Abstract
Objective
Assessment of 12-month safety of ospemifene 60 mg/day for treatment of postmenopausal women with vulvar and vaginal atrophy (VVA).
Methods
In this 52-week, randomized, double-blind, placebo-controlled, parallel-group study, women 40–80 years with VVA and an intact uterus were randomized 6 : 1 to ospemifene 60 mg/day or placebo. The primary objective was 12-month safety, particularly endometrial; 12-week efficacy was assessed. Safety assessments included endometrial histology and thickness, and breast and gynecological examinations. Efficacy evaluations included changes from baseline to week 12 in percentage of superficial and parabasal cells and vaginal pH.
Results
Of 426 randomized subjects, 81.9% (n = 349) completed the study with adverse events the most common reason for discontinuation (ospemifene 9.5%; placebo 3.9%). Most (88%) treatment-emergent adverse events with ospemifene were considered mild or moderate. Three cases (1.0%) of active proliferation were observed in the ospemifene group. For one, active proliferation was seen at end of study week 52, and diagnosed as simple hyperplasia without atypia on follow-up biopsy 3 months after the last dose. This subsequently resolved with progestogen treatment and dilatation and curettage. In six subjects (five ospemifene (1.4%), one placebo (1.6%)) endometrial polyps were found (histopathology); however, only one (ospemifene) was confirmed as a true polyp during additional expert review. Endometrial histology showed no evidence of carcinoma. Statistically significant improvements were seen for all primary and secondary efficacy measures and were sustained through week 52 with ospemifene vs. placebo.
Conclusions
The findings of this 52-week study confirm the tolerance and efficacy of oral ospemifene previously reported in short- and long-term studies.
Keywords: VAGINAL EFFECTS, ENDOMETRIAL SAFETY, DYSPAREUNIA, MATURATION INDEX, EFFICACY, SAFETY
INTRODUCTION
The symptoms of vulvar and vaginal atrophy (VVA) include dyspareunia, dryness, itching and irritation1. These symptoms are a consequence of physiological changes in the superficial cells, parabasal cells and vaginal pH, and are typically progressive and unlikely to resolve without treatment2. VVA affects up to about 50% of postmenopausal women3−9.
It is well established that VVA is a consequence of menopausal hypoestrogenism8,10. Systemic or topical estrogen therapies are effective, although full relief of VVA symptoms is not obtained in all women2,11. Each of these therapies also has other limitations. Inadequately opposed estrogen increases the risks of hyperplasia, polyps and endometrial cancer12,13. Unlike vasomotor symptoms, which tend to abate after several years, symptoms of VVA typically become more apparent and severe over time14. When hormone therapy is considered for treatment of VVA, local vaginal estrogen therapy is generally recommended15. Still, many women have reservations about any estrogen use, even when given as a minimally absorbed vaginal treatment. Vaginal administration and dosing may also be inconvenient and unacceptable for some patients16. Recent results from the REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs (REVIVE) survey in postmenopausal women indicated that patient dissatisfaction with messiness and inconvenience of administration of vaginal over-the-counter (OTC) products may result in suboptimal management of VVA17. Therefore, an oral treatment alternative to current vaginal OTC and estrogen therapies would provide another pharmacologic option in the treatment of VVA symptoms18.
Promising potential developments in therapy options for VVA include compounds known as estrogen agonists/antagonists (i.e. selective estrogen receptor modulators or SERMs). These compounds lack the steroid structure of estrogens19 and can elicit tissue-specific estrogenic responses, such as a positive effect in the vaginal epithelium with a neutral or minimal effect on other estrogen-dependent organs18−21.
Ospemifene, a novel tissue-selective estrogen agonist/antagonist, is a compound that has been shown in preclinical and clinical studies to exert positive effects on the vaginal epithelium and minimal effects on the endometrium22−24. An oral non-estrogen alternative, ospemifene has recently been approved by the US Food and Drug Administration (FDA) for treatment of dyspareunia in women with symptoms of VVA due to menopause. It has a half-life of 26 h and is metabolized by CYP450 enzymes to 4-hydroxyospemifene, its major metabolite. At a daily oral dose of 60 mg, ospemifene has shown efficacy in the treatment of VVA symptoms, such as dyspareunia, and was well tolerated in several short-term and long-term studies in postmenopausal women with or without a uterus25−28. This placebo-controlled study assessed the 12-month safety and efficacy on physiological changes of ospemifene 60 mg/day for the treatment of postmenopausal women with VVA.
METHODS
The protocol, amendments and informed consent forms were reviewed and approved by the Independent Ethics Committee (IEC) before study initiation (NCT00566982). The study, conducted in 23 sites in Europe, was carried out in accordance with the Declaration of Helsinki (2000), current Good Clinical Practice outlined in the International Conference on Harmonization for Good Clinical Practice (E6), and in compliance with local regulatory requirements. All subjects provided written informed consent on IEC-approved forms before study initiation.
This was a 52-week, randomized, double-blind, placebo-controlled, parallel-group study that compared ospemifene 60 mg/day vs. placebo in postmenopausal women (40–80 years of age) with VVA, which was defined as having a proportion of superficial cells ≤ 5% in the vaginal smear and a vaginal pH > 5. Patient advertisement was used as an aid to recruit subjects. Subjects had to have an intact uterus and normal findings (except for atrophic vaginal signs) on pelvic examination, breast palpation and recent mammogram. Subjects were not enrolled based on symptoms (i.e. vaginal dryness or dyspareunia). Subjects were excluded if they had an abnormal endometrial histology other than atrophy based on baseline biopsy, uterine bleeding of unknown origin or clinically significant abnormal gynecological findings. Women were randomized in a 6 : 1 ratio to ospemifene or matching placebo by sequential allocation of randomization number. Randomization was stratified by study center. Study visits included baseline (screening/visit 1), week 12, week 26, week 39, week 52 (end of therapy or early discontinuation) and week 56 (4-week post-treatment follow-up). Ospemifene (or matching placebo) was taken orally each morning with food.
Safety assessments
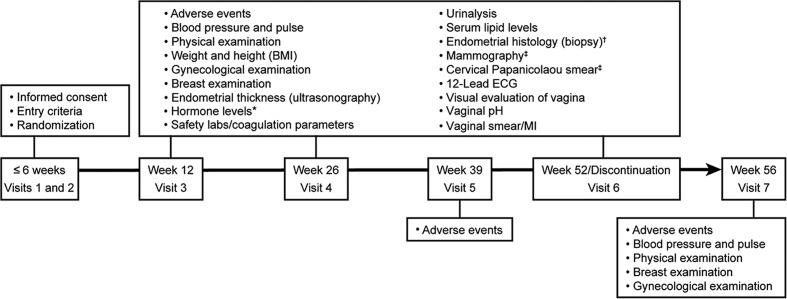
A summary of the safety assessments is presented in Figure 1. Endometrial thickness was measured by transvaginal ultrasound (TVU) at baseline (screening/visit 1) and at weeks 12, 26 and 52/end of therapy. For consistency of data, ultrasound images and videos were read at the central laboratory. Endometrial biopsies were collected at baseline and week 52 and at weeks 12 and 26 if TVU demonstrated endometrial thickness ≥ 4 mm.
Figure 1.
Summary of safety and efficacy assessments (maturation index, vaginal pH, and visual evaluation). BMI, body mass index; ECG, electrocardiogram; MI, maturation index.*, Estradiol, follicle stimulating hormone, luteinizing hormone, and sex hormone binding globulin; †, endometrial biopsy at visit 3 and visit 4 only if the subject's endometrial thickness was ≥ 4 mm; ‡, mammogram and Papanicolaou smear were conducted at week 52
Endometrial biopsies were taken using a suction curette and analyzed by two independent pathologists in the central laboratory who were blinded both to the study treatment and to each other's readings of the histology slides. If there was disagreement about the endometrial histology, a third pathologist evaluated the samples. The final diagnosis was determined by concurrence between two of the three independent pathologists or was reported as the most severe histopathological diagnosis if there was no agreement among the three pathologists. Histology was classified according to Blaustein's criteria29.
Per regulatory agency guidance, endometrial polyps were to be fully characterized histologically. Sites were asked to locally confirm polyps identified by TVU because views of suspected polyps identified by TVU that were sent for central reading were limited compared to the real-time dynamic views available locally. If confirmed, the subject was to be discontinued and a hysteroscopy was performed to obtain tissue for histological diagnosis. In addition, when available, tissue thought to represent a uterine polyp was sent for expert review.
A mammogram was performed at baseline if it had not been performed within 3 months before randomization. Subjects were eligible for the study if the mammogram was negative. A mammogram was also performed at the week-52 visit. Mammograms were read locally by radiologists at each study center.
Efficacy assessments
The primary efficacy assessments were changes from baseline to week 12 (last observation carried forward, LOCF) in the percentages of superficial cells and parabasal cells (maturation index, MI) and in vaginal pH in the vaginal smear sample. Only these objective measures of efficacy were assessed in this study.
Secondary efficacy assessments were changes from baseline at weeks 12, 26 and 52 or end of therapy for observed cases of visual evaluation of the vagina (petechiae, pallor, friability and dryness or redness in vaginal mucosa) and serum hormones (estradiol, follicle stimulating hormone (FSH), luteinizing hormone (LH) and sex hormone binding globulin (SHBG)) in addition to the percentages of superficial cells and parabasal cells in the MI and in vaginal pH.
Statistical methods
The sample size was planned for 350 subjects (50 subjects to be treated with placebo and 300 to be treated with ospemifene 60 mg daily) to be randomized. The number of subjects was based on regulatory requirements to collect sufficient endometrial safety data at 12 months such that the incidence of endometrial hyperplasia/carcinoma was established to be < 1% with an upper limit of the confidence interval < 4. The sample size was chosen to provide evidence of sustained efficacy for the primary efficacy endpoints.
A 6 : 1 randomization ratio was chosen to meet the regulatory requirements to collect endometrial safety in subjects who were exposed to ospemifene for 12 months, while the placebo subjects were enrolled to monitor overall safety.
The method of analysis for the primary efficacy data was analysis of covariance (ANCOVA) with treatment and center as fixed factors and the baseline value as the covariate. If the assumptions of ANCOVA were not met, Cochran–Mantel–Haenszel (CMH) statistics with rank scores were used. The LOCF was used for primary efficacy analyses; secondary efficacy analyses used the observed-cases approach. Visual examination of vaginal characteristics was analyzed using the CMH statistics with rank scores and center as the stratification factor at weeks 12, 26 and 52.
Safety data were reported on the intent-to-treat (ITT) population, which included all subjects who had taken at least one dose of the study drug. Treatment-emergent adverse events (TEAEs) were tabulated by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA, Version 10), causality and severity of event. Adverse events were classified as mild, moderate or severe. Baseline and time point assessments were summarized by descriptive statistics for clinical chemistry and laboratory safety variables. A one-sided 95% confidence interval (upper limit) was calculated to assess serious endometrial outcomes (endometrial hyperplasia and/or cancer).
RESULTS
Subjects in both treatment groups were comparable in demographic details including age, race and body mass index (Table 1).
Table 1.
Subject demographic and baseline clinical characteristics (intent-to-treat population). Data are given as mean ± standard deviation or n (%)
| Variable | Ospemifene 60 mg/day | Placebo |
|---|---|---|
| Number randomized to treatment | 363 | 63 |
| Age (years) | 61.7 ± 6.2 | 62.9 ± 6.5 |
| Body mass index (kg/m2) | 24.7 ± 2.9 | 24.1 ± 2.9 |
| Race | ||
| White | 361 (99.4%) | 63 (100%) |
| Black | 1 (0.3%) | 0 (0%) |
| Asian | 1 (0.3%) | 0 (0%) |
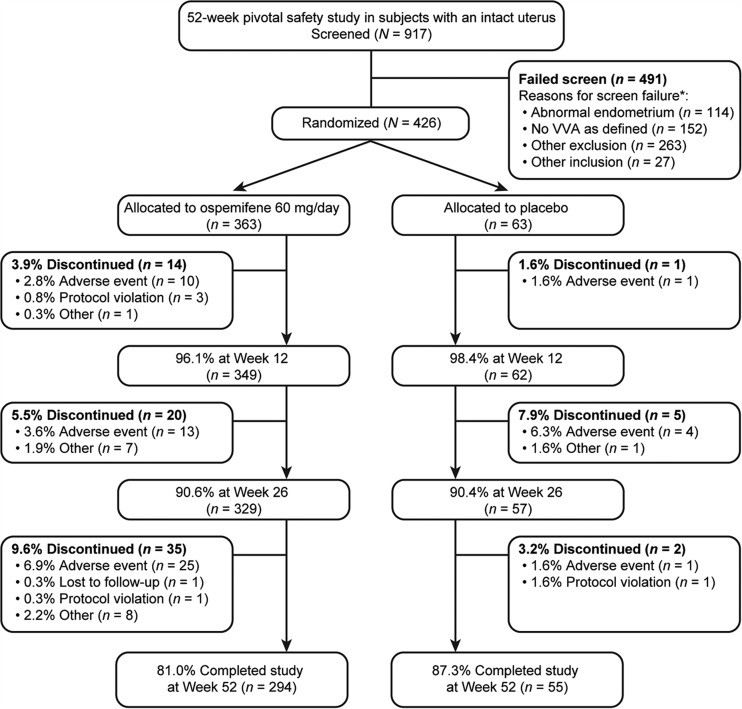
Of 917 subjects screened, 426 were randomized (363 women to ospemifene 60 mg/day and 63 women to placebo) (Figure 2). A total of 349 subjects (81.9%) completed the study: 81.0% (n = 294) in the ospemifene group and 87.3% (n = 55) in the placebo group. The most common reasons for discontinuations were TEAEs (13.2% (n = 48) for ospemifene and 9.5% (n = 6) for placebo), followed by withdrawal of consent by the subject (3.9% (n = 14) for ospemifene and 1.6% (n = 1) for placebo). The most common TEAE that led to withdrawals was hot flushes (2.2% (n = 8) for ospemifene and 0 for placebo).
Figure 2.
Subject disposition. *Subject is counted in more than one inclusion/exclusion criterion if multiple criteria were not met for that subject
A summary of overall TEAEs is shown in Table 2. Treatment-related TEAEs occurred in 42.3% (n = 154) and 35.5% (n = 22) of subjects in the ospemifene and placebo groups, respectively. Among the 308 TEAEs in the ospemifene group, 270 (88%) were considered to be mild or moderate. Serious TEAEs occurred in 4.9% (n = 18) of subjects in the ospemifene group and 6.5% (n = 4) of subjects in the placebo group. Eighteen subjects (4.9%) in the ospemifene group experienced 22 serious TEAEs and four subjects (6.5%) in the placebo group experienced 12 serious TEAEs. The most common TEAE in the ospemifene group was hot flush. While the majority of the cases were mild to moderate, 1.9% (n = 7) of subjects in the ospemifene group reported severe hot flushes. There was a low discontinuation rate (ospemifene 60 mg, 2.2%, n = 8; placebo, n = 0) due to hot flushes.
Table 2.
Summary of treatment-emergent adverse events (TEAEs) occurring in ≥ 5% of subjects in the ospemifene 60 mg/day group. Data are given as n (%). Note: Counts include adverse events that were ongoing from the 12-week pivotal safety study
| Ospemifene 60 mg/day (n = 364)* | Placebo (n = 62)* | |
|---|---|---|
| TEAE | 308 (84.6) | 47 (75.8) |
| Withdrawals due to adverse events | 49 (13.5) | 6 (9.7) |
| Serious TEAEs | 18 (4.9) | 4 (6.5) |
| Most frequent TEAEs | ||
| Urinary tract infection | 61 (16.8) | 15 (24.2) |
| Hot flush | 46 (12.6) | 4 (6.5) |
| Nasopharyngitis | 36 (9.9) | 4 (6.5) |
| Other TEAEs | ||
| Vaginal candidiasis and/or vulvovaginal mycotic infection | 35 (9.6) | 2 (3.2) |
| Headache | 33 (9.1) | 6 (9.7) |
| Muscle spasms | 31 (8.5) | 4 (6.5) |
| Back pain | 24 (6.6) | 2 (3.2) |
| Hyperhidrosis | 22 (6.0) | 5 (8.1) |
| Vaginal discharge | 20 (5.5) | 0 (0) |
| Insomnia | 19 (5.2) | 0 (0) |
| Cystitis | 19 (5.2) | 0 (0) |
*, One subject randomized to placebo was correctly treated until week 39 when by mistake she received ospemifene 60 mg for weeks 39−52. Since placebo was the correct randomized treatment for this subject, but she also received active drug, her data are included in the placebo group for the ITT population and in the ospemifene group for the safety population. Thus, the safety population includes 62 subjects in the placebo group and 364 subjects in the ospemifene 60 mg group, while the ITT population includes 63 subjects in the placebo group and 363 subjects in the ospemifene 60 mg group
Baseline histological characteristics of the endometrium are shown in Table 3. In general, there were no tissue changes observed between baseline and week 52 in histological characteristics of the endometrium. In the ospemifene group, three cases (1.0%) of active proliferation occurred, one of which (0.3%) was identified as simple hyperplasia without atypia. This case occurred approximately 3 months after the last dose of ospemifene, and resolved following progestogen treatment, with the resolution confirmed by dilatation and curettage. This represents an incidence of 0.34% (upper 95% confidence limit, 1.61%) of the subjects who had biopsies at week 52. No cases of active proliferation occurred in the placebo group. No cases of endometrial carcinoma were reported in either treatment group.
Table 3.
Endometrium histological biopsy characteristics: baseline vs. week 52 (last observation carried forward)*. Data are given as n (%)
| Histological characteristics† |
Ospemifene 60 mg/day (n = 364) |
Placebo (n = 62) |
||
|---|---|---|---|---|
| Baseline | Week 52‡ | Baseline | Week 52 | |
| Tissue insufficient for diagnosis | 59 (16.2) | 27 (8.7) | 8 (12.9) | 11 (19.6) |
| Atrophic | 300 (82.4) | 267 (86.1) | 52 (83.9) | 45 (80.4) |
| Inactive | 1 (0.3) | 1 (0.3) | 1 (1.6) | 0 (0) |
| Weakly proliferative | 1 (0.3) | 7 (2.3) | 0 (0) | 0 (0) |
| Active proliferative | 0 (0) | 3 (1.0) | 0 (0) | 0 (0) |
| Hyperplasia | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) |
*, Safety population;
†, assessments of biopsies were made according to predefined and generally accepted microscopic criteria (Blaustein's classification)29;
‡, percentages at week 52 are of the number of subjects with last observation carried forward data; the numbers of women for each parameter at week 52 are different for the calculation of percentage
Additional histological biopsy results: ospemifene, baseline – two cases of atypical epithelial proliferation and one case of endometritis, chronic; ospemifene, week 52 – three cases of endometrial polyp, atrophic type; one case of polyp, functional endometrial type; one case of endocervical polyp. In addition, placebo, baseline – one case of endometrial polyp. Further expert review of the atrophic and functional endometrial polyp slides determined that these did not represent true polyps
Endometrial thickness assessments performed for subjects with endometrial thickness ≥ 4 mm are shown in Table 4. The mean endometrial thickness increased by 0.75 mm between baseline and week 52 in the ospemifene group and by 0.17 in the placebo group. Of note, the mean change in thickness from baseline at 52 weeks was similar to that seen at 12 weeks in other previously published studies26,28. One subject in the placebo group (1.6%) and 22 subjects (6.1%) in the ospemifene group were reported to have endometrial thickness ≥ 5 mm at week 52 or at discontinuation from the study.
Table 4.
Endometrial thickness changes during the 52-week treatment period*. Data are given as mean ± standard deviation. Change from baseline in endometrial thickness defined as thickness at week 26 or week 52 minus thickness at baseline
| Observed value time point | Ospemifene 60 mg/day | Placebo |
|---|---|---|
| Baseline (screening)* | ||
| n | 364 | 62 |
| Endometrial thickness (mm) | 2.1 ± 0.8 | 2.0 ± 0.9 |
| Week 12 | ||
| n | 315 | 58 |
| Endometrial thickness (mm) | 2.5 ± 1.5 | 2.3 ± 1.7 |
| Change from baseline (mm) | 0.44 ± 1.7 | 0.31 ± 1.5 |
| Week 26 | ||
| n | 311 | 57 |
| Endometrial thickness (mm) | 2.6 ± 1.5 | 2.2 ± 1.0 |
| Change from baseline (mm) | 0.51 ± 1.6 | 0.22 ± 1.3 |
| Week 52 | ||
| n | 294 | 55 |
| Endometrial thickness (mm) | 2.8 ± 1.4 | 2.1 ± 1.0 |
| Change from baseline (mm) | 0.75 ± 1.5 | 0.17 ± 1.3 |
*, Observed cases
Five (1.4%) subjects in the ospemifene group reported vaginal bleeding or spotting that was self-limiting and mild to moderate in severity. One of these five subjects was the previously mentioned subject who experienced endometrial hyperplasia approximately 3 months after the last dose of study drug (effects are listed separately but occurred in the same subject); she experienced vaginal bleeding after taking the anticoagulant enoxaparin for a fractured foot. None of the subjects in the placebo group reported bleeding or spotting. No subjects discontinued due to vaginal bleeding or spotting. The remaining subjects showed only postmenopausal atrophic endometrium on biopsies.
In this study, six subjects (five ospemifene (1.4%); one placebo (1.6%)) were reported, on central reading of endometrial biopsy samples, to have a uterine polyp. On further expert review, five were considered artifacts and only one, in the ospemifene group, was confirmed to be a true polyp.
Four subjects (1.1%) in the ospemifene group and none in the placebo group reported breast pain. There were no cases of breast cancer observed in subjects treated with ospemifene or placebo in this population of postmenopausal women.
In the ospemifene group, there were two cerebrovascular accidents: one subject with a significant history of hypercholesterolemia had a non-fatal ischemic stroke considered by the investigator to be possibly treatment-related, and one subject with a history of hypertension, hypercholesterolemia and increased prothrombin time experienced a cerebral hemorrhage that was not considered by the investigator to be treatment-related. Lastly, a 65-year-old subject, who had been on thrombosis prophylaxis for several years prior to enrollment, was diagnosed with a deep vein thrombosis (DVT) that was considered by the investigator to be possibly treatment-related approximately 8 months after initiating therapy. The DVT was treated and resolved without any adverse long-term consequences. No cases of pulmonary embolism, acute coronary events, myocardial infarction, retinal vein thrombosis or new ischemic heart disease were reported for either treatment group. The incidence of urinary tract infection reported in the ospemifene group was 16.8% (61 subjects) vs. 24.2% (15 subjects) in the placebo group.
There were no differences between groups in mean changes from baseline in hematology, clinical chemistry, lipids or coagulation parameters. There was no subject mortality.
Primary efficacy assessments in the ITT population are shown in Table 5. With ospemifene 60 mg/day vs. placebo, statistically significant improvements (p < 0.0001) were observed for all primary efficacy parameters assessed (i.e. increased superficial cells, decreased parabasal cells and decreased vaginal pH).
Table 5.
Maturation index (percentage of superficial and parabasal cells) and vaginal pH: change from baseline to week 12 (last observation carried forward)
|
Ospemifene 60 mg/day (n = 363)
|
Placebo (n = 63) |
p Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Change from baseline to week 12 | Baseline | Week 12 | Change from baseline to week 12 | ||
| % Superficial cells | < 0.0001 | ||||||
| Median (range) | 0 (0, 5) | 5 (0, 60) | 5 (−5, 60.0) | 0 (0, 5) | 0 (0, 30) | 0 (−5, 28) | |
| 95% confidence interval | 5.0 to 7.0 | 0.0 to 0.0 | |||||
| % Parabasal cells | < 0.0001 | ||||||
| Median (range) | 50 (0, 100) | 0 (0, 100) | –40 (−100, 75) | 48 (0, 100) | 70 (0, 100) | 0 (−90, 98) | |
| 95% confidence interval | −55.0 to −30.0 | 0.0 to 5.0 | |||||
| Vaginal pH | < 0.0001 | ||||||
| Mean ± standard deviation | 6.23 ± 0.728 | 5.03 ± 0.715 | –1.21 ± 0.912 | 6.20 ± 0.749 | 6.04 ± 0.887 | –0.16 ± 0.945 | |
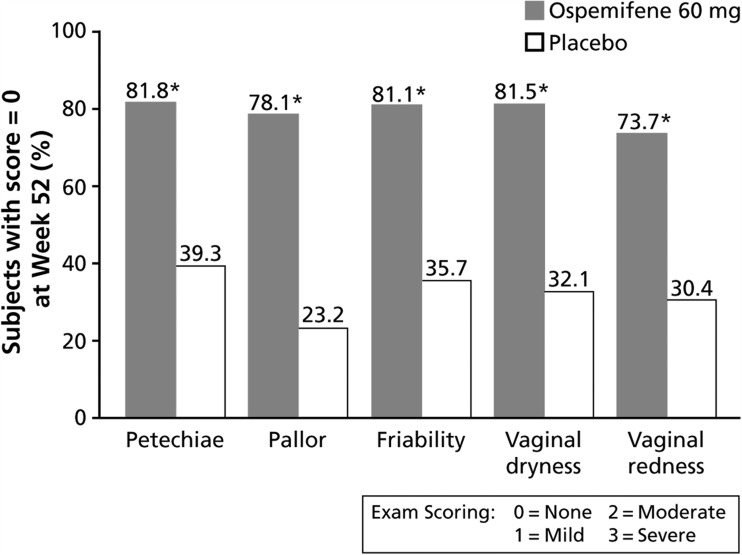
In addition, statistically significant improvements were observed with ospemifene 60 mg/day vs. placebo for all secondary efficacy parameters at every time point assessed (weeks 12, 26 and 52). The median percentage of superficial cells was increased and the median percentage of parabasal cells was decreased at each time point (p < 0.0001). At each time point assessed, greater decreases in vaginal pH were seen with ospemifene 60 mg/day over placebo (p < 0.0001). On visual evaluation of the vagina (Figure 3), ospemifene 60 mg/day demonstrated statistically significant improvements in each of the five parameters (petechiae, pallor, friability, vaginal dryness in the mucosa and vaginal redness in the mucosa) at week 52 (p < 0.0001). Almost 80% of subjects treated with ospemifene had no signs of vaginal atrophy (score of 0) vs. approximately 30% of subjects who received placebo. At 12 and 26 weeks, the proportion of subjects with no atrophic findings for each of the five parameters was significantly greater in the ospemifene 60 mg/day group than in the placebo group (p < 0.0001).
Figure 3.
Visual evaluation of the vagina; percentage of subjects with no abnormalities at week 52 (observed cases; intent-to-treat population). *, p < 0.0001
Estradiol, LH and FSH levels remained well within the postmenopausal range in both the ospemifene group and the placebo group (there were small decreases from baseline in LH and FSH levels in both the ospemifene group and the placebo group). In this study, mean estradiol levels were similar to baseline at week 12. SHBG levels were significantly increased from baseline by approximately 50% throughout the study in the ospemifene group (p < 0.0001) but not in the placebo group (p = 0.7044). An increase in SHBG levels with ospemifene has also been noted in another ospemifene phase-3 study27. Similar increases in SHBG have been reported after treatment with other SERMs30,31.
There were no clinically meaningful differences between groups or changes from baseline to week 52 in lipid levels, including total cholesterol, triglycerides, high density lipoprotein and low density lipoprotein levels.
Overall, compliance was similar in the ospemifene group (approximately 95%) and the placebo group (approximately 99%). Of 426 randomized subjects, 84.7% (n = 361) were exposed to ospemifene 60 mg/day for a mean of 321.5 days and 14.6% (n = 62) were exposed to placebo for a mean of 339.3 days.
DISCUSSION
Estrogen agonists/antagonists (also referred to as SERMs) can selectively elicit different effects depending on the specific tissue19,32,33. It is possible for a suitable compound to have positive effects in vaginal tissue with neutral or minimal effects on estrogen-dependent tissues such as the endometrium and breast. Existing estrogen agonists/antagonists32,34,35 such as tamoxifen, raloxifene, bazedoxifene and lasofoxifene are not indicated for the treatment of VVA.
This study has demonstrated that oral ospemifene, a tissue-selective agonist/antagonist that exerts a positive effect on vaginal epithelial tissue, is well tolerated in the treatment of VVA in postmenopausal women for up to 52 weeks.
In this study, ospemifene demonstrated minimal effects on the endometrial tissue, with only slight mean increases in endometrial thickness. These effects on endometrial tissue are consistent with those observed in other studies with ospemifene25−28 as well as with raloxifene32.
The observed small increases in endometrial thickness after ospemifene treatment were without concomitant cellular proliferation. Such changes in endometrial thickness have been reported with raloxifene and tamoxifen18,36,37. SERMs may induce glandular cystic atrophy that appears heterogeneously thick but is in fact histologically inactive. In this cohort, only one subject using ospemifene was diagnosed with endometrial hyperplasia (simple hyperplasia without atypia). This subject had experienced vaginal bleeding during the study after treatment with an anticoagulant and at study exit was found to have a thickened endometrium; the endometrial hyperplasia was found at follow-up biopsy 3 months after the last dose of study drug.
Endometrial histology findings with ospemifene were similar to those of vaginal estrogens for which rates of endometrial hyperplasia have been reported at approximately 3%38−40. FDA guidance for VVA trials recommended that the clinical trial demonstrate an endometrial hyperplasia rate of less than 1% with an upper bound of less than 4% of the one-sided 95% confidence interval for that rate. Results demonstrated a single case of simple hyperplasia (0.3%), which meets the FDA criteria of ≤ 1% and does not exceed 4%.
Prevalence of endometrial polyps is dependent on age, menopausal state and hormone therapy use41. Endometrial biopsies in the current study indicated similar proportions of subjects with suspected endometrial polyps in the placebo (1.6%, 1/62) and ospemifene (1.4%, 5/364) groups. Upon further expert review, only one of these six cases was found to be a true polyp.
One subject (0.3%) in the current study was diagnosed with a possible new DVT, possibly study drug-related, 248 days after receiving the first dose of ospemifene 60 mg; this subject was believed to have had thrombophlebitis and had been taking thrombosis prophylaxis before and during the study. One subject (0.3%) had a non-fatal ischemic stroke that was considered possibly treatment-related 347 days after starting ospemifene. This subject had a history of hypercholesterolemia, which may have contributed to the stroke.
Breast safety is a key concern among women receiving estrogen therapy for postmenopausal symptoms. Although conclusions cannot be drawn from this study alone, based on other preclinical and clinical data, ospemifene may have a neutral effect or anti-estrogenic effect on breast tissue27,42−44. In this study, after dosing with ospemifene for 1 year, no woman was diagnosed with breast cancer; however, longer-term data with clinical use will continue to characterize the effect of ospemifene on the breast. Four subjects in the ospemifene group and none in the placebo group reported breast pain, but there were no subjects in either treatment group with abnormal clinically significant findings on mammography at baseline or week 52.
Compared with placebo, ospemifene 60 mg/day demonstrated statistically significant (all p < 0.0001) beneficial effects of treatment on the primary and secondary efficacy endpoints of percentage of superficial cells and percentage of parabasal cells and vaginal pH for up to 1 year. Consistent with other published data26, there were clear statistically significant effects of ospemifene 60 mg/day treatment compared with placebo on physiological vaginal parameters (increased proportion of superficial cells, decreased proportion of parabasal cells, reduced vaginal pH and improved visual evaluation), which were sustained throughout the study26,28. SERMs such as tamoxifen and raloxifene have not demonstrated similar favorable agonist effects of ospemifene on vaginal tissue34,45.
Benefits of treatment with ospemifene 60 mg/day were also observed for physical changes associated with VVA. Statistically significant improvement was seen in the secondary efficacy measurement of visual evaluation of the vagina for the five parameters of petechiae, pallor, friability and vaginal dryness and redness of the vaginal mucosa (all p < 0.0001). The primary and secondary efficacy results of this study are consistent with results of previous short-term and long-term studies of ospemifene 60 mg/day26−28.
Levels of LH and FSH were similar though slightly decreased for both the ospemifene and placebo groups and remained well within the postmenopausal range. Decreased LH and FSH levels after ospemifene were also observed in other trials23,27. The effect of estrogen agonist/antagonist compounds on FSH levels is thought to be likely due to effects on the pituitary. In this study, mean estradiol levels remained well within the postmenopausal range and were similar to baseline at week 12, and no increase in systemic estrogen was observed23. The SHBG levels after ospemifene were consistently increased approximately 50% throughout the study. An increase in SHBG levels with ospemifene has also been noted in ospemifene pivotal studies, as well as with several other similar compounds and is thought to be due to direct effects on the liver23,27. Free testosterone levels were not measured in this study. In another study with ospemifene, free testosterone levels remained unchanged despite the increase in SHBG27.
Hot flushes resulted in limited discontinuation (2.2%, n = 8) during treatment with ospemifene in this study. These low discontinuation rates are similar to those seen in a study with lasofoxifene and raloxifene46. However, increased incidence and frequency of hot flushes during treatment, sometimes reaching statistical significance, have been reported after treatment with raloxifene, tamoxifen and toremifene19,37,47,48.
CONCLUSIONS
In this study, treatment with oral ospemifene 60 mg/day for up to 1 year was generally well tolerated and effective in the treatment of VVA in postmenopausal women. Compared with placebo, there were no significant estrogen-related or clinically important adverse effects on the endometrial or breast tissue at this dose of ospemifene over a 1-year duration of treatment. An estrogen agonist/antagonist compound, ospemifene exerts stimulatory action on the vaginal epithelial tissue with minimal effect on endometrial tissue. These tissue-selective effects are consistent with other studies of ospemifene. In addition to the FDA approval of ospemifene, a non-estrogen alternative for the treatment of moderate to severe dyspareunia due to VVA in postmenopausal women, these findings further support the potential of oral ospemifene for the overall treatment of VVA in postmenopausal women.
ACKNOWLEDGEMENTS
The authors would like to thank the study investigators and site staff for their participation in the study, including Anna-Maija Antila, Suomen Terveystalo, Tampere, Finland; Jan Blaakær, Aarhus University Hospital Skejby, Aarhus N, Denmark; Marc Dhont, University Hospital of Gent, Gent, Belgium; Gilbert Donders, Heilig Hart Hospital Campus Mariëndal, Tienen, Belgium; Agneta Ehrenborg, Qvinnolivet – Specialistläkarna Kungsbacka Södra, Kungsbacka, Sweden; Risto Erkkola, Turun Gynekologikeskus, Turku, Finland; Jorma Heikkinen, ODL Terveys Oy/Osteoporoosiklinikka, Oulu, and Suomen Terveystalo/Länsi-Pohjan Lääkärikeskus, Kemi, Finland; Astrid Højgaard, Regionshospitalet i Randers, Randers, Denmark; Dirk Janssens, Gynaecologische Dienst, Turnhout, Belgium; Liisa Karinen, Lääkärikeskus Gyneko Oy, Oulu, Finland; Philippe R. Koninckx, University Hospital of Leuven, Leuven, Belgium; Angelica Lindén-Hirschberg, Kvinnohälsan Karolinska, Stockholm, Sweden; Tord Naessen, Kvinnokliniken, Akademiska, Uppsala, Sweden; Pernille Ravn, Odense Universitetshospital, Odense, Denmark; Serge Rozenberg, UMC Sint Pieter, Brussels, Belgium; Göran Samsioe, Kvinnokliniken Lunds, Lund, Sweden; Peter Sieprath, Ziekenhuis – Oost Limburg ZOL, Genk, Belgium; Philippe Simon, ULB Erasme Hospital, Brussels, Belgium; Marjo Tuppurainen, Lääkäriasema Cantti Oy, Kuopio, Finland; Anne Vanha-Perttula, Itä-Suomen Lääkärikeskus Oy, Joensuu, Finland; and Olavi Ylikorkala, Lääkäriasema Femeda, Helsinki, Finland.
Conflict of interest S. R. Goldstein is Director of and holds stock in SonoSite, Inc, and has received consulting fees, honoraria and/or equipment loans from Amgen Inc; Cook ObGyn; Lilly; Merck & Co, Inc; Novo Nordisk A/S; Pfizer Inc; Philips Ultrasound; Shionogi Inc and Warner Chilcott. He also serves on the Ospemifene Clinical Steering Committee for Shionogi Inc. G. A. Bachmann has received consulting fees, honoraria and/or grant support from Astellas; Barr Laboratories; Bayer; Boehringer Ingelheim GmbH; Duramed Pharmaceuticals, Inc; EndoCeutics Inc; Ferring Pharmaceuticals; Johnson & Johnson; Merck & Co, Inc; Novartis AG; Novo Nordisk A/S; Pfizer Inc; Procter & Gamble (now Warner Chilcott); QuatRx Pharmaceuticals Company; Shionogi Inc; Solvay; Teva and Wyeth (now Pfizer Inc). She also serves on the Ospemifene Clinical Steering Committee for Shionogi Inc. V. H. Lin was an employee of QuatRx Pharmaceuticals Company at the time the study was conducted. She is a consultant for and serves on the Ospemifene Clinical Steering Committee for Shionogi Inc. D. J. Portman has received consulting fees, honoraria and/or grant support from Bayer; Boehringer Ingelheim GmbH; EndoCeutics Inc; Meditrina Pharmaceuticals, Inc; Noven Pharmaceuticals, Inc; Pfizer Inc; QuatRx Pharmaceuticals Company; Shionogi Inc; Teva; Warner Chilcott; Amneal Pharmaceuticals; Watson Pharmaceuticals, Inc.; Palatin Technologies and Sprout Pharmaceuticals. O. Ylikorkala has received lecture fees from Novo Nordisk and Orion Pharma, Ltd. P. R. Koninckx is Emeritus Professor OBGYN, Universities of Leuven, Oxford and Rome (La Cattolicā) and is Chief Executive Officer of EndoSAT, which is a company that addresses peritoneal conditioning.
Source of funding Clinical research for this study was funded by Hormos Medical Ltd, subsidiary of QuatRx Pharmaceuticals. Shionogi Inc., Florham Park, NJ, USA provided financial support to Quintiles, Parsippany, NJ, USA for technical, writing and editing assistance with this manuscript.
References
- 1.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician. 2000;61:3090–6. [PubMed] [Google Scholar]
- 2.North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355–69. doi: 10.1097/gme.0b013e31805170eb. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133–42. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 4.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49:292–303. doi: 10.1016/j.maturitas.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Simon JA, Komi J. Postmenopausal women’s attitudes: vulvovaginal atrophy and its symptoms [abstract LB-10] Menopause. 2007;14:1107. [Google Scholar]
- 6.Freedman M, Reape KZ, Giblin K. Impact of menopausal symptoms on sex lives: a survey evaluation [abstract LB-12] Menopause. 2007;14:1107. [Google Scholar]
- 7.Levine KB, Williams RE, Hartmann KE. Vulvovaginal atrophy is strongly associated with female sexual dysfunction among sexually active postmenopausal women. Menopause. 2008;15:661–6. doi: 10.1097/gme.0b013e31815a5168. [DOI] [PubMed] [Google Scholar]
- 8.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85:87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gass ML, Cochrane BB, Larson JC, et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women’s Health Initiative. Menopause. 2011;18:1160–71. doi: 10.1097/gme.0b013e3182227ebd. [DOI] [PubMed] [Google Scholar]
- 10.Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91–105. doi: 10.1080/13697130802585576. [DOI] [PubMed] [Google Scholar]
- 11.Barlow DH, Samsioe G, van Geelen JM. A study of European womens’ experience of the problems of urogenital ageing and its management. Maturitas. 1997;27:239–47. doi: 10.1016/s0378-5122(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 12.Beral V, Bull D, Reeves G Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 13.Effects of hormone replacement therapy on endometrial histology in postmenopausal women: The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 14.Kagan R. Therapeutic options for the treatment of vaginal atrophy. OBG Management. 2010;22:8–13. [Google Scholar]
- 15.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 16.Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. 2002;9:179–87. doi: 10.1097/00042192-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: Findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10:1790–9. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 18.Nath A, Sitruk-Ware R. Pharmacology and clinical applications of selective estrogen receptor modulators. Climacteric. 2009;12:188–205. doi: 10.1080/13697130802657896. [DOI] [PubMed] [Google Scholar]
- 19.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators − mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 20.Ball L, Levy N, Zhao X, et al. Cell type- and estrogen receptor-subtype specific regulation of selective estrogen receptor modulator regulatory elements. Mol Cell Endocrinol. 2009;299:204–11. doi: 10.1016/j.mce.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 22.Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809–20. doi: 10.1210/endo.141.2.7342. [DOI] [PubMed] [Google Scholar]
- 23.Voipio SK, Komi J, Kangas L, Halonen K, DeGregorio MW, Erkkola RU. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas. 2002;43:207–14. doi: 10.1016/s0378-5122(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 24.Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol. 2005;97:230–40. doi: 10.1016/j.jsbmb.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause. 2003;10:433–9. doi: 10.1097/01.GME.0000063609.62485.27. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480–6. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 27.Simon JA, Lin VH, Radovich C, Bachmann GA. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause. 2012;20:418–27. doi: 10.1097/gme.0b013e31826d36ba. [DOI] [PubMed] [Google Scholar]
- 28.Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623–30. doi: 10.1097/gme.0b013e318279ba64. [DOI] [PubMed] [Google Scholar]
- 29.Mutter GL, Ferenczy A. Anatomy and histology of the uterine corpus. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. 5th edn. New York: Springer-Verlag; 2002. pp. 383–420. [Google Scholar]
- 30.Ellmén J, Hakulinen P, Partanen A, Hayes DF. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat. 2003;82:103–11. doi: 10.1023/B:BREA.0000003957.54851.11. [DOI] [PubMed] [Google Scholar]
- 31.Plouffe L, Jr, Siddhanti S. The effect of selective estrogen receptor modulators on parameters of the hypothalamic-pituitary-gonadal axis. Ann N Y Acad Sci. 2001;949:251–8. doi: 10.1111/j.1749-6632.2001.tb04029.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinkerton JV, Goldstein SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause. 2010;17:642–53. doi: 10.1097/gme.0b013e3181c4f1d6. [DOI] [PubMed] [Google Scholar]
- 33.Taylor HS. Designing the ideal selective estrogen receptor modulator − an achievable goal? Menopause. 2009;16:609–15. doi: 10.1097/gme.0b013e3181906fa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmanto A, Nahas-Neto J, Nahas EA, de Oliveira ML, Fernandes CE, Traiman P. Effect of raloxifene on the vaginal epithelium of postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2008;139:187–92. doi: 10.1016/j.ejogrb.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325–7. doi: 10.1097/01.gme.0000222332.31101.42. [DOI] [PubMed] [Google Scholar]
- 37.Marttunen MB, Cacciatore B, Hietanen P, Pyrhönen S, Wahlström T, Ylikorkala O. Prospective study on gynaecological effects of two antioestrogens tamoxifen and toremifene in postmenopausal women. Br J Cancer. 2001;84:897–902. doi: 10.1054/bjoc.2001.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rioux JE, Devlin C, Gelfand MM, Steinberg WM, Hepburn DS. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156–61. doi: 10.1097/00042192-200007030-00005. [DOI] [PubMed] [Google Scholar]
- 39.Suckling JA, Kennedy R, Lethaby A, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(Issue 4) doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Vagifem [package insert] Princeton, NJ: Novo Nordisk Inc; 2012. [Google Scholar]
- 41.Dreisler E, Sorensen SS, Ibsen PH, Lose G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet Gynecol. 2009; 33:102–8. doi: 10.1002/uog.6259. [DOI] [PubMed] [Google Scholar]
- 42.Burich RA, Mehta NR, Wurz GT, et al. Ospemifene and 4-hydroxyospemifene effectively prevent and treat breast cancer in the MTag. Tg transgenic mouse model. Menopause. 2012;19:96–103. doi: 10.1097/gme.0b013e318223e82a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wurz GT, Soe LH, DeGregorio MW. Ospemifene, vulvovaginal atrophy, and breast cancer. Maturitas. 2013 Jan 15 doi: 10.1016/j.maturitas.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol. 2001;77:271–9. doi: 10.1016/s0960-0760(01)00066-8. [DOI] [PubMed] [Google Scholar]
- 45.Polin SA, Ascher SM. The effect of tamoxifen on the genital tract. Cancer Imaging. 2008;8:135–45. doi: 10.1102/1470-7330.2008.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006;13:377–86. doi: 10.1097/01.gme.0000188736.69617.4f. [DOI] [PubMed] [Google Scholar]
- 47.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 48.Johnston CC, Jr, Bjarnason NH, Cohen FJ, et al. Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three-year data from 2 double-blind, randomized, placebo-controlled trials. Arch Intern Med. 2000;160:3444–50. doi: 10.1001/archinte.160.22.3444. [DOI] [PubMed] [Google Scholar]