Abstract
Animal alarm calls can encode information about a predator's category, size, distance or threat level. In non-human primates, alarm calls typically refer to broad classes of disturbances, in some instances to specific predators. Here, we present the results of a field experiment with a New World primate, the black-fronted titi monkey (Callicebus nigrifrons), designed to explore the information conveyed by their alarm call system. Adults produced sequences consisting of two main alarm call types that conveyed, in different parts of the utterance, information about a predator's type and location. In particular, sequence compositions differed depending on whether the predator was a mammalian carnivore or a raptor, and whether it was detected in a tree or on the ground. This is the first demonstration of a sequence-based alarm call system in a non-human animal that has the capacity to encode both location and type of predatory threat.
Keywords: alarm calls, sequences, New World monkeys, predator models
1. Introduction
Many species of mammals and birds produce alarm calls that convey information about predator type [1–3], size [4], distance [5], location [6] or threat level [7]. Where alarm calls encode predator type, threat level and location generally appear to be less important, although there are some systems that vary simultaneously with both predator type and risk-urgency [4,8]. In non-human primates, alarm calls typically refer to broad classes of disturbances, in some instances to specific predators.
Callicebus monkeys are small, diurnal Neotropical primates hunted by raptors, terrestrial carnivores, snakes and other primates [9,10]. Groups usually consist of a breeding pair and their immediate offspring [11]. Black-fronted titi monkeys (Callicebus nigrifrons) produce three types of unusually high-pitched, quiet calls in response to predators. Two of these are common and produced in context-specific sequences [12]. To raptors, monkeys usually produce series of A-calls, whereas terrestrial predators and other disturbances on the ground trigger series of B-calls. However, pilot observations suggested additional complexities. For example, A-calls were not only given to raptors but also, in combination with B- and C-calls, to predatory capuchin monkeys within the canopy [12]. This and other findings suggested that the two alarm call types do not function simply as predator-specific warning signals. To explore the communicative function of these monkeys’ alarm call system, we conducted a field experiment by systematically presenting models of terrestrial and aerial predators to different groups, either on the ground or within the canopy. We were interested in whether monkey vocalizations encoded predator type, elevation or both.
2. Material and methods
Experiments were conducted with five habituated groups of titi monkeys living in a 11 000 ha private reserve area, Serra do Caraça, Minas Gerais (20°05′ S; 43°29′ W). The study site and composition of groups are described elsewhere [12,13]. An oncilla (Leopardus tigrinus) model represented a threat from a mammalian ground predator, and a caracara (Caracara plancus) model represented that from an aerial raptor (see electronic supplementary material, figure S1; courtesy of PUC Minas Natural History Museum, Pontifícia Universidade Católica)
More detailed information on methodology can be found in the electronic supplementary material.
3. Results
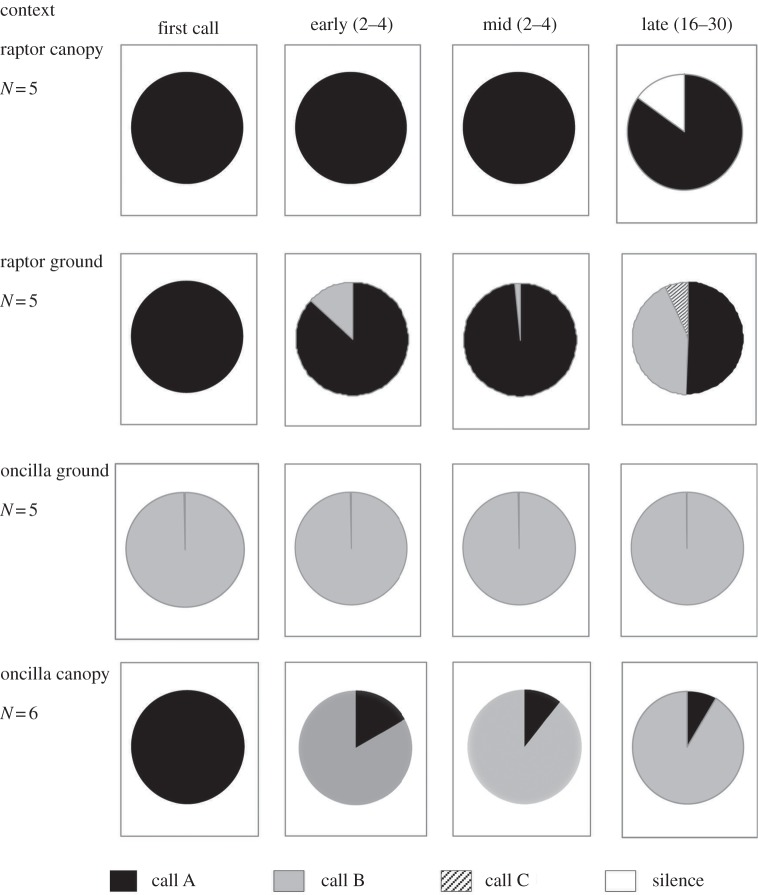
We tested the five study groups with model predators; all produced calls in response to both models (table 1). If the raptor was discovered within the canopy, monkeys produced pure A-call series, whereas raptor discoveries on the ground elicited sequences of A-calls interspersed with B-calls (figure 1 and electronic supplementary material, figure S2 and table S1), usually after an initial A-call sequence of at least four calls (G-test Williams: Gadjusted = 6.2639; d.f. = 2; p = 0.0436). If the second trial of raptor on the ground from group D (with a single A-call) is included, the result becomes non-significant (G-test Williams: Gadjusted = 6.9357; d.f. = 3; p = 0.074). If the oncilla was discovered on the ground, sequences of B-calls were given, whereas encounters in the canopy elicited B-call series that were always introduced by single A-calls (figure 1); again, the difference was significant (G-test Williams: Gadjusted = 11.3894; d.f. = 2; p = 0.0034). Finally, we found that the four main call sequences elicited by the different experimental condition differed in frequency across conditions (AAA; AA-B; A-BB; BBB; G-test Williams: Gadjusted = 37.073; d.f. = 9; p < 0.0001; electronic supplementary material, table S2).
Table 1.
Age/sex class of the first individual to detect and call in response to a predator model. Numbers in brackets indicate, firstly the number of individuals producing the first 30 calls, and secondly the ordinal call number at which a second individual joined in with calling. AM, adult male; AF, adult female; AU, adult sex unidentified; JM, Juvenile male; question mark (?), unknown.
| group | raptor canopy | raptor ground | oncilla ground | oncilla canopy |
|---|---|---|---|---|
| GA | AF (≥2/22) | JM (1/none) | AM (1/185) | AM (1/84) |
| GD | AM (?/?) | AF (1/none) | AM (1/33) | AF (1/50) |
| GM | AM (≥2/20) | AM (1/none) | AF (≥2/21) | ? (≥2/17) |
| GP | AF (?/?) | AU (1/50) | AM (1/41) | AM (≥/217) |
| GR | AF (1/69) | AF (1/39) | AM (≥2/10) | AF (≥2/3) |
| GB | — | — | — | AM (≥2/6) |
Figure 1.
Sequential analyses of the first 30 calls produced by black-fronted titi monkeys after encountering two species of predators in the canopy or on the ground.
The duration and call rate of vocal responses varied with the nature of the threat, that is raptor versus oncilla and/or canopy versus ground (total call duration: χ2 = 12.120, d.f. = 3, exact p = 0.001; call rate during first minute: χ2 = 12.120, d.f. = 3, exact p = 0.001; Friedman tests, two-tailed; figure 2a,b). From the figure, it can be seen that this effect appeared to derive entirely from the type of predator; indeed, vocal responses did not vary for either predator type (total call duration and call rate during first minute = Wilcoxon: z = 15; n1 = n2 = 5; p = 0.059 for both comparisons). We therefore combined location data for each predator, and found that predator type affected the duration and call rate of vocal responses (total call duration and call rate during first minute = Wilcoxon: z = 55; n1 = n2 = 10; p = 0.006; figure 2a,b). Distance of detection did not differ between predator types, regardless of location (all comparisons: χ2 = 3.607, d.f. = 4, p = 0.462).
Figure 2.
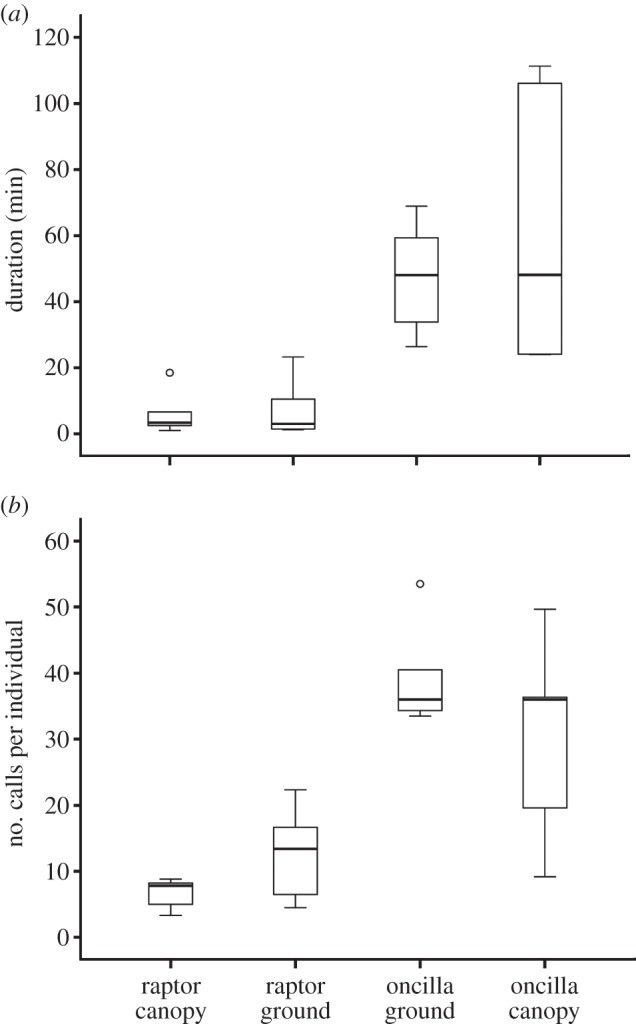
Histograms indicating the (a) median duration of calling behaviour when encountering two species of predators in the canopy or on the ground (b) median number of calls produced per individual. Box plots represent medians and upper and lower quartiles. Outliers are marked with circles.
Although all raptor encounters first triggered series of A-calls, the interval between first and second call was always longer when the raptor was on the ground than in the canopy (Wilcoxon: z = −2.023, n1 = n2 = 5, p = 0.043 or exact p = 0.063; electronic supplementary material, figure S3). For oncilla encounters, intervals between first and second calls (regardless of type) were longer when discovered in the canopy compared with the ground (Wilcoxon: z = −2.023, n1 = n2 = 5, p = 0.043 or exact p = 0.063; electronic supplementary material, figure S3).
4. Discussion
Black-fronted titi monkeys produced alarm call series that were characteristic of predator type and location, with A-series given mainly during raptor encounters, and B-series in oncilla encounters. However, if a raptor was encountered on the ground, callers tended to intersperse a small number of B-calls within the main A-series. Correspondingly, if an oncilla was encountered in the canopy, callers tended to produce B-series introduced by single A-calls. Thus, in both cases, an uncharacteristic location was indicated by a modified sequence, although the sequences remained predator-specific. We concluded that this primate species uses sequences of calls combined in predictable ways that potentially allow listeners to extract accurate information about the type of predator present and its location.
In previous studies, the typical finding for mammals has been that alarm calls are associated with a single category of information (primates [14,15], marmots [7] and squirrels [5]). Combined messages have been reported, but only rarely: meerkat (Suricata suricatta) alarms differ acoustically according to both predator type and distance of detection [8], and chickadee (Poecile atricapilla) alarms appear to encode something about the locomotion (flying versus perched), size and manoeuvrability of a raptor [4]. Here, we have shown that titi monkey alarms encode information about a predator's type and its location in terms of ground versus canopy, as combinations of two call types within the same sequence.
Interestingly, monkeys did not use the same patterns of combinations for both predators. During raptor encounters, spatial information was conveyed by presence/absence of a series of B-calls inserted within the raptor-typical A-series. A series beginning with multiple A-calls, in other words, provides reliable information to other monkeys that the caller has spotted a predator within the canopy, typically a raptor or, less often, capuchin monkeys (see electronic supplementary material, figure S2). If the caller then switches to B-calls, this indicates that the event is taking place on the ground. By contrast, during oncilla encounters, monkeys give B-series with spatial information conveyed by an optional introductory single A-call (see the electronic supplementary material, figure S2 for two exceptions to this pattern). Inter-call intervals also differed systematically with experimental condition. In raptor encounters, the interval between the first two calls was longer for terrestrial than arboreal encounters, whereas the opposite was found for oncilla encounters, although the overall call rates remained the same.
These and previous results [12] demonstrate that titi monkeys are potentially able to convey a range of information at the call sequence level. Some of this is demonstrably meaningful to listeners: with field experiments [13], we have shown that monkeys respond to the two basic sequences (A- and B-series) in adaptive ways, implying that their alarm call system also functions referentially [16]. However, as mentioned, differences in call intervals and sequence composition may also convey information concerning the location of a predator. Whether recipients are able to make inferences from different sequences will require further research.
The structural rules of this unusual communication system are relatively complex. Interestingly, A-call series were more common and primarily given in response to raptors (both falcons and eagles), regardless of their behaviour (perched, flying and calling); but they were also given in response to typical terrestrial predators, such as the oncilla or predatory capuchin monkeys, provided they were encountered in trees ([12]; electronic supplementary material, figure S2). By contrast, B-call series were produced not only to felid predators and to tayra (Eira barbara), but also to non-predatory disturbances on the ground and when monkeys were feeding/foraging near or descending to the ground [12]. Thus, hearing a series of B-calls does not seem to carry much referential specificity, suggesting that listeners will have to take additional information into account before deciding on how to respond, although we cannot rule out that there are subtle acoustic differences in the structure of B-calls that reveal something about the external context. Although there are some indications that locational information may be also incorporated in other primate alarm calls or call combinations [17–19], the results presented here go beyond anything previously described for call sequences [20], by suggesting the ability to convey information in sequential patterning on both predator type and location simultaneously.
In sum, the alarm calling behaviour of black-fronted titi monkeys is remarkably versatile, based on complex, structurally organized sequences that have the potential to convey information about both location and type of a predator. The fact that such rich information can be indicated, by changing the order and number of calls, raises interesting questions about the evolution of communication in this species, and also how these monkeys categorize different aspects of their environment. The study also demonstrates that event-specific call combinations are not specific to Old World monkeys and apes [20,21], suggesting that this type of vocal behaviour has evolved before the phylogenetic split or independently in the two lineages.
Acknowledgements
We thank the Fathers Marcos, Lauro, Wilson and Sebastião for permission to work on their reserve, Aline Abreu, Vandilso Farias, Aryanne Clyvia Martins Moreira and Rafaela Vale dos Santos for support in the field and Simon Townsend, Cat Hobaiter and three anonymous referees for comments on the manuscript.
The research reported in this article was conducted in compliance with all relevant local and international laws.
Data accessibility
Data submitted to dryad: http://dx.doi.org/10.5061/dryad.sd1sr.
Funding statement
The research was financially supported by FAPEMIG-Brazil, CAPES-Brazil, the Leakey Trust and the University of St Andrews.
References
- 1.Seyfarth RM, Cheney DL, Marler P. 1980. Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science 210, 801–803 (doi:10.1126/science.7433999) [DOI] [PubMed] [Google Scholar]
- 2.Zuberbühler K, Noë R, Seyfarth RM. 1997. Diana monkey long-distance calls: messages for conspecifics and predators. Anim. Behav. 53, 589–604 (doi:10.1006/anbe.1996.0334) [Google Scholar]
- 3.Kirchhof J, Hammerschmidt K. 2006. Functionally referential alarm calls in tamarins (Saguinus fuscicollis and Saguinus mystax): evidence from playback experiments. Ethology 112, 346–354 (doi:10.1111/j.1439-0310.2006.01165.x) [Google Scholar]
- 4.Templeton CN, Greene E, Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 (doi:10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 5.Owings DH, Virginia RA. 1978. Alarm calls of California ground squirrels (Spermophilus beecheyi). Z. Tierpsychol. 46, 58–70 (doi:10.1111/j.1439-0310.1978.tb01438.x) [Google Scholar]
- 6.Evans CS, Evans L, Marler P. 1993. On the meaning of alarm calls: functional reference in an avian vocal system. Anim. Behav. 46, 23–38 (doi:10.1006/anbe.1993.1158) [Google Scholar]
- 7.Blumstein DT. 2007. The evolution, function, and meaning of marmot alarm communication. Adv. Study Behav. 37, 371–400 (doi:10.1016/S0065-3454(07)37008-3) [Google Scholar]
- 8.Manser MB. 2001. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. Lond. B 268, 2315–2324 (doi:10.1098/rspb.2001.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari SF. 2009. Predation risk and antipredator strategies. In South American primates developments in primatology: progress and prospects (eds Ferrari SF, Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB.), pp. 251–277 New York, NY: Springer [Google Scholar]
- 10.Miller LE, Treves A. 2011. Predation on primates: past studies, current challenges, and directions for the future. In Primates in perspective (eds Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder SK.), pp. 535–547 Oxford, UK: Oxford University Press [Google Scholar]
- 11.Kinzey W, Becker M. 1983. Activity pattern of the masked titi monkey, Callicebus personatus. Primates 24, 337–343 (doi:10.1007/BF02381979) [Google Scholar]
- 12.Cäsar C, Byrne RW, Young RJ, Zuberbühler K. 2012. The alarm call system of wild black-fronted titi monkeys, Callicebus nigrifrons . Behav. Ecol. Sociobiol. 66, 653–667 (doi:10.1007/s00265-011-1313-0) [Google Scholar]
- 13.Cäsar C, Byrne RW, Hoppitt W, Young RJ, Zuberbühler K. 2012. Evidence for semantic communication in Titi monkey alarm calls. Anim. Behav. 84, 405–411 (doi:10.1016/j.anbehav.2012.05.010) [Google Scholar]
- 14.Zuberbühler K. 2009. Survivor signals: the biology and psychology of animal alarm calling. Adv. Study Behav. 40, 277–322 [Google Scholar]
- 15.Cäsar C, Zuberbühler K. 2012. Referential alarm calling behaviour in New World primates. Curr. Zool. 58, 680–697 [Google Scholar]
- 16.Macedonia JM, Evans CS. 1993. Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93, 177–197 (doi:10.1111/j.1439-0310.1993.tb00988.x) [Google Scholar]
- 17.Zuberbühler K. 2000. Referential labelling in Diana monkeys. Anim. Behav. 59, 917–927 (doi:10.1006/anbe.1999.1317) [DOI] [PubMed] [Google Scholar]
- 18.Schel AM, Candiotti A, Zuberbühler K. 2010. Predator-deterring alarm call sequences in Guereza colobus monkeys are meaninfgul to cosnpecifics. Anim. Behav. 80, 799–808 (doi:10.1016/j.anbehav.2010.07.012) [Google Scholar]
- 19.Norris JC. 1990. The semantics of Cebus olivaceus alarm calls: object designation and attribution. PhD dissertation, University of Florida, Gainesville, FL, USA [Google Scholar]
- 20.Arnold K, Zuberbühler K. 2006. Semantic combinations in primate calls. Nature 441, 303 (doi:10.1038/441303a) [DOI] [PubMed] [Google Scholar]
- 21.Clay Z, Zuberbühler K. 2011. Bonobos extract meaning from call sequences. PLoS ONE 6, e18786 (doi:10.1371/journal.pone.0018786) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data submitted to dryad: http://dx.doi.org/10.5061/dryad.sd1sr.