding species that employ generalized food deception (see electronic supplementary material, figure S2). In terms of the consequences of reward transitions for overall diversification of lineages, our analyses do not support previous suggestions that floral deception promotes speciation [12] or that it increases vulnerability to extinction [7].
The general finding of repeated independent evolution of nectar production in orchids is corroborated by the identity of their nectar-producing organs. Nectar in most orchids is produced by the labellum (from which spur is usually derived), whereas in other Asparagales it is produced by septal nectaries. In Disa, nectar is usually produced by the median sepal, providing further evidence that nectaries in Disa evolved independently from those in other orchids.
Although it would be expected that the evolution of spurs and nectar production are coupled in orchids, we found no evidence for an association between these traits. Spurs evolved and were maintained in lineages with non-rewarding flowers (see electronic supplementary material, figure S1) and must therefore have other functions unrelated to nectar storage, such as mechanical fit between long-proboscid insects and flowers [30]. Indeed, strong pollinator-mediated selection on spur length has been documented in both nectar-producing and deceptive orchids [31–33].
Understanding of transitions between deception and nectar production in orchids would be greatly enhanced if the genetic architecture of orchid nectaries were to be elucidated. For example, it would be useful to know how much of the genetic architecture of orchid nectaries is shared with other Asparagales that have septal nectaries, whether the sepal-derived nectaries of Disa species involve essentially the same set of genes as those of the petal-derived nectaries of other orchids and whether these genes are present in all deceptive orchids, with only simple mutational steps required to activate nectar production.
References
- 1.Sprengel CK. 1793. Das entdeckte Geheimnis der Natur im Bau und in der Befruchtung der Blumen. Berlin, Germany: Friedrich Vieweg dem aeltern [Google Scholar]
- 2.Darwin CR. 1862. On the various contrivances by which British and foreign orchids are fertilized by insects. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- 3.Jersáková J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 81, 219–235 (doi:10.1017/S1464793105006986) [DOI] [PubMed] [Google Scholar]
- 4.Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 28, 307–315 (doi:10.1016/j.tree.2013.01.019) [DOI] [PubMed] [Google Scholar]
- 5.Neiland MRM, Wilcock CC. 1998. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 85, 1657–1671 (doi:10.2307/2446499) [PubMed] [Google Scholar]
- 6.Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2005. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol. J. Linn. Soc. 84, 1–54 (doi:10.1111/j.1095-8312.2004.00400.x) [Google Scholar]
- 7.Gill DE. 1989. Fruiting failure, pollinator inefficiency, and speciation in orchids. In Speciation and its consequences (eds Otte D, Endler JA.), pp. 458–481 Sunderland, MA: Sinauer [Google Scholar]
- 8.Johnson SD, Nilsson LA. 1999. Pollen carryover, geitonogamy and the evolution of deceptive pollination systems in orchids. Ecology 80, 2607–2619 (doi:10.1890/0012-9658(1999)080[2607:PCGATE]2.0.CO;2) [Google Scholar]
- 9.Johnson SD, Peter CI, Agren J. 2004. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proc. R. Soc. Lond. B 271, 803–809 (doi:10.1098/rspb.2003.2659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jersáková J, Johnson SD. 2006. Lack of floral nectar reduces self-pollination in a fly-pollinated orchid. Oecologia 147, 60–68 (doi:10.1007/s00442-005-0254-6) [DOI] [PubMed] [Google Scholar]
- 11.Smithson A, Gigord LDB. 2001. Are there fitness advantages in being a rewardless orchid? Reward supplementation experiments with Barlia robertiana. Proc. R. Soc. Lond. B 268, 1435–1441 (doi:10.1098/rspb.2001.1705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cozzolino S, Widmer A. 2005. Orchid diversity: an evolutionary consequence of deception?. Trends Ecol. Evol. 20, 487–494 (doi:10.1016/j.tree.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 13.Cozzolino S, Aceto S, Caputo P, Widmer A, Dafni A. 2001. Speciation processes in Eastern Mediterranean Orchis s.l. species: molecular evidence and the role of pollination biology. Israel J. Plant Sci. 49, 91–103 (doi:10.1560/QV6M-E7A0-QFC7-G6BL) [Google Scholar]
- 14.Smithson A. 2009. A plant's view of cheating in plant-pollinator mutualisms. Israel J. Plant Sci. 57, 151–163 (doi:10.1560/ijps.57.3.151) [Google Scholar]
- 15.Inda LA, Pimentel M, Chase MW. 2012. Phylogenetics of tribe Orchideae (Orchidaceae: Orchidoideae) based on combined DNA matrices: inferences regarding timing of diversification and evolution of pollination syndromes. Ann. Bot 110, 71–90 (doi:10.1093/aob/mcs083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell AK, Roberts DL, Hawkins JA, Rudall PJ, Box MS, Bateman RM. 2009. Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae). Bot. J. Linnean Soc. 160, 369–387 (doi:10.1111/j.1095-8339.2009.00985.x) [Google Scholar]
- 17.Indsto JO, Weston PH, Clements MA, Dyer AG, Batley M, Whelan RJ. 2007. Generalised pollination of Diuris alba (Orchidaceae) by small bees and wasps. Aust. J. Bot. 55, 628–634 (doi:10.1071/BT06207) [Google Scholar]
- 18.Johnson SD, Linder HP, Steiner KE. 1998. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). Am. J. Bot 85, 402–411 (doi:10.2307/2446333) [PubMed] [Google Scholar]
- 19.Bytebier B, Bellstedt DU, Linder HP. 2007. A molecular phylogeny for the large African orchid genus Disa. Mol. Phylogenet. Evol. 43, 75–90 (doi:10.1016/j.ympev.2006.08.014) [DOI] [PubMed] [Google Scholar]
- 20.Bytebier B, Bellstedt DU, Linder HP. 2008. A new phylogeny-based sectional classification for the large African orchid genus Disa. Taxon 57, 1233–1251 [Google Scholar]
- 21.Maddison WP, Maddison DR.2011. Mesquite: a modular system for evolutionary analysis (2.75 ed.). See: http://mesquiteproject.org .
- 22.Pirie MD, Humphreys AM, Antonelli A, Galley C, Linder HP. 2012. Model uncertainty in ancestral area reconstruction: a parsimonious solution?. Taxon 61, 652–664 [Google Scholar]
- 23.Pagel M. 1999. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 48, 612–622 (doi:10.1080/106351599260184) [Google Scholar]
- 24.Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158 (doi:10.1080/10635150390192780) [DOI] [PubMed] [Google Scholar]
- 25.Harvey PH, Pagel M. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 26.Pagel M. 1994. Detecting correlated evolution on phylogenies - a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 (doi:10.1098/rspb.1994.0006) [Google Scholar]
- 27.Maddison WP, Midford PE, Otto SP. 2007. Estimating a binary character's effect on speciation and extinction. Syst. Biol. 56, 701–710 (doi:10.1080/10635150701607033) [DOI] [PubMed] [Google Scholar]
- 28.Hobbhahn N. 2012. Correlates and consequences of repeated nectar evolution in the ancestrally rewardless orchid genus Disa. PhD Thesis. Calgary, Canada: University of Calgary [Google Scholar]
- 29.Johnson SD. 1994. Evidence for Batesian mimicry in a butterfly-pollinated orchid. Biol. J. Linnean Soc. 53, 91–104 [Google Scholar]
- 30.Anderson B, Johnson SD. 2009. Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants. New Phytol. 182, 533–540 (doi:10.1111/j.1469-8137.2009.02764.x) [DOI] [PubMed] [Google Scholar]
- 31.Nilsson LA. 1988. The evolution of flowers with deep corolla tubes. Nature 334, 147–149 (doi:10.1038/334147a0) [Google Scholar]
- 32.Johnson SD, Steiner KE. 1997. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51, 45–53 (doi:10.2307/2410959) [DOI] [PubMed] [Google Scholar]
- 33.Ellis AG, Johnson SD. 2010. Gender differences in the effects of floral spur length manipulation on fitness in a hermaphrodite orchid. Int. J. Plant. Sci. 171, 1010–1019 (doi:10.1086/656351). [Google Scholar]