Abstract
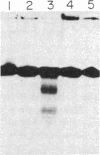
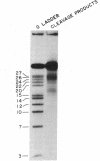
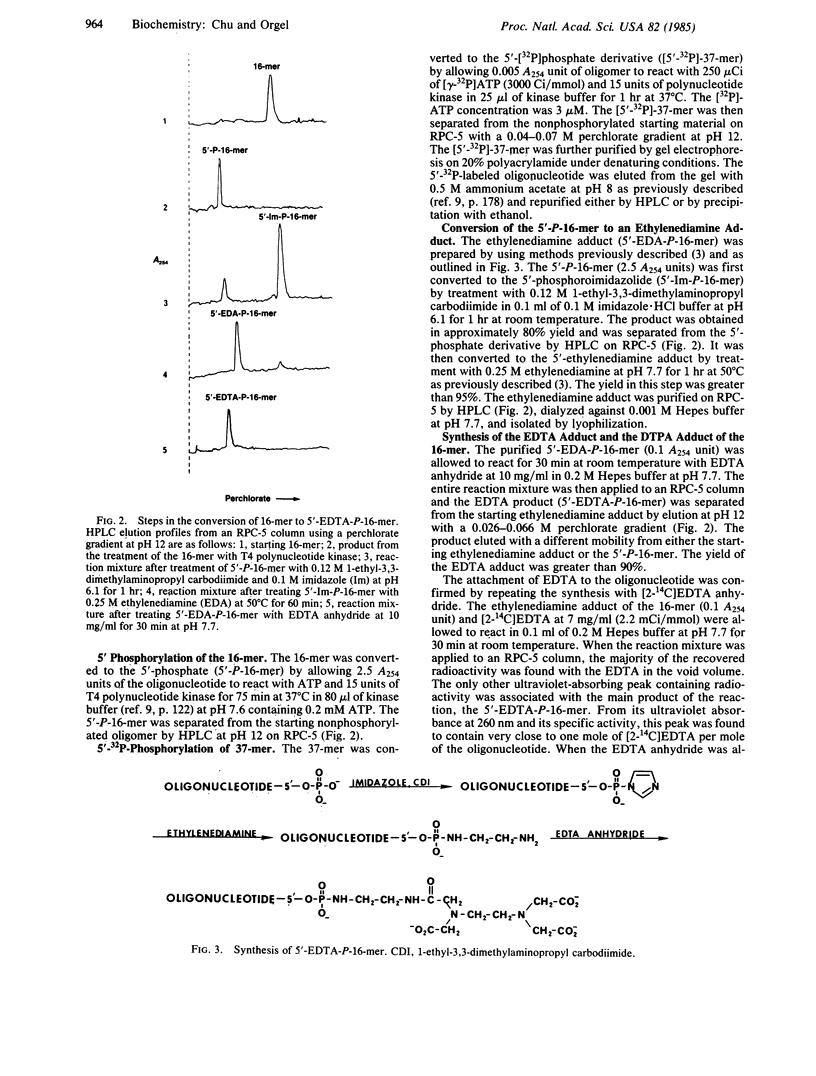
Ethylenediaminetetraacetic acid and diethylenetriaminepentaacetic acid have been attached covalently to the 5' terminus of the deoxynucleotide sequence C-A-C-A-A-T-T-C-C-A-C-A-C-A-A-C (16-mer) via an ethylenediamine linker. In the presence of Fe2+ and dithiothreitol, these reagents bring about the hybridization-dependent cleavage of the sequence T-C-G-T-A-T-G-T-T-G-T-G-T-G-G-A-A-T-T-G-T-G-A-G-C-G-G-A-T-A-A-C-A-A-T-T- T (37-mer), a sequence that contains an internal subsequence complementary to the 16-mer. The principal cleavage sites on the 37-mer are about four residues on each side of the terminal phosphate group of the 16-mer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridson P. K., Orgel L. E. Catalysis of accurate poly(C)-directed synthesis of 3'-5'-linked oligoguanylates by Zn2+. J Mol Biol. 1980 Dec 25;144(4):567–577. doi: 10.1016/0022-2836(80)90337-x. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grineva N. I., Karpova G. G., Kuznetsova L. M., Venkstern T. V., Bayev A. A. Complementary addressed modification of yeast tRNA Val 1 with alkylating derivative of d(pC-G)-A. The positions of the alkylated nucleotides and the course of the alkylation in the complex. Nucleic Acids Res. 1977;4(5):1609–1631. doi: 10.1093/nar/4.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnatowich D. J., Layne W. W., Childs R. L. The preparation and labeling of DTPA-coupled albumin. Int J Appl Radiat Isot. 1982 May;33(5):327–332. doi: 10.1016/0020-708x(82)90144-2. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. New ways to "mutate" genes. Science. 1984 Aug 24;225(4664):819–819. doi: 10.1126/science.6474154. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz P. G., Dervan P. B. Sequence-specific double-strand cleavage of DNA by penta-N-methylpyrrolecarboxamide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1983 Nov;80(22):6834–6837. doi: 10.1073/pnas.80.22.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]