Abstract
Fatty acid composition of eggs affects development, growth and ecological performance of fish embryos and larvae, with potential consequences for recruitment success. Essential fatty acids in eggs derive from the maternal diet, and the time between ingestion and deposition in eggs is ecologically important but unknown. We examined the dynamics of diet–egg transfer of arachidonic acid (ARA) in the batch-spawning fish, red drum (Sciaenops ocellatus), by measuring ARA concentrations in eggs after a single diet shift and during a period of irregular variations in diet. ARA concentrations in eggs changed within 2–16 days of a diet shift. The rate of change was proportional to the magnitude of the shift, with no evidence of equilibration. These results are not consistent with eggs being assembled entirely from accumulated body stores. The immediate source of ARA in eggs appears to be the recent diet. We propose that batch spawning produces rapid diet–egg transfer of ARA because it removes large amounts of fatty acids from the body and prevents equilibration. The immediacy of the diet–egg connection suggests that spawning migration combined with short-interval batch spawning may have evolved to take advantage of nutrients critical for offspring survival that are available at the spawning site.
Keywords: egg composition, lipid deposition, reproductive strategies, batch spawning
1. Introduction
Use of food resources to fuel reproduction ranges from capital breeders, which accumulate and store energy and nutrients prior to the breeding season, to income breeders, which use recently acquired resources [1,2]. These strategies can have very different transgenerational effects [1], which are especially important for populations that are controlled by larval survival. If eggs are assembled from accumulated reserves, those body stores could serve as a buffer that maintains egg quality when maternal food resources are poor. By contrast, if somatic reserves are low or they are not a major source for oogenesis, egg composition could fluctuate with diet over short-time scales, with potentially important consequences for offspring survival and recruitment.
Fishes require certain long-chain, highly unsaturated fatty acids (HUFAs) for normal physiological function, and these HUFAs must be obtained from dietary sources because they cannot be synthesized in sufficient quantities to meet these requirements [3,4]. As embryos and early larvae obtain all energy and materials from maternally derived yolk and oil droplets, the ultimate source of essential HUFAs in eggs is the maternal diet [5,6]. The immediate source is generally thought to be somatic reserves. Three HUFAs—arachidonic acid (ARA; 20 : 4ω-6), eicosapentaenoic acid (20 : 5ω-3) and docosahexaenoic acid (22 : 6ω-3)—are especially important for growth and development of marine fish embryos and larvae [7,8], and concentrations of certain HUFAs (including ARA) in eggs are correlated with anti-predator performance of larvae [9]. This implies a direct connection between maternal diet and offspring survival, and the possibility that recruitment may be linked to the match or mismatch between spawning adults and high-quality food resources [9]. The time frame over which this diet–egg connection operates has important ecological implications but is not known.
We report on experiments that clarify the dynamics of diet–egg relationships for red drum (Sciaenops ocellatus), using ARA as a representative HUFA. We established different starting levels of ARA in eggs, then imposed an abrupt shift in dietary intake of ARA and measured concentrations in eggs spawned over the subsequent 30 days. We also measured ARA concentrations in eggs after irregular variations in dietary intake. We expected a lag in the response of egg ARA concentrations to a diet shift if somatic reserves were the immediate source (capital breeding), and the duration of the lag to be positively related to the starting level of ARA in eggs. A small or absent lag would indicate that the immediate source of ARA in eggs was the recent diet (income breeding).
2. Material and methods
(a). Diet-shift experiments
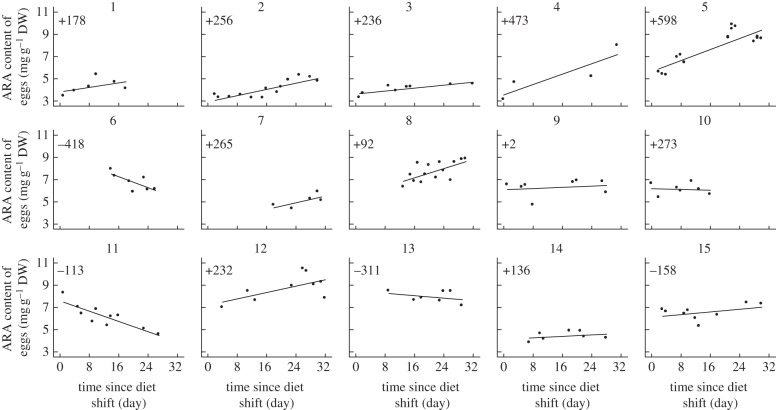
Fifteen experiments were conducted using nine separate tanks of adult fish (length: 90–100 cm, weight: 9–15 kg) that were fed three times per week (Monday, Wednesday and Friday). In each experiment, the diet was switched from one level of ARA (ARApre, mg ARA wk−1 fish−1) to another (ARApost; table 1) by changing the proportions of shrimp (Litopenaeus setiferus or Farfantepenaeus aztecus), Atlantic mackerel (Scomber scombrus), Spanish sardine (Sardinella aurita), squid (Loligo opalescens), beef liver, dry commercial feed and an ARA supplement in the diet. This produced a wide range of starting levels of ARA in eggs and a wide range of magnitudes of dietary change (ΔARA = ARApost − ARApre; figure 2). Values for ARApre and ARApost were calculated as 28-day averages of dietary ARA intake preceding and succeeding the diet shift, respectively. On average, fish spawned every 4 (±1) days but not always immediately after the diet shift, and eggs were sampled from almost every spawn. ARA content of eggs and diet components was analysed by gas chromatography (see the electronic supplementary material). These diet-shift experiments simulate conditions that might occur in species that migrate for spawning.
Table 1.
Conditions for 15 diet-shift experiments and results from time courses (figure 1). (ARA0 is the estimated ARA content of eggs at the time of the diet shift. IARA is the rate of incorporation of ARA into eggs after the diet shift. s.e. is standard error for the estimated coefficient.)
| experiment | tank no. | ARA in diet (mg wk−1 fish−1) |
no. females | ARA0 (mg g−1 DW) |
IARA (mg g−1 DW d−1) |
|||
|---|---|---|---|---|---|---|---|---|
| ARApre | ARApost | ARA0 | s.e. | IARA | s.e. | |||
| 1 | 1 | 374 | 552 | 1 | 3.82 | 0.497 | 0.051 | 0.045 |
| 2 | 2 | 523 | 779 | 1 | 2.93 | 0.243 | 0.070 | 0.013 |
| 3 | 3 | 615 | 851 | 1 | 3.63 | 0.149 | 0.033 | 0.009 |
| 4 | 3 | 851 | 1324 | 1 | 3.55 | 0.875 | 0.117 | 0.044 |
| 5 | 4 | 256 | 854 | 1 | 5.64 | 0.425 | 0.124 | 0.023 |
| 6 | 4 | 854 | 436 | 1 | 9.24 | 1.053 | −0.124 | 0.051 |
| 7 | 5 | 260 | 525 | 1 | 3.11 | 1.139 | 0.075 | 0.043 |
| 8 | 5 | 525 | 617 | 1 | 5.51 | 0.775 | 0.103 | 0.035 |
| 9 | 6 | 673 | 675 | 1 | 6.09 | 0.476 | 0.014 | 0.027 |
| 10 | 6 | 675 | 948 | 1 | 6.18 | 0.387 | −0.008 | 0.040 |
| 11 | 6 | 948 | 835 | 1 | 7.59 | 0.344 | −0.115 | 0.024 |
| 12 | 7 | 764 | 996 | 3 | 7.19 | 0.832 | 0.072 | 0.035 |
| 13 | 8 | 764 | 453 | 3 | 8.50 | 0.694 | −0.028 | 0.032 |
| 14 | 9 | 211 | 347 | 2 | 4.13 | 0.394 | 0.017 | 0.022 |
| 15 | 5 | 558 | 400 | 1 | 6.12 | 0.390 | 0.030 | 0.024 |
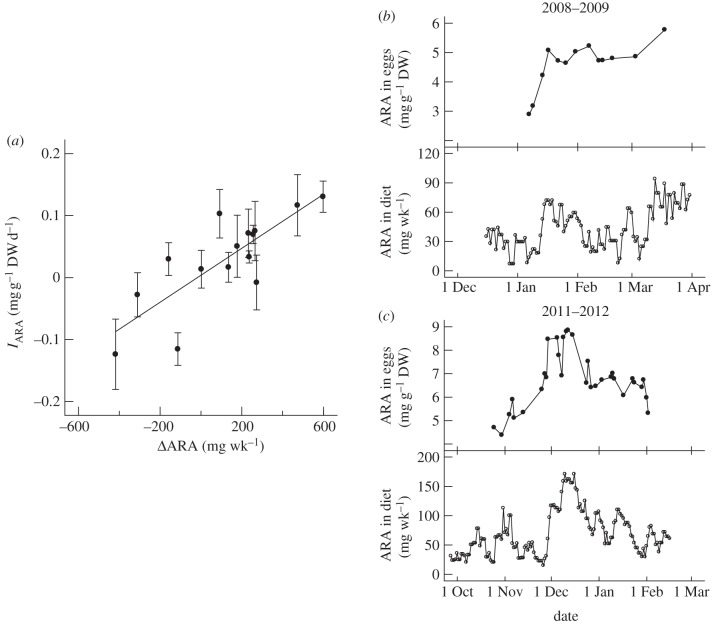
Figure 2.
(a) Diet-shift experiments. Relationship between rate of incorporation of ARA into eggs (IARA) and the change in ARA content of diets (ΔARA). IARA was estimated as the slope of a linear regression fitted to each time course (figure 1) and plotted ±1 s.e. Regression line represents equation (3.2). (b,c) Irregular variations in diet experiments. ARA content of eggs (upper panels) and 7-day mean daily ARA intake by adults (lower panels). Data for eggs were shifted to the left by the calculated lags of (b) 6 days and (c) 2 days to highlight the correspondence between trends.
(b). Irregular variations in diet
Two additional experiments were conducted, one in 2008–2009 and one in 2011–2012, to determine whether egg fatty acid composition is sensitive to multiple, irregular variations in dietary intake and to estimate the time lag between ARA intake and its appearance in eggs. In these experiments, spawns were sampled from two separate tanks of adults (two females, three males each) that were fed a constant wet weight of food 3 days per week, but proportions of the diet components (shrimp, squid, sardine and mackerel) varied irregularly. Intake of ARA (mg d−1 fish−1) was calculated for each feeding.
(c). Statistical analyses
ARA concentrations in eggs from each of the diet-shift experiments constituted a time course for the incorporation of ARA into eggs. Each time course was characterized by two parameters: ARA content of eggs at the time of the diet shift (regression intercept, ARA0, in mg g−1 dry weight (DW)), and rate of incorporation of ARA in eggs (regression slope, IARA, mg g−1 DW d−1). The lag between ARA intake and deposition in eggs was estimated using data from the two tanks with irregular variations in diet. For that analysis, mean ARA intake for each day of the spawning period was estimated as the total ARA in the feedings during the seven preceding days divided by seven, as the feeding schedule repeated weekly. Correlation coefficients were calculated between mean daily intake and egg ARA content for lags of 0–10 days (see the electronic supplementary material).
3. Results
(a). Diet-shift experiments
The diet shifts (ΔARA = −418 to +598 mg wk−1 fish−1) produced time courses for ARA concentration of eggs that were approximately linear for at least one month (figure 1). Initial concentrations (ARA0) varied threefold among experiments, from 2.9 to 9.2 mg g−1 DW (table 1). Observable changes in egg ARA concentrations occurred within 4–16 days after the diet shift (figure 1). Only one experiment (number 2) provided evidence of a lag (approx. 15 days), and that occurred when ARA0 was low and a pronounced lag would not be expected because somatic reserves of ARA should have been low.
Figure 1.
Time courses for incorporation of ARA into eggs after a diet shift. Numbers above each panel identify experiments and correspond to diets and results in table 1. Numbers within panels denote magnitude of diet shift (ΔARA). Each data point represents an individual spawn.
(b). Diet–egg dynamics
Rate of incorporation of ARA in eggs (IARA) was influenced by both ARApre and ARApost. Together, they explained 68% (R2) of the total variance in ARA content of eggs (p = 0.001). Both diets made significant contributions to the overall regression model (p = 0.001 for ARApre; p = 0.002 for ARApost), and they were not significantly correlated with each other (r = 0.40, p = 0.14).
| 3.1 |
The nearly equal but opposite values for the coefficients of ARApre and ARApost indicate that the relationship could be expressed more simply as a function of ΔARA. In fact, the regression of IARA on ΔARA (R2 = 0.64, p < 0.001; figure 2a) was statistically indistinguishable from equation (3.1):
| 3.2 |
(c). Irregular variations in diet
ARA content of eggs from adults that experienced irregular variations in diet showed a striking correspondence with averaged ARA intake (figure 2b,c). For the 2008–2009 data, the strongest correlation between ARA intake and ARA content of eggs was for a 6-day lag (r = 0.72, p = 0.006, n = 13 spawns). For the 2011–2012 data, a 2-day lag offered the strongest correlation (r = 0.56, p < 0.001, n = 32 spawns).
4. Discussion
Only two prior studies provide insight on the short-term dynamics of the diet–egg relationship in marine fishes. Fatty acid composition of gilthead seabream (Sparus aurata) eggs equilibrated within 15 days after a diet shift from a low level of ω-3 HUFAs to a high-level or the reverse shift [10]. ARA concentration in red drum eggs decreased steadily for up to 100 days after diet was shifted from high levels of ARA to low levels [9]. Using a wide range of starting concentrations in eggs and magnitudes of diet shifts, we found that egg composition responded quickly to dietary fluctuations. Incorporation rates of ARA remained constant for at least one month and were a linear function of the magnitude of the diet shift. Separate experiments confirmed the rapid link between dietary intake and egg composition, showing a lag of 2–6 days rather than weeks or months. Taken together, these results are not consistent with eggs being assembled from accumulated somatic reserves (capital breeders), and they demonstrate that red drum lack the capacity to buffer short-term fluctuations in dietary supply of ARA.
A simple dilution model has been shown to adequately describe the change in fatty acid composition of somatic tissues after a diet shift for many fish species [11,12]. Under this model, the rate of change in dietary fatty acid content is directly proportional to the difference between current and final tissue levels of fatty acids. The rate diminishes over time as the fatty acid content of the body approaches the equilibrated level. In our study, ARApre and ARApost are proxies for the initial and final levels, respectively. The fact that IARA is directly proportional to ΔARA (figure 2a) is consistent with the dilution model. However, the model predicts that IARA would decrease with time, tending towards zero (equilibration), yet IARA remained unchanged for at least one month (figure 1). We suggest that this is because equilibration is prevented by frequent spawning (every 4 days on average), which eliminates large amounts of fatty acids from the body and maintains a high differential between intake and tissue levels. This results in continuous, rapid transfer of essential nutrients from the diet to the eggs (income breeders).
It has been suggested that variations in the concentrations of fatty acids in eggs may play a role in the highly variable recruitment of marine fish populations [13], and that a portion of these variations may be caused by changes in food resources that result from migrations, regime shifts and exploitation of marine communities [9]. For migrations to have consequences for fish populations through this mechanism, egg composition must respond quickly to dietary intake. Our results confirm this for ARA, and if these dynamics hold true for other essential nutrients that are critical for larval survival, the immediacy of the diet–egg connection may be part of the motivation for some spawning migrations. Like other species, red drum migrate from offshore waters to more productive near shore habitats and spawn at short intervals for a period of two to three months. This migration exposes reproductive adults to a different prey field, and the composition of their eggs should quickly reflect these new food resources. If the near shore diet confers a survival advantage on their offspring, then this reproductive strategy of migration followed by short-interval batch spawning may have evolved, in part, to take advantage of nutrients available at the spawning site that are critical for offspring survival. The same may be true for other batch-spawning species that migrate to a spawning habitat.
Acknowledgements
We thank Robert Vega and Texas Parks and Wildlife Department staff for assistance with experiments at their facilities, and staff at our Fisheries and Mariculture Laboratory.
Animal procedures were approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin.
Data accessibility
Data deposited in dryad http://dx.doi.org/10.5061/dryad.056r5.
Funding statement
This research was supported by the National Science Foundation (OCE-0425241), Texas Sea Grant College Program, and by the Guy Harvey Ocean Foundation. This publication is contribution 1673 of the University of Texas Marine Science Institute.
References
- 1.Stearns SC. 1989. Trade-offs in life history evolution. Funct. Ecol. 3, 259–268 (doi:10.2307/2389364) [Google Scholar]
- 2.Jönsson KI. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66 (doi:10.2307/3545800) [Google Scholar]
- 3.Sargent JR, Henderson RJ, Tocher DR. 2002. The lipids In Fish nutrition (ed. Halver JE.), pp. 154–219 San Diego, CA: Academic Press [Google Scholar]
- 4.Tocher DR. 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107–184 (doi:10.1080/713610925) [Google Scholar]
- 5.Kjorsvik E, Mangor-Jensen A, Holmefjord I. 1990. Egg quality in fishes. Adv. Mar. Biol. 26, 71–113 [Google Scholar]
- 6.Wiegand MD. 1996. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish Biol. Fish. 6, 259–286 (doi:10.1007/BF00122583) [Google Scholar]
- 7.Izquierdo MS, Socorro J, Arantzamendi L, Hernández-Cruz CM. 2000. Recent advances in lipid nutrition in fish larvae. Fish Physiol. Biochem. 22, 97–107 (doi:10.1023/A:1007810506259) [Google Scholar]
- 8.Johnson RB. 2009. Lipid deposition in oocytes of teleost fish during secondary oocyte growth. Rev. Fish. Sci. 17, 79–99 (doi:10.1080/10641260802590004) [Google Scholar]
- 9.Fuiman LA, Ojanguren AF. 2011. Fatty acid content of eggs determines antipredator performance of fish larvae. J. Exp. Mar. Biol. Ecol. 407, 155–165 (doi:10.1016/j.jembe.2011.06.004) [Google Scholar]
- 10.Harel M, Tandler A, Kissil GW. 1994. The kinetics of nutrient incorporation into body tissues of gilthead seabream (Sparus aurata) females and the subsequent effects on egg composition and egg quality. Br. J. Nutr. 72, 45–58 (doi:10.1079/BJN19940008) [DOI] [PubMed] [Google Scholar]
- 11.Robin JH, Regost C, Arzel J, Kaushik SJ. 2003. Fatty acid profile of fish following a change in dietary fatty acid source: model of fatty acid composition with a dilution hypothesis. Aquaculture 225, 283–293 (doi:10.1016/S0044-8486(03)00296-5) [Google Scholar]
- 12.Jobling M. 1996. Are modifications in tissue fatty acid profiles following a change in diet the result of dilution? Test of a simple dilution model. Aquaculture 232, 551–562 (doi:10.1016/j.aquaculture.2003.07.001) [Google Scholar]
- 13.Bell MV, Sargent JR. 1996. Lipid nutrition and fish recruitment. Mar. Ecol. Prog. Ser. 134, 315–316 (doi:10.3354/meps134315) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in dryad http://dx.doi.org/10.5061/dryad.056r5.