Abstract
Variation in behaviour occurs at multiple levels, including between individuals (personality) and between situations (plasticity). Behaviour also varies within individuals, and intra-individual variation (IIV) in behaviour describes within-individual residual variance in behaviour that remains after the effects of obvious external and internal influences on behaviour have been accounted for. IIV thus describes how predictable an individual's behaviour is. Differences in predictability, between individuals and between situations, might be biologically significant. For example, behaving unpredictably under predation threat might reduce the chance of capture. Here, we investigated the duration of startle responses in hermit crabs, in the presence and absence of a predator cue. Individuals differed in startle response duration (personality) and while individuals also varied in their sensitivity to risk, mean response time was greater in the presence of a predator (plasticity). Moreover, IIV was greater in the presence of a predator, providing some of the first evidence that the facultative injection of unpredictability into behaviour might represent a strategy for dealing with risk.
Keywords: personality, intra-individual variation, boldness, risk, hermit crab
1. Introduction
Consistent between-individual variation in behaviour (‘animal personality’) is currently receiving a huge amount of interest, and evidence from a wide range of taxa [1] supports the notion that it can be evolutionarily significant [2–4]. But even when between-individual variation in behaviour is accounted for, residual variance remains. Some of this residual variance may remain even when systematic trends, for instance habituation, are accounted for [5]. This remaining ‘intra-individual variation (IIV) in behaviour’ [5] describes how predictable an individual's behaviour is. As in the case of individual-mean (observations in an individual averaged across several trials) and population-mean (observations in a sample of individuals averaged across individuals) levels of behaviour, IIV in behaviour has the potential to vary between individuals and between situations. Individuals might also vary in how sensitive they are to a change in situation [6] so that the behaviour of some individuals changes markedly across conditions but others change little. When detected at the individual-mean level, these differences in behavioural reaction norms are referred to as the ‘individual × environment (I × E) interaction’ [7]; with sufficient data, it is theoretically possible to detect differences in ‘reaction norms of IIV’ [8] as well.
One explanation for unpredictable behaviour is that, under conditions of predation threat, behaving too predictably might increase the risk of capture [9]. For example, ‘protean’ behaviour, where prey show random locomotion patterns, might enhance their ability to evade predators [10]. On the other hand, random behaviour could cause departures from otherwise optimal ways of behaving [11]. Therefore, we might expect to see behavioural plasticity in IIV, such that individuals adjust their predictability, behaving less predictably under conditions of high risk [10].
Significant between-individual differences in IIV have been recently demonstrated in hermit crabs, Pagurus bernhardus, and damsel fish [5,8] and in zebra finches [12]. Residuals from relationships between observation number and behaviour quantify IIV that has been corrected for any systematic changes that may occur over time. The variance associated with these residuals then gives a measure of predictability, the ‘residual individual standard deviation’ (riSD), high riSD representing low predictability [5]. In both hermit crabs and damselfish, between-individual differences in IIV occurred in the presence of consistent between-individual differences in mean behavioural responses [2–4, 6–7]. In hermit crabs, the behaviour concerned was the latency of re-emergence from a gastropod shell following a rapid ‘startle response’, of withdrawing into the shell (hereafter referred to as ‘latency’). At the population mean level, the duration of this response increases in the presence of predators [13]. While there is significant behavioural plasticity in the duration of these responses at the sample mean level, it is not yet known whether there are significant between-individual differences in sensitivity to risk in hermit crabs. Furthermore, if IIV is a plastic aspect of phenotype, adjusted to match the level of risk, we would expect to see elevated riSD in the presence of a predator [10]. I also ask whether individuals differ from one another in their riSD, i.e. are some individuals more predictable than others.
2. Material and methods
Pagurus bernhardus were collected intertidally from Hannafore Point, Cornwall, UK between September and December 2008. They were transported back to the laboratory and transferred to 125 l holding tanks containing constantly filtered aerated seawater at 15°C. Apart from the period 1 h prior to startle response induction (see below), crabs were fed ad libitum on cubes of white fish. Crabs were removed from their gastropod shell (using in a bench vice) and sexed. Only male crabs free from parasites, appendage loss or recent moult were used (see the electronic supplementary material, S1). Fifty crabs were placed individually into 12 cm crystallizing dishes containing aerated seawater and supplied with a Littorina littorea shell, of optimal mass [14]. Crabs were left to acclimatize to their new surroundings for 24 h. One hour before induction of the startle response, the seawater was drained from the crab's crystallizing dish and replaced with either fresh seawater (predator cue absent) or seawater that was taken from 30 l of seawater containing 25 shore crabs, Carcinus maenas (predator cue present). To induce a startle response, a crab is removed from its dish by hand, held in an inverted position for 5 s and replaced in the dish in this inverted position [13]. Latency is timed from the point where the crab is replaced to the point where the crab emerges from the shell and makes contact with the base of the dish with its pereopods [5,8,13,14]. Startle responses were induced on the second and sixth day of each week over a 26-day period, providing eight latencies. Each crab underwent four trials in each situation. To avoid the possibility of confounding treatment order effects, the order of cue exposure was varied between crabs (see the electronic supplementary material, S1). During the experiment, four crabs moulted reducing the sample size to 46. For the analysis of IIV, we followed the same approach used in recent studies [5,8], described in the electronic supplementary material, S2.
3. Results
(a). Mean level behaviour: plasticity, personality and I×E
There was no situation × trial interaction (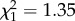 , p = 0.25) or situation × weight interaction (
, p = 0.25) or situation × weight interaction (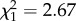 , p = 0.1). Latency did not vary with weight (
, p = 0.1). Latency did not vary with weight (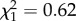 , p = 0.42) or between trials (
, p = 0.42) or between trials (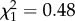 , p = 0.49) but latencies were greater in the presence of the predator cue than in its absence (
, p = 0.49) but latencies were greater in the presence of the predator cue than in its absence (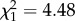 , p = 0.034; figure 1). As variance differed between individuals (see below), these likelihood ratio tests should be interpreted cautiously [5]. Individuals differed in intercept (the absence of the predator cue) (
, p = 0.034; figure 1). As variance differed between individuals (see below), these likelihood ratio tests should be interpreted cautiously [5]. Individuals differed in intercept (the absence of the predator cue) (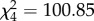 , p < 0.0001) and in slope, such that some were significantly more sensitive to the cue than others (
, p < 0.0001) and in slope, such that some were significantly more sensitive to the cue than others (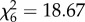 , p = 0.002; figure 1). There was a significant positive correlation between intercept and slope, indicating that less bold individuals with higher intercepts were also more sensitive to the presence of the predator (
, p = 0.002; figure 1). There was a significant positive correlation between intercept and slope, indicating that less bold individuals with higher intercepts were also more sensitive to the presence of the predator (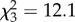 , r = 0.437, p = 0.002; figure 1). To check that this correlation was not biased by a floor effect, I tested for a correlation in the between-situation difference in latency (present − absent) and average duration (present + absent/2) across situations [12] (Spearman correlation: ρ46 = 0.395, p = 0.008).
, r = 0.437, p = 0.002; figure 1). To check that this correlation was not biased by a floor effect, I tested for a correlation in the between-situation difference in latency (present − absent) and average duration (present + absent/2) across situations [12] (Spearman correlation: ρ46 = 0.395, p = 0.008).
Figure 1.
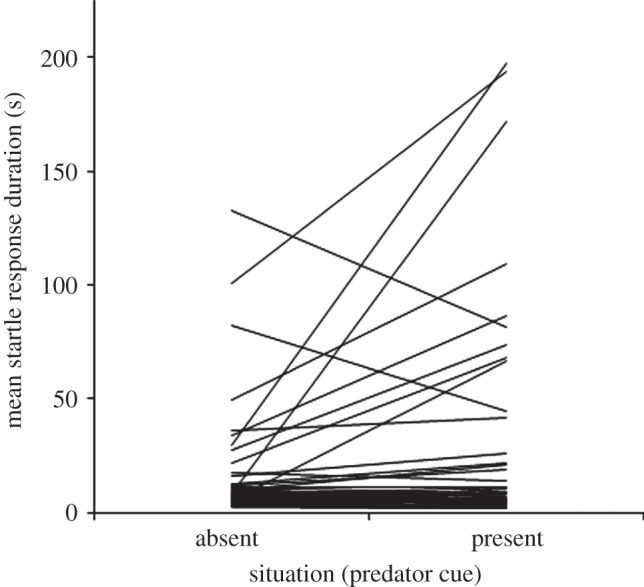
Inter-individual variation in reaction norms across situations. For each crab, a line links the mean startle response durations in the presence and absence of the predator cue.
(b). Intra-individual variation in behaviour: plasticity and personality
As latencies differed between situations, and as the effect of situation differed between individuals, I performed two additional analyses of latency from which to quantify situation-specific repeatability and riSD for each individual (see the electronic supplementary material, S2). In the absence of the predator cue, there was no effect of trial (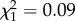 , p = 0.78) and no slope effect (
, p = 0.78) and no slope effect (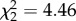 , p = 0.11), but individuals differed in intercept (
, p = 0.11), but individuals differed in intercept (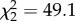 , p < 0.0001), and slope and intercept were negatively correlated (
, p < 0.0001), and slope and intercept were negatively correlated (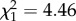 , r = −0.76, p = 0.035). In the presence of the predator cue, there was no effect of trial (
, r = −0.76, p = 0.035). In the presence of the predator cue, there was no effect of trial (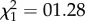 , p = 0.26), no slope effect (
, p = 0.26), no slope effect (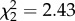 , p = 0.3) and no correlation between intercept and slope (
, p = 0.3) and no correlation between intercept and slope (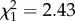 , p = 0.12), but individuals differed in intercept (
, p = 0.12), but individuals differed in intercept (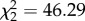 , p < 0.0001). Repeatability for each situation was RM = 0.76 (absent) and RM = 0.70 (present). While repeatability was similar, predictability was reduced (i.e. riSD was greater) in the presence of the predator cue (Z45 = 2.43, p = 0.015, r = 0.25; figure 2), an effect that remained marginally significant when riSD was standardized with respect to mean latency (see the electronic supplementary material, S2) (Z45 = 1.95, p = 0.051, r = 0.21). Further, there were significant differences in IIV among individuals within in each situation (absent; W45,139 = 2.3, p < 0.0001, present; W45,139 = 3.3, p < 0.0001). This indicates that within both situations, individuals significantly differed from one another in predictability. There was also a significant correlation in riSD between situations (ρ46 = 0.44, p = 0.003).
, p < 0.0001). Repeatability for each situation was RM = 0.76 (absent) and RM = 0.70 (present). While repeatability was similar, predictability was reduced (i.e. riSD was greater) in the presence of the predator cue (Z45 = 2.43, p = 0.015, r = 0.25; figure 2), an effect that remained marginally significant when riSD was standardized with respect to mean latency (see the electronic supplementary material, S2) (Z45 = 1.95, p = 0.051, r = 0.21). Further, there were significant differences in IIV among individuals within in each situation (absent; W45,139 = 2.3, p < 0.0001, present; W45,139 = 3.3, p < 0.0001). This indicates that within both situations, individuals significantly differed from one another in predictability. There was also a significant correlation in riSD between situations (ρ46 = 0.44, p = 0.003).
Figure 2.
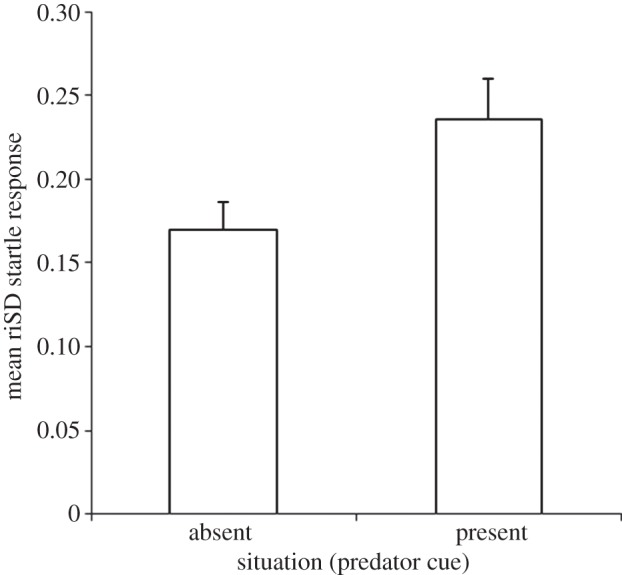
Mean riSD (±s.e.) of startle response durations in the presence and absence of the predator cue. In the presence of the predator cue, riSD was greater, meaning that startle response durations were less predictable.
4. Discussion
Compared to consistent between-individual differences, the underlying causes of IIV are less well understood and evidence for its presence in non-human animals only recently obtained (although see studies in the human literature, for example [15]). In a previous study on hermit crabs, IIV of startle response duration was greater at higher temperatures [7]. This result was consistent with the idea that animals should behave unpredictably in the presence of elevated risk [9,16] because metabolic rate, and the need to search for food, increase with temperature in poikilotherms. The current data directly link IIV to external predator cues. Not only are startle responses of greater duration in the perceived presence of risk, but they are also less predictable. Thus, in addition to changing with environmentally driven variation in metabolism, IIV also increases in response to extrinsic information about risk.
For P. bernhardus faced with the presence of C. maenas, unpredictable re-emergence times could be adaptive. Although C. maenas can crush L. littorea shells [17], the opportunity to consume P. bernhardus will be enhanced when the crab is exposed. When the crab is withdrawn, more of the shell must be crushed until edible body parts are reached; crushing begins with material around the margin of the aperture and then moves back towards the apex. Uncertainty about when re-emergence will occur might reduce the willingness of the predator to persist with shell crushing. Certainly, re-emergence after a predictable hiding time would not be a good anti-predator strategy. At times of low risk, on the other hand, it would not make sense to deviate from the optimal re-emergence time, which presumably depends on proximate factors such as the encounter rate of resources (e.g. food, mates and empty gastropod shells), internal state (e.g. hunger level, reproductive condition and hormonal status) and perhaps shell quality [14]. Note that high IIV would not necessarily be adaptive in all contexts. For example, signals of individual quality are often dependent on a consistent performance.
In other animals reliant on the protection of a shelter, it also seems likely that unpredictable emergence would be advantageous in the presence of risk. However, individuals might also differ in their sensitivity to predation risk and show different behavioural reaction norms [6], as demonstrated here (figure 1). What is not yet clear, is whether individuals differ in the amount by which IIV varies between situations. To elucidate this would require a ‘multiple-burst’ [5,8] experiment, whereby each individual is subjected to several bursts of observations under each situation (in this study, one set of observations was collected per situation). Nevertheless, this study shows for the first time that nested within the patterns of (i) population level plasticity (ii) an individual by environment interaction across the presence or absence of a predator, there is another pattern of plasticity in IIV in behaviour. Although predation experiments will be needed to confirm the fitness consequences of IIV, this plasticity in IIV may represent a strategy for coping with risk in diverse organisms.
Acknowledgements
I am grateful for the insightful comments of Morgan David and three anonymous reviewers.
References
- 1.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dall SRX, Houston AI, McNamara JM. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- 3.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584 (doi:10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 4.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959–3968 (doi:10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamps JA, Briffa M, Biro PA. 2012. Unpredictable animals: individual differences in intra-individual variability (IIV). Anim. Behav. 83, 1325–1334 (doi:10.1016/j.anbehav.2012.02.017) [Google Scholar]
- 6.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 7.Dingemanse NJ, Kazem AJ, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 8.Briffa M, Bridger D, Biro PA. 2013. How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim. Behav. 86, 47–54 (doi:10.1016/j.anbehav.2013.04.009) [Google Scholar]
- 9.Brembs B. 2011. Towards a scientific concept of free will as a biological trait: spontaneous actions and decision-making in invertebrates. Proc. R. Soc. B 278, 930–939 (doi:10.1098/rspb.2010.2325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones KA, Jackson AL, Ruxton GD. 2012. Prey jitters; protean behaviour in grouped prey. Behav. Ecol. 22, 831–836 (doi:10.1093/beheco/arr062) [Google Scholar]
- 11.Ydenberg RC, Dill LM. 1986. The economics of fleeing from predators. Adv. Stud. Behav. 16, 229–249 (doi:10.1016/s0065-3454(08)60192-8) [Google Scholar]
- 12.David M, Auclair Y, Cézilly F. 2012. Assessing short- and long-term repeatability and stability of personality in captive zebra finches using longitudinal data. Ethology 118, 932–942 (doi:10.1111/j.1439-0310.2012.02085.x) [Google Scholar]
- 13.Briffa M, Rundle SD, Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briffa M, Bibost A-L. 2009. Effects of shell size on behavioural consistency and flexibility in hermit crabs. Can. J. Zool. 87, 597–603 (doi:10.1139/Z09-047) [Google Scholar]
- 15.Salthouse TA, Nesselroade JR. 2010. Dealing with short-term fluctuation in longitudinal research. J. Gerontol. B Psychol. Sci. Soc. Sci. 65, 698–705 (doi:10.1093/geronb/gbq060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maye A, Hsieh C-H, Sugihara G, Brembs B. 2007. Order in spontaneous behaviour. PLoS ONE 2, e443 (doi:10.1371/journal.pone.0000443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotton PA, Rundle SD, Smith KE. 2004. Trait compensation in marine gastropods: shell shape, avoidance behavior, and susceptibility to predation. Ecology 85, 1581–1584 (doi:10.1890/03-3104) [Google Scholar]


