Summary
Protein synthesis and degradation are essential processes that allow cells to survive and adapt to their surrounding milieu. In nucleated cells, the degradation and/or cleavage of proteins is required to eliminate aberrant proteins. Cells also degrade proteins as a mechanism for cell signaling and complex cellular functions. Although the last decade has convincingly shown that platelets synthesize proteins, the roles of protein degradation in these anucleate cytoplasts are less clear. Here we review what is known about protein degradation in platelets placing particular emphasis on the proteasome and the cysteine protease calpain.
Keywords: platelets, protein degradation, proteasome, calpain
Introduction
Numerous studies have now revealed that anucleate platelets use a variety of sophisticated mechanisms to translate mRNA into protein (1–4). Platelets constitutively synthesize actin, GPIIbIIIa, and likely P-Selectin (5–7) and signal-dependent synthesis generates new proteins important for inflammation, clot retraction, and matrix remodeling (8). In order to maintain protein homeostasis, cells balance protein synthesis with degradation. Protein degradation pathways are well-characterized in nucleated cells and required to eliminate old and misfolded proteins. Protein degradation also generates free amino acids that are recycled into newly-synthesized proteins. In addition, the proteasome is involved in antigen processing, transcriptional control, and apoptosis (9–10). Genetic deletion of key proteasomal subunits is lethal for mice (11) and impairments in proteasomal function have been linked to Alzheimer’s, Parkinson’s, and other neurodegenerative diseases (9). Despite these critical functions, there is a paucity of research describing protein degradation pathways in anucleate platelets. Below we briefly review our current understanding of protein degradation pathways in platelets.
The Proteasome in Platelets
The proteasome is a large protein complex that consists of a 19S regulatory and a 20S core component. In depth descriptions of the proteasome are described elsewhere (12–14). Next-generation RNA-sequencing demonstrates that platelets express mRNAs for genes known to be part of the ubiquitin-proteasome pathway (15) and proteomic profiling has shown that platelets possess key proteasomal components (16). Several groups have also detected proteasomal-like activity in platelets (17–22). Yukawa and coworkers were the first to purify proteasome-like activity from the cytoplasmic fraction of platelets. The same group later demonstrated chymotrypsin- and trypsin-like proteasome activity in platelets. Specific proteins, such as c-Mpl, pro-GPIIb and Syk, are also ubiquinated and degraded in megakaryocytes or platelets by the proteasome (17, 23–24). Cumulatively, these studies strongly suggest that platelets possess an active proteasome.
Even though the proteasome appears to be present, its function(s) in platelets is not yet known. One intriguing possibility is that the proteasome regulates events related to platelet formation and/or the destruction. In this regard, thrombocytopenia is commonly observed when multiple myeloma patients are treated with proteasome inhibitors (e.g. bortezomib) (25). Whether or not bortezomib induces thrombocytopenia by directly inhibiting proteasomal activity in platelets or through in-direct mechanisms is unclear. An argument for the former is that bortezomib inhibits platelet aggregation in vitro (26). Recent work from Nayak and colleagues also demonstrates that the calcium ionophore A23187 augments proteasome activity in platelets (18). This suggests that the platelet proteasome is responsive to environmental cues and, in particular, changes in intracellular calcium levels. Consistent with this notion, endogenous activators of the platelet proteasome have been identified (19–22). Initially, Yukawa and colleagues purified a 170kDa polypeptide complex that activated chymotrypsin- and trypsin-like catalytic subunits of the proteasome (21–22). Later the proteasome activator PA28, which activates the chymotryptic activity of the proteasome, was discovered in platelets (19). PA28 had previously been shown to modulate antigen peptide production by the proteasome in other cells (27).
Necchi and coworkers found that platelets from patients with ANKRD26-related thrombocytopenia contain intracellular vesicles that are enriched for proteosome-like structures and ubiquinated proteins, which they referred to as ubiquitin/proteasome-rich particulate cytoplasmic structures (PaCSs) (28). In addition to decreased platelet counts, patients with this syndrome have compromised platelet aggregatory responses, leukocyte adhesion, and are at increased risk for developing cancer. Furthermore, the proteasome proteins PSB8 (29) and PSB1 (30) are decreased in platelets from patients with coronary artery disease and acute myocardial infarction. In contrast, our group has observed increased expression of proteasome subunits and proteasomal activity in platelets isolated from patients diagnosed with sepsis (unpublished observations). These reports suggest that acute and chronic diseases alter proteasome activity in platelets. It remains to be determined if alterations in platelet proteasome activity regulates the development of disease or is a consequence of the disease process. Regardless, these observations suggest that the proteasome is differentially regulated in human disease and protein degradation pathways affect platelet function.
Functional role of Calpain in Platelets
Calpains are calcium-dependent, lysosomal cysteine proteases that are rapidly activated by stimuli that induce intracellular calcium fluxes, including calcium ionophores, pore forming toxins such as shiga-toxin (31), streptolysin (32) or porB (33), and thrombin (34). Talin (35), filamin, fodrin (36), vinculin (37), kindlin-3 (38), and myosin-light chain kinase (39) are well known substrates of calpain. Of note, these substrates are critical cytoskeletal and membrane proteins, which may explain why calpain activation in platelets regulates granule secretion and cell spreading (40) and genetic deletion of μ-calpain attenuates platelet aggregation and clot retraction (41). Microparticle shedding is similarly linked to calpain activity in platelets (42).
Because calpains display widespread proteolytic activity, they regulate a variety of diseases (43). Platelet dysfunction in diabetes results in increased calpain activity and subsequent cleavage of septin-5, which promotes the release of CCL5 and TGF-β from α-granules (44). Thus, calpain activation in platelets induces the release of atherosclerosis promoting cytokines that effect vascular responses in diabetes patients. Through cleavage of SNAP-23, calpain also regulates local release of α-granular constituents at areas of thrombus formation (45) and calpain-dependent proteolysis of vWF promotes platelet aggregation in thrombocytopenic patients (46).
Calpains have central roles in cell death pathways and emerging evidence demonstrates that anucleate platelets undergo apoptosis. The apoptotic cascade is a classic example of how protein cleavage triggers enzymatic activation of downstream proteases that drive intracellular signaling events. Calpains are capable of enzymatically cleavaging pro-caspases such as caspase-7 (47) or caspase-12 (48) into their active forms. Calpains also cleave the anti-apoptotic protein Bcl-xL (49). We recently demonstrated that Bcl-xL undergoes calpain-mediated degradation in human platelets (50). Bcl-xL degradation is induced by bacteria or calcium ionophores and rescued by specific calpain inhibitors. Degradation is more rapid and robust when platelets are exposed to bacterial strains that secrete toxins. In this regard, non-virulent E. coli gained degrading properties when they were forced to express α-hemolysin. Conversely, genetic deletion of α-hemolysin in virulent E. coli strains abrogated cleavage of Bcl-xL. These observations suggest that calpain-dependent cleavage of Bcl-xL, a pro-survival protein that enhances survival in platelets (51), contributes to thrombocytopenia that is commonly observed in patients with bacterial sepsis.
We also found that traditional platelet agonists such as thrombin or platelet-activating factor (PAF) do not induce cleavage of Bcl-xL. This is surprising because thrombin activates calpain in platelets (34). One simple explanation is that pore-forming bacteria and calcium ionophores induce greater calcium fluxes than thrombin or PAF. Whatever the mechanism, these data indicate that platelet activation and apoptosis are distinct processes (52–53).
Conclusion
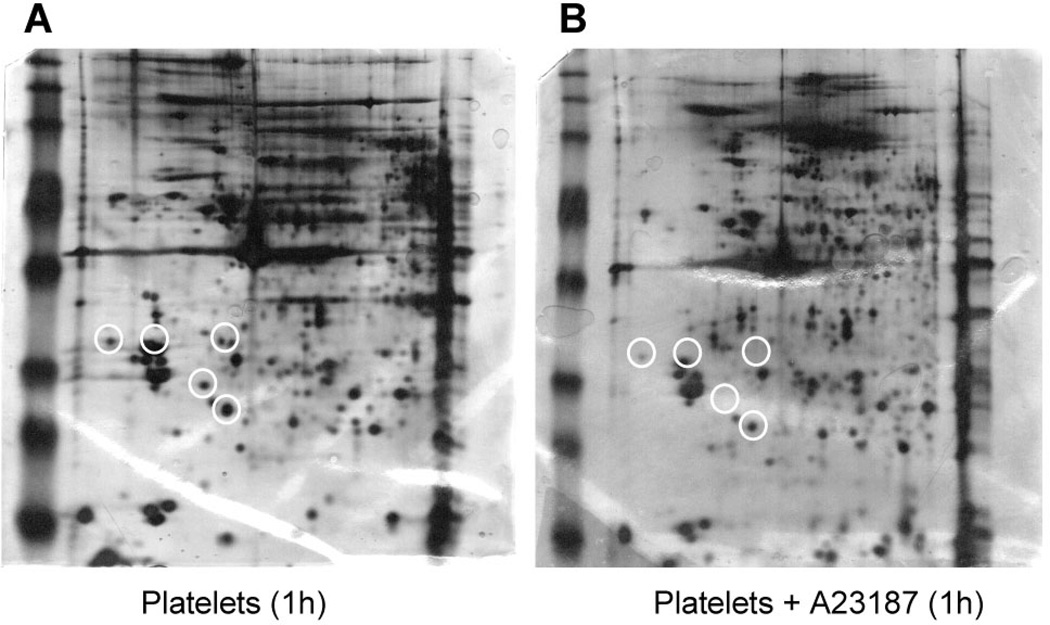
Despite their anucleate stature, there is a growing appreciation that platelets have complex biosynthetic and degradation systems. Understanding how these systems counterbalance one another will shed considerable insight into platelet function in health and disease. In regards to degradation, it is clear that platelets possess at least two protein degradation pathways (Figure 1): the proteasome, which seems critical for steady and gradual degradation of ubiquinated proteins that may accumulate as platelets age or are exposed to chronic environmental challenges such as the diabetic milieu; and, calpain-dependent degradation that mediates cleavage of a defined set of substrates that modulate rapid cell signaling events in platelets (54). Although the responsible pathway(s) needs to be determined, preliminary results from our group demonstrate that protein degradation is widespread in calcium-ionophore treated platelets (Figure 2). As we move forward, it will be important to identify the complete repertoire of degraded proteins in stimulated platelets, the signals that induce degradation, and determine how these degradation processes regulate platelet function.
Figure 1. Protein degradation pathways in human platelets.
The schematic highlights potential functions of the proteasome and calpain protein degradation pathways in human platelets. The proteasome degrades polyubiquitinated (Ub) proteins that have been marked for degradation. Calcium-dependent activation of calpain cleaves a variety of intracellular proteins including Bcl-xLwhich is followed by translocation of pro-apoptotic bax into mitochondria and platelet apoptosis. Interplay between the calpain and proteasome degradation pathways has also been observed.
Figure 2. Calcium-dependent protein degradation in human platelets.
Platelets were left alone (A) or stimulated with A23187 (B) for 1 hour and intracellular proteins were separated by 2-D gel electrophoresis. The white circles identify proteins that disappear after calcium ionophore stimulation.
References
- 1.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent premRNA splicing in anucleate platelets. Cell. 2005 Aug 12;122(3):379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001 Aug 6;154(3):485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwertz H, Tolley ND, Foulks JM, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006 Oct 30;203(11):2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyrich AS, Schwertz H, Kraiss LW, et al. Protein synthesis by platelets: historical and new perspectives. J Thromb Haemost. 2009 Feb;7(2):241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booyse F, Rafelson ME., Jr In vitro incorporation of amino-acids into the contractile protein of human blood platelets. Nature. 1967 Jul 15;215(5098):283–284. doi: 10.1038/215283a0. [DOI] [PubMed] [Google Scholar]
- 6.Thon JN, Devine DV. Translation of glycoprotein IIIa in stored blood platelets. Transfusion. 2007 Dec;47(12):2260–2270. doi: 10.1111/j.1537-2995.2007.01455.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Lang S, Zhai Z, et al. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood. 2009 Jul 9;114(2):425–436. doi: 10.1182/blood-2008-03-145821. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):s17–s24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlmann B. Role of proteasomes in disease. BMC Biochem. 2007;8(Suppl 1):S3. doi: 10.1186/1471-2091-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jentsch S, Schlenker S. Selective protein degradation: a journey's end within the proteasome. Cell. 1995 Sep 22;82(6):881–884. doi: 10.1016/0092-8674(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 11.Bedford L, Hay D, Devoy A, et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008 Aug 13;28(33):8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochtler M, Ditzel L, Groll M, et al. The proteasome. Annu Rev Biophys Biomol Struct. 1999;28:295–317. doi: 10.1146/annurev.biophys.28.1.295. [DOI] [PubMed] [Google Scholar]
- 13.Nandi D, Tahiliani P, Kumar A, et al. The ubiquitin-proteasome system. J Biosci. 2006 Mar;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 14.Sorokin AV, Kim ER, Ovchinnikov LP. Proteasome system of protein degradation and processing. Biochemistry (Mosc) 2009 Dec;74(13):1411–1442. doi: 10.1134/s000629790913001x. [DOI] [PubMed] [Google Scholar]
- 15.Rowley JW, Oler AJ, Tolley ND, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011 Oct 6;118(14):e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brophy TM, Raab M, Daxecker H, et al. RN181, a novel ubiquitin E3 ligase that interacts with the KVGFFKR motif of platelet integrin alpha(IIb)beta3. Biochem Biophys Res Commun. 2008 May 16;369(4):1088–1093. doi: 10.1016/j.bbrc.2008.02.142. [DOI] [PubMed] [Google Scholar]
- 17.Dangelmaier CA, Quinter PG, Jin J, et al. Rapid ubiquitination of Syk following GPVI activation in platelets. Blood. 2005 May 15;105(10):3918–3924. doi: 10.1182/blood-2004-09-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayak MK, Kumar K, Dash D. Regulation of proteasome activity in activated human platelets. Cell Calcium. 2011 Apr;49(4):226–232. doi: 10.1016/j.ceca.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowska H, Ostrowska JK, Worowski K, et al. Human platelet 20S proteasome: inhibition of its chymotrypsin-like activity and identification of the proteasome activator PA28. A preliminary report. Platelets. 2003 May;14(3):151–157. doi: 10.1080/0953710031000092802. [DOI] [PubMed] [Google Scholar]
- 20.Ostrowska H, Wojcik C, Worowski K. Cathepsin A and chymotrypsin-like activity of the proteasome in human blood platelets. Folia Histochem Cytobiol. 1999;37(2):153–154. [PubMed] [Google Scholar]
- 21.Yukawa M, Sakon M, Kambayashi J, et al. Proteasome and its novel endogeneous activator in human platelets. Biochem Biophys Res Commun. 1991 Jul 15;178(1):256–262. doi: 10.1016/0006-291x(91)91807-o. [DOI] [PubMed] [Google Scholar]
- 22.Yukawa M, Sakon M, Kambayashi J, et al. Purification and characterization of endogenous protein activator of human platelet proteasome. J Biochem. 1993 Sep;114(3):317–323. doi: 10.1093/oxfordjournals.jbchem.a124174. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell WB, Li J, French DL, et al. alphaIIbbeta3 biogenesis is controlled by engagement of alphaIIb in the calnexin cycle via the N15-linked glycan. Blood. 2006 Apr 1;107(7):2713–2719. doi: 10.1182/blood-2005-07-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saur SJ, Sangkhae V, Geddis AE, et al. Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood. 2010 Feb 11;115(6):1254–1263. doi: 10.1182/blood-2009-06-227033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005 Dec 1;106(12):3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avcu F, Ural AU, Cetin T, et al. Effects of bortezomib on platelet aggregation and ATP release in human platelets, in vitro. Thromb Res. 2008;121(4):567–571. doi: 10.1016/j.thromres.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Kuehn L, Dahlmann B. Structural and functional properties of proteasome activator PA28. Mol Biol Rep. 1997 Mar;24(1–2):89–93. doi: 10.1023/a:1006897801858. [DOI] [PubMed] [Google Scholar]
- 28.Necchi V, Balduini A, Noris P, et al. Ubiquitin/proteasome-rich particulate cytoplasmic structures (PaCSs) in the platelets and megakaryocytes of ANKRD26-related thrombo-cytopenia. Thromb Haemost. 2012 Dec 6;109(2) doi: 10.1160/TH12-07-0497. [DOI] [PubMed] [Google Scholar]
- 29.Banfi C, Brioschi M, Marenzi G, et al. Proteome of platelets in patients with coronary artery disease. Exp Hematol. 2010 May;38(5):341–350. doi: 10.1016/j.exphem.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Farre AJ, Zamorano-Leon JJ, Azcona L, et al. Proteomic changes related to"bewildered" circulating platelets in the acute coronary syndrome. Proteomics. 2011 Aug;11(16):3335–3348. doi: 10.1002/pmic.201000708. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Lee MS, Cherla RP, et al. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008 Mar;10(3):770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 32.Fettucciari K, Fetriconi I, Mannucci R, et al. Group B Streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006 Jun 15;176(12):7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- 33.Muller A, Gunther D, Dux F, et al. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999 Jan 15;18(2):339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii H, Suzuki Y, Kuboki M, et al. Activation of calpain I in thrombin-stimulated platelets is regulated by the initial elevation of the cytosolic Ca2+ concentration. Biochem J. 1992 Jun 15;284(Pt 3):755–760. doi: 10.1042/bj2840755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox JE, Reynolds CC, Phillips DR. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- 36.Vanags DM, Porn-Ares MI, Coppola S, et al. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996 Dec 6;271(49):31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 37.Serrano K, Devine DV. Vinculin is proteolyzed by calpain during platelet aggregation: 95 kDa cleavage fragment associates with the platelet cytoskeleton. Cell Motil Cytoskeleton. 2004 Aug;58(4):242–252. doi: 10.1002/cm.20011. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Malinin NL, Meller J, et al. Regulation of cell adhesion and migration by Kindlin-3 cleavage by calpain. J Biol Chem. 2012 Nov 16;287(47):40012–40020. doi: 10.1074/jbc.M112.380469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito M, Tanaka T, Nunoki K, et al. The Ca2+ -activated protease (calpain) modulates Ca2+/calmodulin dependent activity of smooth muscle myosin light chain kinase. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1321–1328. doi: 10.1016/0006-291x(87)91582-8. [DOI] [PubMed] [Google Scholar]
- 40.Croce K, Flaumenhaft R, Rivers M, et al. Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J Biol Chem. 1999 Dec 17;274(51):36321–36327. doi: 10.1074/jbc.274.51.36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azam M, Andrabi SS, Sahr KE, et al. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol Cell Biol. 2001 Mar;21(6):2213–2220. doi: 10.1128/MCB.21.6.2213-2220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur J Biochem. 1996 Aug 1;239(3):647–654. doi: 10.1111/j.1432-1033.1996.0647u.x. [DOI] [PubMed] [Google Scholar]
- 43.Zatz M, Starling A. Calpains and disease. N Engl J Med. 2005 Jun 9;352(23):2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 44.Randriamboavonjy V, Isaak J, Elgheznawy A, et al. Calpain inhibition stabilizes the platelet proteome and reactivity in diabetes. Blood. 2012 Jul 12;120(2):415–423. doi: 10.1182/blood-2011-12-399980. [DOI] [PubMed] [Google Scholar]
- 45.Lai KC, Flaumenhaft R. SNARE protein degradation upon platelet activation: calpain cleaves SNAP-23. J Cell Physiol. 2003 Feb;194(2):206–214. doi: 10.1002/jcp.10222. [DOI] [PubMed] [Google Scholar]
- 46.Moore JC, Murphy WG, Kelton JG. Calpain proteolysis of von Willebrand factor enhances its binding to platelet membrane glycoprotein IIb/IIIa: an explanation for platelet aggregation in thrombotic thrombocytopenic purpura. Br J Haematol. 1990 Apr;74(4):457–464. doi: 10.1111/j.1365-2141.1990.tb06335.x. [DOI] [PubMed] [Google Scholar]
- 47.Gafni J, Cong X, Chen SF, et al. Calpain-1 cleaves and activates caspase-7. J Biol Chem. 2009 Sep 11;284(37):25441–25449. doi: 10.1074/jbc.M109.038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000 Aug 21;150(4):887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002 Jul 26;277(30):27217–27226. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- 50.Kraemer BF, Campbell RA, Schwertz H, et al. Bacteria differentially induce degradation of BclxL, a survival protein, by human platelets. Blood. 2012 Dec 13;120(25):5014–5020. doi: 10.1182/blood-2012-04-420661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007 Mar 23;128(6):1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 52.Schoenwaelder SM, Yuan Y, Josefsson EC, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009 Jul 16;114(3):663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 53.Vogler M, Hamali HA, Sun XM, et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011 Jun 30;117(26):7145–7154. doi: 10.1182/blood-2011-03-344812. [DOI] [PubMed] [Google Scholar]
- 54.Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol. 2007;8(6):218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]