Abstract
Telomeres are regarded as markers of biological or cellular ageing because they shorten with the degree of stress exposure. Accordingly, telomere lengths should show different rates of change when animals are faced with different intensities of environmental challenges. However, a relationship between telomere length and the environment has not yet been tested within a natural setting. Here, we report longitudinal telomere dynamics in free-living, black-tailed gulls (Larus crassirostris) through the recapture of birds of a known age over 2–5 consecutive years. The rate of change in telomere lengths differed with respect to year but not sex or age. The years when gulls showed stable telomere lengths or increases in telomere lengths (from 2009 to 2010) and decreases in telomere lengths (from 2010 to 2011) were characterized by El Niño and the Great Japan Earthquake, respectively. Both events are suspected to have had long-lasting effects on food availability and/or weather conditions. Thus, our findings that telomere dynamics in long-lived birds are influenced by dramatic changes in environmental conditions highlight the importance of environmental fluctuations in affecting stress and lifespan.
Keywords: annual change, environmental effects, longitudinal data, seabird, telomere dynamics
1. Introduction
Telomere dynamics (the rate at which the lengths of telomeres change over time) have been the subject of intense investigation as a molecular marker for individual quality [1,2] and life expectancy [3–5]. There is growing evidence that oxidative damage [6,7], which is caused by internal factors, such as psychological [8] and reproductive stress [9], as well as external factors, such as stressful social contact [10] and diet [11], affects telomere dynamics more than the degree of shortening that occurs routinely during each round of somatic-cell division. Accordingly, telomeres are expected to show different dynamics when animals are faced with different intensities of environmental challenges in their natural environments. Although very few studies, that have involved animals of known age living under natural conditions, have measured the lengths of telomeres more than twice, those that have done so show substantial variation in changes of telomere length between individuals and suggest the importance of extrinsic environmental factors on telomere dynamics [5,12]. Of these extrinsic environmental changes, long-lasting environmental changes would affect life-history costs and cause chronic stress, which will considerably affect annual variations in telomere dynamics.
In this study, we examined telomere dynamics over five years in adult black-tailed gulls (Larus crassirostris). In this species, no relationship was observed between telomere length and chronological age, although adults had shorter telomeres than chicks [13]. We repeatedly measured telomere lengths of birds of known age (4–28 years old) that returned to the same breeding colony every year over the course of five consecutive years to investigate the effects of year-round environmental change on telomere lengths.
2. Material and methods
This study was conducted between 2007 and 2011 on Kabushima Island, Japan (40°32′ N, 141°33′ E). Each year, we collected blood samples from birds of known ages between May and mid-June; therefore, we refer to the change in telomere length between one year and the next as the ‘annual change’. For example, 2007/2008 is the sampling interval between May 2007 and May 2008.
Blood samples (30–50 µl) were collected by puncturing the brachial vein. Genomic DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen) and involved the experimental procedure described by Pauliny et al. [1] with the modifications described by Mizutani et al. [13]. Briefly, we used Southern blotting and DNA hybridization to measure absolute telomere lengths in kilobases with telomere restriction fragment analysis. Longitudinal data obtained from the same bird was analysed using the same gel to avoid the effects of interstitial telomeric repeats and to detect the within-individual changes. Sex determination was done using a PCR-based method [14].
Changes in telomere lengths between the two years were entered as a dependent variable, and year, sex, age and their interaction effects were treated as fixed factors in linear mixed models. Bird identity was set as a random factor. We selected the most parsimonious model among all possible candidate models based on Akaike's information criterion corrected for small sample size (AICc) and the associated Akaike's weights [15]. Moreover, we performed model averaging for the fixed effects across the top candidate models with an AICc difference (ΔAICc) less than 4. We then examined pairwise differences between groups in the best model using Tukey's multiple comparisons tests. To investigate the relationship between telomere length and age, which has been one of the most active areas in telomere biology in recent years [16], we used a random regression model to quantify the extent of within- and between-individual effects [17]. The methodological details are provided in the electronic supplementary material.
3. Results
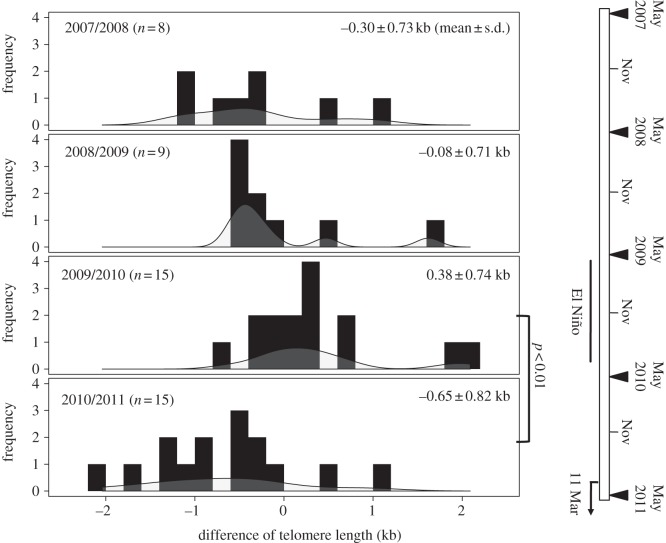
We sampled a total of 25 individuals (18 males and seven females) over multiple years (recaptured every year from two years up to five years). In total, we measured telomeres in 72 samples that provided 47 instances of changes in telomere lengths for statistical analysis (see electronic supplementary material, figure S1). The best model predicting telomere changes included only ‘year’ (table 1), and the other models did not receive substantial support because of their ΔAICc values were greater than or equal to 3 [15]. More specifically, the telomere change in 2010/2011 was significantly less than in 2009/2010 (Tukey's multiple comparison tests: p < 0.01; figure 1; electronic supplementary material, figure S2). Further support of this trend between the two years was provided by the coefficient of model-averaged estimates (see electronic supplementary material, table S1). Values for the relative variable importance derived from the model averaging were 0.87 and 0.16 for year and sex, respectively. We found no evidence of a significant change of telomere with age at either the individual or population level (see electronic supplementary material, table S2).
Table 1.
Model selection of linear mixed models with telomere change in response to the variables (sex, year and age as well as the interactions between these variables) using Akaike's information criterion corrected for small sample size (AICc) and Akaike weights. d.f. is the number of parameters. ΔAICc is the difference in AICc values between the models, with the best-candidate model having the lowest AICc. The evidence ratio is the ratio of the Akaike weight of each model to that of best model, indicating the extent to which the best model is better than the model. All models with ΔAICc values of less than 10 are listed. The null model includes only the intercept and the random effect (i.e. individual ID).
| variables | d.f. | AICc | ΔAICc | Wi | evidence ratio |
|---|---|---|---|---|---|
| year | 6 | 122.1 | 0 | 0.66 | 1.0 |
| year, sex | 7 | 125.1 | 3.0 | 0.15 | 4.4 |
| null | 3 | 125.5 | 3.4 | 0.12 | 5.4 |
| sex | 4 | 128.0 | 5.9 | 0.04 | 18.9 |
| year, sex, year : sex | 10 | 129.5 | 7.4 | 0.02 | 38.8 |
| year, age | 7 | 130.6 | 8.5 | 0.01 | 66.0 |
Figure 1.
Frequency distribution of the rate of telomere change between each year included in the study. Each solid line indicates the density distribution. The right panel shows the chronology of major events that were likely to affect the behaviour and physiology of the gulls: the bars represent the El Niño events from summer 2009 to spring 2010 [15] and the Great Japan Earthquake and subsequent massive tsunami on 11 Mar 2011. The triangles represent sampling dates.
4. Discussion
We found that telomere dynamics differed between years (table 1), with the length more likely to be unchanged or even increase from 2009 to 2010, but more likely to decrease from 2010 to 2011 (see figure 1 and electronic supplementary material, S2 and table S1). Thus, telomere dynamics might reflect different exposure to different stress conditions. Although it remains unclear how long-term exposure to environmental stressors affects telomere dynamics, chronic stress may influence telomere dynamics within several months [10]. The two-year duration coincided with large-scale environmental changes: El Niño events occurred from summer 2009 to spring 2010, [18] and the Great Japan Earthquake and subsequent tsunami occurred in March 2011. The El Niño (El Niño Modoki) brought mild weather and low sea surface temperatures (SSTs) near Japan [19]. Given that seabirds are known to adjust foraging behaviours to adapt to changes in the habitat of their prey associated with changes in SST [20], the climate during El Niño may have provided ideal weather and foraging conditions for the gulls and may have mitigated their physiological stress. Conversely, since March 2011, the fishery and meat processing plants along the Pacific coast, which are the preferred foraging sites of the gulls [21], have not been available to them. Accordingly, the gulls were forced to forage at sea, which was associated with higher energetic costs owing to the need to travel and search for prey. Food availability is one of the most important factors that determine levels of nutrition stress and survival [22], and the metabolic costs associated with the increased need for foraging change the oxidative status of tissues [23]. Given that gulls are omnivores that forage for food over large areas, it was extremely difficult either to identify precisely which environmental stressor(s) affected telomere length or to predict what effects these stressor(s) might have. Nonetheless, our results strongly suggest that long-lasting environmental changes affect telomere length in gulls, and that the same might apply to other wild animals.
Males were more likely to have increased telomere lengths than females (see electronic supplementary material, figure S1), although sex was not a predictor in the best model (table 1). Sex-specific changes in telomeres have been reported [24] (but see [5]), probably because reproduction (especially egg production) can increase oxidative stress [25] and accelerate telomere loss. In fact, laboratory experiments suggest that sex-specific changes in telomere lengths might be triggered by differential reproductive costs and stresses between males and females [9]. In addition, the black-tailed gulls included in this study showed sex-related differences in foraging areas during the breeding season (Y. Mizutani 2011, unpublished data), which may also link behaviour and physiological stress to different telomere dynamics.
In conclusion, we found that relatively gradual and sustained environmental changes (e.g. El Niño and SSTs) as well as catastrophes (e.g. tsunamis), and their subsequent effects might impact telomere dynamics. Telomere loss and extension is mediated by changes in the relative rates of telomere shortening (associated with cell division and stress intensity) and telomere lengthening (associated with telomerase activation [26]), and low stress may increase the relative effect of telomere restoration by telomerase. Environmental conditions may influence this mechanism and lead to the high age-independent individual variation in telomere lengths (see electronic supplementary material, table S2), which might complicate reliable estimation of chronological age using telomere length [16]. Alternatively, telomeres could be new indicators of ‘biological age’, which is affected by exposure to environmental challenges and chronicle annual fluctuations in levels of environmental stress. Nonetheless, further work would be required to judge the usefulness of telomere length as an indicator of ‘biological age’.
Acknowledgements
We are grateful to A. Narita, K. Narita, H. Fujii, R. Sugiura, H. Takahashi, C. Zavalaga, M. Muller and the staff of the observation post at Kabushima Island for their assistance, as well as two anonymous reviewers for their constructive comments. We used the banding data with permission from the Yamashina Institute for Ornithology and the Ministery of the Environment.
The procedures used in this study were approved by the Animal Experimental Committee of Nagoya University and the Ministry of the Environment and the Agency for Cultural Affairs, Japan.
Funding statement
This work was supported by grants from the Co-operation Research Programme of the Wildlife Research Centre, Kyoto University and the Japan Society for the Promotion of Science (24681006).
References
- 1.Pauliny A, Wagner RH, Augustin J, Szép T, Blomqvist D. 2006. Age-independent telomere length predicts fitness in two bird species. Mol. Ecol. 15, 1681–1687 (doi:10.1111/j.1365-294X.2006.02862.x) [DOI] [PubMed] [Google Scholar]
- 2.Plot V, Criscuolo F, Zahn S, Georges J-Y. 2012. Telomeres, age and reproduction in a long-lived reptile. PLoS ONE 7, e40855 (doi:10.1371/journal.pone.0040855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683 (doi:10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165 (doi:10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 22, 249–259 (doi:10.1111/mec.12110) [DOI] [PubMed] [Google Scholar]
- 6.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (doi:10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 7.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic. Biol. Med. 44, 235–246 (doi:10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 8.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315 (doi:10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748 (doi:10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotrschal A, Ilmonen P, Penn DJ. 2007. Stress impacts telomere dynamics. Biol. Lett. 3, 128–130 (doi:10.1098/rsbl.2006.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demerath EW, Cameron N, Gillman MW, Towne B, Siervogel M. 2004. Telomeres and telomerase in the fetal origins of cardiovascular disease: a review. Hum. Biol. 76, 127–146 (doi:10.1353/hub.2004.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B 271, 1571–1576 (doi:10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizutani Y, Tomita N, Kazama K, Takahashi H, Hasegawa O, Niizuma Y. 2009. Relationship between telomere length and age in black-tailed gull. Jpn. J. Ornithol. 58, 192–195 (doi:10.3838/jjo.58.192) [Google Scholar]
- 14.Fridolfsson AK, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121 (doi:10.2307/3677252) [Google Scholar]
- 15.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer [Google Scholar]
- 16.Horn T, Robertson BC, Gemmell NJ. 2010. The use of telomere length in ecology and evolutionary biology. Heredity 105, 497–506 (doi:10.1038/hdy.2010.113) [DOI] [PubMed] [Google Scholar]
- 17.van de Pol M, Wright J. 2009. A simple method for distinguish within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758 (doi:10.1016/j.anbehav.2008.11.006) [Google Scholar]
- 18.Japan Meteorological Agency 2012. See http://www.data.kishou.go.jp/shindan/sougou/html/2.3.html
- 19.Ratnam JV, Behera SK, Masumoto Y, Takahashi K, Yamagata T. 2012. Anomalous climatic conditions associated with the El Niño Modoki during boreal winter of 2009. Clim. Dyn. 39, 227–238 (doi:10.1007/s00382-011-1108-z) [Google Scholar]
- 20.Kappes MA, Shaffer SA, Tremblay Y, Foley DG, Palacios DM, Robinson PW, Bograd SJ, Costa DP. 2010. Hawaiian albatrosses track interannual variability of marine habitats in the North Pacific. Prog. Oceanogr. 86, 246–260 (doi:10.1016/j.pocean.2010.04.012) [Google Scholar]
- 21.Yoda K, Tomita N, Mizutani Y, Narita A, Niizuma Y. 2012. Spatio-temporal responses of black-tailed gulls to natural and anthropogenic food resources. Mar. Ecol. Prog. Ser. 466, 249–259 (doi:10.3354/meps09939) [Google Scholar]
- 22.Kitaysky AS, Piatt JF, Hatch SA, Kitaiskaia EV, Benowitz-Fredericks ZM, Shultz MT, Wingfield JC. 2010. Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct. Ecol. 24, 625–637 (doi:10.1111/j.1365-2435.2009.01679.x) [Google Scholar]
- 23.Beaulieu M, Reichert S, Le Maho Y, Ancel A, Criscuolo F. 2011. Oxidative status and telomere length in a long-lived bird facing a costly reproductive event. Func. Ecol. 25, 577–585 (doi:10.1111/j.1365-2435.2010.01825.x) [Google Scholar]
- 24.Barrett ELB, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell 10, 913–921 (doi:10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996 (doi:10.1111/j.1365-2435.2010.01750.x) [Google Scholar]
- 26.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53 (doi:10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]