Abstract
Although ambient temperature has diverse effects on disease dynamics, few studies have examined how temperature alters pathogen transmission by changing host physiology or behaviour. Here, we test whether reducing ambient temperature alters host foraging, pathology and the potential for fomite transmission of the bacterial pathogen Mycoplasma gallisepticum (MG), which causes seasonal outbreaks of severe conjunctivitis in house finches (Haemorhous mexicanus). We housed finches at temperatures within or below the thermoneutral zone to manipulate food intake by altering energetic requirements of thermoregulation. We predicted that pathogen deposition on bird feeders would increase with temperature-driven increases in food intake and with conjunctival pathology. As expected, housing birds below the thermoneutral zone increased food consumption. Despite this difference, pathogen deposition on feeders did not vary across temperature treatments. However, pathogen deposition increased with conjunctival pathology, independently of temperature and pathogen load, suggesting that MG could enhance its transmission by increasing virulence. Our results suggest that in this system, host physiological responses are more important for transmission potential than temperature-dependent alterations in feeding. Understanding such behavioural and physiological contributions to disease transmission is critical to linking individual responses to climate with population-level disease dynamics.
Keywords: disease ecology, house finch, fomite, climate change
1. Introduction
Ambient temperature can influence disease dynamics through diverse mechanisms such as expanded vector habitat and differential pathogen persistence [1–4]. However, less is known about how temperature alters pathogen transmission by changing host physiology or behaviour. Revealing these links is critical for understanding the dynamics of infectious diseases of humans and wildlife, particularly in the face of global climate change [2].
Here, we investigated how ambient temperature influences pathogen deposition via changes in foraging and pathology among North American house finches (Haemorhous mexicanus) infected with Mycoplasma gallisepticum (MG). This bacterial pathogen causes conjunctivitis [5] and is spread by indirect contact at bird feeders, which serve as fomites [6]. Epidemics occur during autumn and winter, driven in part by seasonal flocking behaviour and influxes of naive juveniles [7,8]. Given the fomite-based transmission of MG, seasonal epidemics may be exacerbated, particularly during the late autumn and winter, if increased foraging during cold weather (to offset metabolic costs of thermoregulation [9–11]) augments pathogen deposition onto bird feeders. In addition, the degree of conjunctival exudate or swelling may increase pathogen deposition onto feeders during foraging [5]. Our prior work found that finches housed at lower temperatures showed lower conjunctival pathology during MG infection, despite similar pathogen loads [9]. Therefore, manipulation of ambient temperature should allow both the alteration of energy expenditure (and compensatory food intake) and host pathology in response to infection.
To determine the relative importance of foraging versus pathology on MG deposition, we housed finches at two temperatures: one within and one below the thermoneutral zone, defined as the temperature range (22–30°C for house finches) within which an animal's metabolic rate does not vary with ambient temperature [10,11]. Based on prior work, we predicted that finches housed below the thermoneutral zone would increase food intake to compensate for thermoregulatory costs but would exhibit reduced conjunctival pathology [9]. If food intake is more important than pathology in driving MG deposition, we predicted that deposition would be higher in the sub-thermoneutral group and that individual variation in food intake within treatments would predict pathogen deposition. If pathology is more important than food intake, we predicted that deposition would be lower in the sub-thermoneutral group and that variation in pathology within temperature treatments would predict the extent of MG deposition.
2. Material and methods
The electronic supplementary material contains additional methods. Ten days before experimental inoculation, 27 wild-caught female house finches were placed into individual cages with a single-port tube feeder and housed in one of two temperature-controlled rooms. Temperatures were initially 22°C and were adjusted over 6 days to final ranges (night–day) of 22–28°C (thermoneutral) and 13–19°C (sub-thermoneutral) [9–11]. On day 0, birds were inoculated bilaterally in the palpebral conjunctiva with 40 µl of MG in Frey's media or media alone (see electronic supplementary material). Infections were monitored for four weeks (see electronic supplementary material, table S1).
Conjunctival pathogen load was measured by swabbing conjunctival sacs for 5 s with a sterile cotton swab pre-dipped in tryptose phosphate broth (TPB). Swabs were swirled and wrung out into a single tube of 300 μl TPB, which was frozen at −20°C. At 16.00 on the day before sampling, feeder ports were wiped for 10 s with Vircon S disinfectant (DuPont Disinfectants, Sudbury, Suffolk, UK) to remove MG. After 24 h, feeders were sampled for MG by rotating a sterile, TPB-dipped cotton swab inside the port for 10 s, then processing as above. The amount of MG in all samples was determined by quantitative PCR using published methods [12,13]. Conjunctival lesions were scored on a scale of 0–3 per eye (see electronic supplementary material).
At 16.00 on the day before sampling, feeders were weighed and cage floors cleaned. After 24 h, feeders were reweighed and spilled food collected from cage floors. Faecal matter was removed and the amount of food consumed was calculated. On the same day, video of all birds was recorded from 08.00 to 16.00. We calculated total time spent within one body width of the feeder and number of pecks at food during two 15 min focal sampling periods, one between 08.45 and 09.15, and one between 14.45 and 15.15 (see electronic supplementary material).
Conjunctival pathogen load, conjunctival lesions, food consumption and foraging behaviours were analysed in R [14] using linear mixed effects models with individual as a random effect and these fixed effects: MG treatment, temperature treatment, time since inoculation and their interactions, which were removed if p > 0.05. Mass taken 2–4 days before each dependent variable's measurement was also included as a fixed effect (see electronic supplementary material). Data on pathogen load and deposition were log10 transformed.
We assessed the effect of ambient temperature on pathogen deposition, controlling for individual conjunctival pathogen load, mass and foraging behaviours using a general additive mixed effects model [15] (see electronic supplementary material). To determine whether conjunctival pathology and food consumption (which were excluded from the additive model because of colinearity assumptions) affected pathogen deposition, we performed a mixed effects model on residuals from the additive mixed model, using total eye score and food consumption as main effects.
Experiments were conducted under all appropriate permits (see electronic supplementary material).
3. Results
As predicted, birds housed below the thermoneutral zone consumed more food per day than birds housed within the thermoneutral zone (on average 0.8 g±0.2 s.e. or 4.1%±1.0 s.e. of pre-infection body mass; table 1). Regardless of temperature, MG-treated birds consumed less food than controls (on average 0.5 g±0.2 s.e. or 2.2%±1.0 s.e. of pre-infection body mass), indicating infection-induced anorexia (table 1). Surprisingly, while both metrics of foraging behaviour were lower in MG-treated birds, neither varied with temperature (table 1).
Table 1.
Statistical models on responses to infection.
| dependent variables | independent variablesa |
|||||
|---|---|---|---|---|---|---|
| temperature treatment | MG treatment | time | mass | MG × time | temperature × time | |
| pathogen load | F1,24 = 0.14, p = 0.71 | F1,24 = 136.2, p < 0.001* | F3,72 = 6.5, p < 0.001* | F1,72 = 0.2, p = 0.65 | F3,72 = 5.5, p = 0.002* | n.s. |
| eye lesion severity | F1,24 = 0.33, p = 0.57 | F1,24 = 20.7, p < 0.001* | F3,74 = 7.3, p = 0.001* | F1,74 = 0.9, p = 0.35 | F3,74 = 6.4, p = 0.001* | n.s. |
| food consumed (g) | F1,24 = 23.45, p < 0.001* | F1,24 = 5.7, p = 0.03* | F3,73 = 41.9, p < 0.001* | F1,73 = 0.7, p = 0.39 | n.s. | F3,73 = 5.1, p = 0.003* |
| time spent at feeder (min) | F1,24 < 0.001, p = 0.99 | F1,24 = 4.4, p = 0.047* | F3,77 = 3.0, p = 0.04* | F1,77 = 1.5, p = 0.23 | n.s. | n.s. |
| pecks at food (per min) | F1,24 = 0.95, p = 0.34 | F1,24 = 15.5, p = 0.001* | F3,77 = 2.3, p = 0.09 | F1,77 = 2.4, p = 0.12 | n.s. | n.s. |
Bold typeface and *, p < 0.05; n.s. p > 0.05, removed from model.
aThe following were n.s. in all models and are not listed above: temperature × MG and temperature × MG × time.
Contrary to predictions, temperature did not influence eye lesion severity (table 1). Pathogen load followed a similar pattern, with no effect of temperature (table 1).
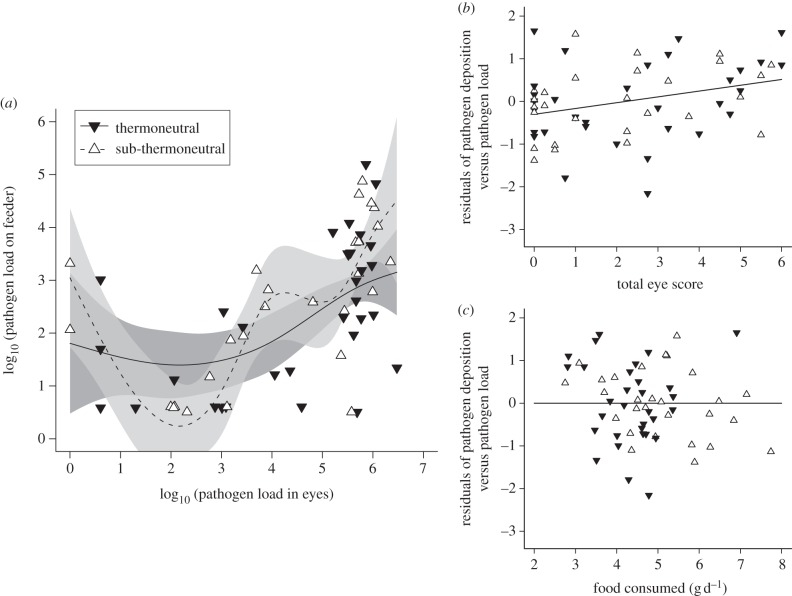
MG deposition on feeders increased nonlinearly with individual pathogen load, but this relationship was unaffected by temperature, foraging behaviours or mass (figure 1a and table 2). For a given pathogen load, birds with more severe eye lesions deposited more MG (figure 1b; F1,42 = 6.66, p = 0.01), but food consumption did not influence deposition (figure 1c; F1,42 = 0.06, p = 0.81).
Figure 1.
(a) Deposition of Mycoplasma gallisepticum (MG) onto bird feeders increased with pathogen load in the eyes of experimentally infected house finches, but ambient temperature did not influence this relationship. (b) Even when controlling for temperature, pathogen load, mass and foraging behaviours, deposition increased with the severity of eye lesions (c) but not with food consumption. Lines: predictions from (a) a general additive mixed model and (b,c) linear mixed model. Shading: ±1 s.e. around predictions.
Table 2.
Statistical models of pathogen deposition. S, cubic spline smoothing function; TN, birds housed within the thermoneutral zone; sub-TN, birds housed below the thermoneutral zone.
| model | AICc | ΔAICc |
|---|---|---|
| S(pathogen load)all birds | 191.0 | 0 |
| mass + S(pathogen load)all birds | 193.2 | 2.2 |
| pathogen load (linear model) | 193.6 | 2.6 |
| mass + foraging behavioursa + S(pathogen load)all birds | 198.9 | 7.9 |
| intercept only | 201.6 | 10.6 |
| temperature + mass + foraging behavioursa + S(pathogen load)all birds | 207.1 | 16.1 |
| temperature + mass + foraging behavioursa + S(pathogen load)TN + S(pathogen load)sub-TN | 240.4 | 94.4 |
aPC1 of a principal components analysis including time at feeder and pecks at food (see electronic supplementary material).
4. Discussion
We show that temperature-driven changes in food consumption do not cause house finches infected with MG to deposit more pathogen on bird feeders, which act as fomites in this system. Because conjunctival pathology did not differ significantly between temperature treatments, as it had in previous experiments [9], we could not assess the contribution of pathology to pathogen deposition at the group level. However, at the individual level, conjunctival pathology significantly increased pathogen deposition, whereas foraging behaviours had no effect. Overall, our results suggest that in this system, differences in within-host pathogen replication and pathology may be more important for transmission dynamics than temperature-dependent changes in food intake. Distinguishing between such contributions to disease transmission is critical for linking individual responses to climate with population-level disease dynamics [2].
Our prior work showed modest, but significant, reductions in pathology at sub-thermoneutral temperatures [9]. While mean conjunctival pathology exhibited a similar pattern here, this trend was not significant. One explanation for this discrepancy is that this study used a less virulent isolate of MG. Complex interactions between host and parasite strains are common, and abiotic factors can further complicate such relationships [16]. Future work on multiple MG isolates could yield informative results regarding complex relationships between temperature and MG disease dynamics. Despite similarities in pathology between temperature treatments here, at the individual level, pathology had a positive effect on pathogen deposition (figure 1b). This pattern indicates that conjunctival exudate and/or swelling likely facilitates pathogen deposition onto feeders. This result suggests that MG isolates that cause higher virulence, independent of pathogen load, may achieve higher fitness by increasing deposition onto feeders.
As food intake was higher in sub-thermoneutral conditions, it is at first counterintuitive that pathogen deposition on feeders was equivalent between temperatures. However, behavioural observations indicate that foraging efficiency—not frequency—was higher under sub-thermoneutral conditions (table 1). Therefore, interactions with the feeder, and opportunities to deposit pathogen, did not necessarily increase with food consumption. It remains unclear whether this pattern holds true under the more extreme winter temperatures in the wild, when MG prevalence peaks (minimum temperatures of −3 to 15°C for populations at comparable latitudes, well below thermoneutral) [7]. In addition, because MG deposition is only one component of transmission, additional work is needed to understand how temperature influences other aspects of transmission, including pathogen persistence on feeders and host susceptibility. Regarding susceptibility, while our data and previous results showed no difference in pathogen load between temperature treatments, suggesting that sub-thermoneutral temperatures do not alter host resistance [9], complex interactions between season (e.g. physiological changes with daylength) and temperature could influence this pattern [17]. For example, in spring or summer, when birds are breeding or moulting, immunological and energetic constraints [18] could alter trade-offs between thermoregulation and pathogen defence, potentially increasing foraging behaviours or decreasing host resistance at lower temperatures.
Our data show that in this system, physiological processes underlying pathology and pathogen replication are important predictors of fomite deposition. These patterns probably have important consequences for MG transmission and evolution. First, nonlinear relationships between pathogen load and deposition (figure 1a) suggest that transmission is more likely above some threshold of pathogen load. Theory predicts such nonlinear relationships can facilitate persistence of acute, high-pathogen-load infections in host populations [19]. Second, the observed increase in pathogen deposition with pathology may help explain why MG has evolved towards more virulent infections since its jump to finches in the mid-1990s [20]. Overall, these results suggest that revealing the mechanisms underlying transmission variation is crucial to understanding the effects of abiotic factors, like climate, on pathogen dynamics and evolution.
Acknowledgements
We thank David Ley for providing Mycoplasma gallisepticum, and Mark Hepner, Ashley Love, Ghazi Majhoub, Megan Mis, Casey Setash and Cari Lynn Squibb for sampling. Andy Davis and two anonymous referees provided helpful comments.
Experiments were conducted under the following permits: Virginia Tech IACUC (10-059-BIOL), Alabama Department of Conservation and Natural Resources (5436), United States Fish and Wildlife Service (MB158404-1), United States Geological Survey Bird Banding Lab (23513).
Funding statement
The National Science Foundation supported this work (IOS-1054675 to D.M.H, and IOB-0615361 to W.A.H.).
References
- 1.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900 (doi:10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 2.Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. 2009. Climate change and wildlife diseases: when does the host matter the most? Ecology 90, 912–920 (doi:10.1890/08-0616.1) [DOI] [PubMed] [Google Scholar]
- 3.Ostfeld RS. 2009. Climate change and the distribution and intensity of infectious diseases. Ecology 90, 903–905 (doi:10.1890/08-0659.1) [DOI] [PubMed] [Google Scholar]
- 4.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484 (doi:10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 5.Luttrell MP, Stallknecht DE, Fischer JR, Sewell CT, Kleven SH. 1998. Natural Mycoplasma gallisepticum infection in a captive flock of house finches. J. Wildl. Dis. 34, 289–296 [DOI] [PubMed] [Google Scholar]
- 6.Dhondt AA, Dhondt KV, Hawley DM, Jennelle CS. 2007. Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36, 205–208 (doi:10.1080/03079450701286277) [DOI] [PubMed] [Google Scholar]
- 7.Altizer S, Hochachka WM, Dhondt AA. 2004. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J. Anim. Ecol. 73, 309–322 (doi:10.1111/j.0021-8790.2004.00807.x) [Google Scholar]
- 8.Hosseini PR, Dhondt AA, Dobson A. 2004. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. Lond. B 271, 2569–2577 (doi:10.1098/rspb.2004.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley DM, DuRant SE, Wilson AF, Adelman JS, Hopkins WA. 2012. Additive metabolic costs of thermoregulation and pathogen infection. Funct. Ecol. 26, 701–710 (doi:10.1111/j.1365-2435.2012.01978.x) [Google Scholar]
- 10.Dawson WR, Buttemer WA, Carey C. 1985. A reexamination of the metabolic response of house finches to temperature. Condor 87, 424–427 (doi:10.2307/1367228) [Google Scholar]
- 11.Root TL, Oconnor TP, Dawson WR. 1991. Standard metabolic level and insulative characteristics of eastern house finches, Carpodacus mexicanus (Müller). Physiol. Zool. 64, 1279–1295 [Google Scholar]
- 12.Grodio JL, Dhondt KV, O'Connell PH, Schat KA. 2008. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathol. 37, 385–391 (doi:10.1080/03079450802216629) [DOI] [PubMed] [Google Scholar]
- 13.Hawley DM, Grodio J, Frasca S, Jr, Kirkpatrick L, Ley DH. 2011. Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian Pathol. 40, 321–327 (doi:10.1080/03079457.2011.571660) [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team 2011. R: a language and environment for statistical computing, v. 2.13.1. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 15.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 16.Lazzaro BP, Little TJ. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26 (doi:10.1098/rstb.2008.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegemann A, Matson KD, Both C, Tieleman BI. 2012. Immune function in a free-living bird varies over the annual cycle, but seasonal patterns differ between years. Oecologia 170, 605–618 (doi:10.1007/s00442-012-2339-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin LB, Weil ZM, Nelson RJ. 2008. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B 363, 321–339 (doi:10.1098/rstb.2007.2142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King AA, Shrestha S, Harvill ET, Bjørnstad ON. 2009. Evolution of acute infections and the invasion-persistence trade-off. Am. Nat. 173, 446–455 (doi:10.1086/597217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley DM, Osnas EE, Dobson AP, Hochachka WM, Ley DH, Dhondt AA. 2013. Parallel patterns of increased virulence in a recently emerged wildlife pathogen. PLoS Biol. 11, e1001570 (doi:10.1371/journal.pbio.1001570) [DOI] [PMC free article] [PubMed] [Google Scholar]