Abstract
Objective
Activation and dimerization of the ERBB family play a role in the pathogenesis and progression of ovarian cancer. We conducted a phase II trial to evaluate the activity and tolerability of lapatinib in patients with recurrent or persistent epithelial ovarian cancer (EOC) and to explore the clinical value of expression levels of epidermal growth factor receptors (EGFR), phosphorylated EGFR, HER-2/neu, and Ki-67, and the presence of EGFR mutations.
Methods
Eligible patients had recurrent or persistent EOC or primary peritoneal carcinoma, measurable disease, and up to 2 prior chemotherapy regimens for recurrent disease. Patients were treated with lapatinib 1500 mg/day. The primary endpoint of efficacy was 6-month progression free survival (PFS).
Results
Twenty-five of 28 patients were eligible and evaluable for analysis of efficacy and toxicity. Two (8.0%) were alive and progression-free at 6 months. No objective responses were observed. There were 1 grade 4 toxicity (fatigue) and few grade 3 toxicities. Associations between Ki-67 with prior platinum-free interval, PFS, and a polymorphism in EGFR were suggested.
Conclusions
Lapatinib has minimal activity in recurrent ovarian cancer. Ki-67 expression may be associated with prior PFS and a polymorphism in EGFR exon 20 (2361G>A, Q787Q).
Keywords: Epidermal growth factor receptor (EGFR), Ki-67, Lapatinib, Ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of mortality among gynecological malignancies [1]. After conventional frontline therapy the majority of patients relapse. Chemotherapy offers limited and temporary benefit in the setting of recurrent disease [2]. Incomplete understanding of the pathways driving tumor growth has limited the expansion of efficient targeted therapies for patients with EOC.
Up-regulation, activation, and dimerization of the ERBB family (i.e., epidermal growth factor receptor [EGFR] and HER-2) have been implicated in the pathogenesis and progression of ovarian cancer [3–9]. Co-expression of HER-1 and HER-2 is seen more frequently in EOC than in normal ovarian epithelium [6], and overexpression of both receptors correlates with poor prognosis [7–9]. In ovarian cancer cells dual inhibition of EGFR and ERBB2 results in more effective anti-tumor activity [10–13]. Therefore, targeting this group of receptors appears to be a reasonable approach for EOC therapy.
Lapatinib is an orally administered selective EGFR/HER-2 tyrosine kinase inhibitor which is thought to bind to the ATP binding site of protein kinases and compete with the ATP substrate [14]. This results in inhibition of EGFR/HER-2 autophosphorylation. Lapatinib inhibits the proliferation of a large range of tumor cells in vitro [15], and in xenograft models [16]. It has activity as single agent in HER-2 positive metastatic breast cancer and a favorable toxicity profile where skin rash and diarrhea were the most common toxicities [17]. Based on this, the Gynecologic Oncology Group (GOG) decided to evaluate the clinical activity of lapatinib in recurrent ovarian cancer. Expression levels of EGFR, phosphorylated EGFR (pEGFR), HER-2/neu, and Ki-67 and presence of EGFR mutations were evaluated against clinical outcomes.
Materials and methods
Eligibility
Eligible patients had histological diagnoses of recurrent or persistent EOC or primary peritoneal carcinoma as documented on the pathology report. Patients were required to be 18 years of age or older and have measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) [18], a GOG performance status of 0 to 2, and adequate bone marrow (absolute neutrophil count≥1500/μL, platelet count≥100,000/μL), renal (serum creatinine ≤1.5× the upper limit of normal), and hepatic function (total bilirubin≤1.5× the upper limit of normal, and transaminases and alkaline phosphatase≤2× the upper limit of normal). Eligible patients were permitted to have up to 2 prior cytotoxic regimens including 1 required prior platinum-based chemotherapeutic regimen for management of primary disease and were required to have a platinum-free interval of less than 12 months. Patients with prior treatment for recurrent disease with a non-cytotoxic agent, prior radiation to more than 25% of marrow bearing areas, therapeutic warfarin treatment, inability to take oral medications, or concurrent use of CYP3A4 inducers or inhibitors were ineligible.
Patients provided written informed consent consistent with federal, state, and local institutional requirements and authorization permitting release of personal health information. The protocol was approved by the institutional review board at each participating GOG institution and performed in accordance with assurances filed with and approved by the Department of Health and Human Services.
Treatment plan and dose modifications
Oral lapatinib was started at a dose of 1500 mg daily until progression of disease or adverse effects prohibited further therapy. A cycle equaled 28 days. Lapatinib was supplied by the Cancer Treatment Evaluation Program of the National Cancer Institute (NCI).
Toxicity was graded using the NCI Common Toxicity Criteria for Adverse Events (CTCAE) v3.0. Patients who experienced neutropenic fever, grade 4 neutropenia lasting more than 7 days, or grade 4 thrombocytopenia had treatment delayed until toxicity resolved to grade 1 or less, and subsequently their dose was reduced 1 dose level to 1250 mg daily. If the same toxicity recurred, then the dose level was reduced to 1000 mg daily, with no further reductions permitted. Use of erythroid growth factors was permitted after the hemoglobin dropped below 10 g/dL. Patients experiencing grade 2 or worse non hematologic toxicity had therapy held until resolution to grade 1 and subsequent doses were reduced 1 dose level. Specific dose modifications were followed for diarrhea and skin toxicity. Loperamide was initiated in the event of grade 2 diarrhea and lapatinib could be held until recovery to grade 1 or less. In the event of diarrhea grade 3 or higher, despite optimal use of loperamide, lapatinib was interrupted until recovery to grade 1 or less and subsequent doses were reduced by 1 dose level. In the event of grade 2 skin rash lapatinib could be interrupted until recovery to grade 1 or less and treatment with minocycline, topical antibiotics, diphenhydramine, or oral prednisone was allowed. Treatment was interrupted in the event of grade 3 or higher skin rash and treatment was reassumed with 1 dose level reduction when recovered to grade 1 or less. In the event of confirmed decreases of more than 20% in left ventricular ejection fraction (LVEF) from baseline and below the institution's lower limit of normal, lapatinib was interrupted. If the LVEF recovered within 3 weeks, lapatinib could be restarted with 1 dose reduction. Treatment was discontinued in patients with grade 3 or 4 ventricular systolic dysfunction or grade 3 or 4 interstitial pneumonitis. If a patient's dose was reduced for non hematologic toxicity, it was not subsequently increased. Treatment delays of more than 14 days for non hematologic toxicities required removal from protocol treatment (with the exception for LVEF stated above).
Response assessment
Patients were evaluated clinically every 4 weeks, and radiologically every 8 weeks for the first 6 months, then every 6 months thereafter. The same evaluation modality was used throughout for each patient on study. Response criteria used were as defined by RECIST [18]. Progression free survival (PFS) was the period of time from study entry until death, disease progression, or date of last contact (whichever occurs first). Overall survival (OS) was the period of time from entry until death or last contact. If a patient survived progression-free for at least 6 months, they were deemed to be 6-month PFS.
Translational research methodologies
Recurrent or persistent ovarian or primary peritoneal tumor tissue was obtained by a pre-treatment core biopsy or fine needle aspiration. Tumor tissue was formalin-fixed and paraffin-embedded (FFPE). Unstained sections of tumor tissue were used for immunohistochemistry (IHC; e.g., EGFR, pEGFR, HER2-neu, and Ki-67). DNA isolated from FFPE sections was used for PCR, sequencing, and mutation analyses of EGFR.
Immunohistochemistry
FFPE tumor tissue was tested for expression of selected bio-markers in the GOG Core Laboratory for Receptors and Targets using previously published methods [19]. A positive and negative control and optimization of antibody dilution and antigen retrieval protocols were performed for each assay. The tested biomarkers included EGFR (Zymed, San Francisco, CA), Tyr1173 phospho-EGFR (Zymed, San Francisco, CA), Ki-67 (Ventana, Tucson, AZ), and HER-2/neu (Ventana, Tucson, AZ).
FFPE slides were de-paraffinized through 3 changes of xylene to alcohol to water. Antigen retrieval was performed by microwaving slides in 1 mM EDTA in a pressure cooker (Nordicware, MN) for 30 min. Following a cooling period at room temperature for 20 min, tissue was incubated with 3% hydrogen peroxide in methanol for 30 min to quench endogenous peroxidases. Slides were rinsed in Tris buffer pH 7.6 for 10 min. Specimens were incubated with the primary monoclonal antibodies listed above. Following a 10 minute rinse, specimens were incubated with biotinylated antibody (anti-rabbit, anti-mouse) for 30 min. Slides were rinsed in Tris buffer pH 7.6 for 10 min, and incubated with streptavidin-peroxidase for 30 min. Following the final washes, the specimens were incubated with DAB solution (the chromogen substrate). Slides were rinsed and counterstained with hematoxylin. The proportion of malignant epithelial cells, surrounding non-malignant epithelial cells, and stromal cells staining, the sub cellular location of the signal, and the overall intensity of staining were evaluated in each case by an experienced pathologist (EF).
Positivity for each biomarker was defined as membranous or intracellular staining above background (negative control) level; for Ki-67, and EGFR, staining of the nuclear compartment also was assessed. Absence of staining was reported as negative. All slides were submitted to the GOG for an independent pathologic review. The pathologist (EGF) was blinded to clinical outcome and response status and evaluated the slides to assess staining intensity (0–4+) and percent of tumor cells stained. Absence of staining within the tumor was scored as 0. Positive staining was defined as any staining of the tumor cell membrane or intracellular/nuclear compartment and was further characterized as 1+, 2+, 3+, or 4+. The immunostaining intensity was evaluated by standard semi-quantitative techniques. An experienced pathologist compared each tumor specimen to a strongly positive control. Slides scoring 1+ had approximately one quarter of the intensity of the positive control, those scoring 2+ were approximately half of the intensity, and those scoring 3+ were three quarters of the intensity of the positive control.
EGFR mutation analysis
Genomic DNA was extracted from FFPE tumor tissue using a Trimgen DNA purification kit (Trimgen Corp, Sparks, MD) according to the kit instructions. EGFR exons 18–21 were amplified by polymerase-chain-reaction (PCR) as published previously [20] using primer sequences listed in Table 5. PCR amplicons were cleaned with QIA-quick® PCR Purification Kit (Qiagen Inc., Valencia, CA) and were subjected to direct sequencing using same primers for PCR amplification and ABI BigDye Terminator kit v1.1 (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Sequence variations were determined by using Sequencher software 4.7 (Gene Codes Corporation, Ann Arbor, MI) compared with GenBank genomic sequences for each gene. All of the sequence variations were confirmed by multiple, independent PCR amplifications and repeated sequencing reactions. Mutational analyses were performed without knowledge of clinical outcome including tumor response.
Table 5.
Primer sequences for mutational analysis of EGFR.
| Gene | Exon | Primer sequence | Annealing temperature |
|---|---|---|---|
| EGFR (sense) | 18 | 5′-GTG TCC TGG CAC CCA AGC-3′ | 60 |
| EGFR (anti-sense) | 18 | 5′-CCC CAC CAG ACC ATG AGA-3′ | 60 |
| EGFR (sense) | 19 | 5′-CAG CAT GTG GCA CCA TCT C-3′ | 62 |
| EGFR (anti-sense) | 19 | 5′-CAG AGC AGC TGC CAG ACA T-3′ | 62 |
| EGFR (sense) | 20 | 5′-CAT TCA TGC GTC TTC ACC TG-3′ | 60 |
| EGFR (anti-sense) | 20 | 5′-CAT ATC CCC ATG GCA AAC TC-3′ | 60 |
| EGFR (sense) | 21 | 5′-AGC CAT AAG TCC TCG ACG TG-3′ | 60 |
| EGFR (anti-sense) | 21 | 5′-ACC CAG AAT GTC TGG AGA GC-3′ | 60 |
Statistical methods
The primary objectives of the study were to assess the efficacy and toxicity of lapatinib. The primary endpoint used to evaluate efficacy was 6-month PFS. Patients with 6-month PFS were classified as positive outcomes. All others were considered treatment failures even if their follow-up time was less than 6 months.
The clinical trial utilized a 2 stage optimal and flexible group sequential design [21]. The specific decision rules and operating characteristics of the design can be found in the statistical methods of Schilder et al. [20]. Briefly, 22–29 patients were entered to the first stage of accrual. If more than 4 of 25 patients were 6-month PFS, then a cumulative of 53–60 patients was targeted. The treatment would be considered worthy of further investigation if more than 11 of 56 patients were 6-month PFS. If the true probability of being 6-month PFS is 15%, the average probability of designating the treatment as active was 10%, and the average probability of early termination was 59%. If the true probability of being a 6-month PFS was 30%, then the average probability of classifying the treatment as active was 90%.
Secondary clinical objectives included the characterization of the distribution of PFS and OS with Kaplan–Meier plots [22]. The proportion of patients responding (partial and complete) was also calculated with 90% confidence intervals (CI) assuming a binomial distribution.
Evaluation of translational research was conducted with biomarker data (IHC and mutation analyses) against patient demographics and clinical outcome including 6-month PFS, tumor response, PFS, and OS. IHC data was expressed in 3 forms for each biomarker (intensity, percent staining positive, and a product of the 2 called a modified H-value or histoscore). These values were further dichotomized into high and low values (greater or equal to versus less than the observed median value, respectively). Depending on the nature of the data, associations were examined with Kendall's or Spearman's correlation, Fisher's Exact Test, or an Exact Chi-Square Test [23–26]. Kaplan–Meier plots and Cox proportional hazards models were used to assess the associations between the dichotomized biomarkers with PFS and OS [22,27]. Suggested associations were provided by any test with a p-value<0.05, which are used to generate hypotheses in future studies.
Results
Twenty-eight patients were enrolled into the trial and 25 (89%) were evaluable: 2 patients were found to be ineligible due to a second primary and wrong primary, while another patient was inevaluable for never receiving any treatment.
Patient characteristics are presented in Table 1. Most patients had serous carcinomas and over half of the patients had been pretreated with 2 chemotherapy regimens.
Table 1.
Patient characteristics.
| Characteristic | Number of cases |
|---|---|
| Age | |
| 40–49 | 3 |
| 50–59 | 4 |
| 60–69 | 12 |
| 70–79 | 4 |
| 80–89 | 2 |
| Performance status | |
| 0 | 18 |
| 1 | 6 |
| 2 | 1 |
| Race | |
| White | 23 |
| Black | 2 |
| Cell type | |
| Endometrioid | 3 |
| Serous | 20 |
| Undifferentiated | 2 |
| Grade | |
| 2 | 10 |
| 3 | 14 |
| Not graded | 1 |
| Prior chemotherapy | |
| 1 regimen | 12 |
| 2 regimens | 13 |
Toxicity
Treatment was well tolerated (Table 2). There were no treatment related deaths and only 1 grade 4 toxicity (fatigue). The most common toxicity was gastrointestinal which was observed in 20 patients; however, only 4 patients experienced grade 3 toxicity (nausea, diarrhea, dehydration, and anorexia). Mild to moderate grade nausea and diarrhea were common (>50% of patients). Other grade 3 toxicities included fatigue in 2 patients, metabolic in 2, and musculoskeletal, pain, and neuropathy each in 1 patient, respectively.
Table 2.
Toxicity.
| Adverse event | Grade
|
Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Leukopenia | 23 | 2 | 0 | 0 | 0 | 25 |
| Thrombocytopenia | 24 | 1 | 0 | 0 | 0 | 25 |
| Anemia | 12 | 10 | 3 | 0 | 0 | 25 |
| Other hematologic | 24 | 1 | 0 | 0 | 0 | 25 |
| Hearing | 21 | 0 | 4 | 0 | 0 | 25 |
| Constitutional | 12 | 9 | 1 | 2 | 1 | 25 |
| Dermatologic | 15 | 6 | 4 | 0 | 0 | 25 |
| Gastrointestinal | 5 | 11 | 5 | 4 | 0 | 25 |
| Hemorrhage | 23 | 2 | 0 | 0 | 0 | 25 |
| Infection | 24 | 0 | 1 | 0 | 0 | 25 |
| Lymphatics | 24 | 1 | 0 | 0 | 0 | 25 |
| Musculoskeletal | 22 | 1 | 1 | 1 | 0 | 25 |
| Metabolic | 13 | 8 | 2 | 2 | 0 | 25 |
| Neuropathy | 21 | 2 | 1 | 1 | 0 | 25 |
| Ocular | 24 | 1 | 0 | 0 | 0 | 25 |
| Pain | 17 | 7 | 0 | 1 | 0 | 25 |
| Pulmonary | 23 | 2 | 0 | 0 | 0 | 25 |
Treatment response
There were 2 patients who survived progression-free for 6 months (8.0%; 2-sided 90% CI: 1.4–23.1%). No objective responses were observed (0.0%; one-sided 90% CI: 0.0–8.8%). Best response was stable disease in 4 patients (16.0%), increasing disease in 20 (80.0%) and 1 patient was indeterminate (4.0%). Eight patients (32.0%) received 1 cycle of therapy, 13 patients (52.0%) received 2, and 4 patients (16.0%) received 4 or more cycles. PFS and OS Kaplan Meir curves are shown in Fig. 1. The median PFS and OS were 1.8 and 10.5 months, respectively.
Fig. 1.
Overall survival (Surv) and progression-free survival (PFS).
Translational research endpoints
FFPE recurrent or persistent tumor, collected prior to starting study drug, was received from 22 evaluable patients and used for EGFR, pEGFR, HER2/neu, and Ki-67 expression by IHC and EGFR gene sequencing. The results of the IHC analyses are presented in Tables 3 and 4. CI for the hazards of progression and death could not be provided because of the small sample sizes. pERK and Ki-67 were expressed in 58% and 100% of pre-treatment tumors analyzed, respectively. The distribution of the pERK intensity scores of 0+, 1+, and 2+ was 8, 4, and 7 patients, respectively. There was limited EGFR and HER2/neu expression as analyzed by IHC, with 6% and 15% expression, respectively. Categorized pERK and Ki-67 expression (by intensity, percentage staining positive, nor H-value) were not notably associated with tumor response, PFS for at least 6 months, performance status, age, or platinum sensitivity. However, there was a suggested association between Ki-67 H-value and prior platinum-free interval (Spearman's correlation, 51%). Higher levels of pre-treatment Ki-67 were suggestively associated with longer PFS, but not OS (Fig. 2). There was a trend for longer PFS among patients classified as platinum sensitive and a suggested association between platinum sensitive patients and OS. Due to the low expression of EGFR and HER2/neu these markers were not correlated with patient outcome.
Table 3.
Expression of biomarkers in pre-treatment tumor specimens.a
| Biomarker | n | Lower quartile | Median | Upper quartile |
|---|---|---|---|---|
| pERK (H-value) | 19 | 0.00 | 0.05 | 0.70 |
| Ki-67 (H-Value) | 19 | 1.40 | 1.80 | 2.10 |
| pERK (Percentage) | 19 | 0% | 5% | 60% |
| pERK (Percentage) | 19 | 60% | 60% | 70% |
No significant EGFR or Her/neu was detected.
Immunohistochemistry H-value or percentage staining positive.
Table 4.
Association of biomarkers with survival.
| Biomarker | Progression free survival
|
Overall survival
|
|
|---|---|---|---|
| HR | Adjusted HRa | HR | |
| pERK (intensity)b | 1.36 | 0.99 | 0.94 |
| pERK (H-value) | 1.45 | 0.74 | 0.88 |
| pERK (percentage) | 1.45 | 0.74 | 0.88 |
| Ki-67 (H-value) | 0.28c | 2.00 | 0.72 |
| Ki-67 (percentage) | 0.63 | 0.56 | 0.39 |
HR = unadjusted hazard ratio for the relevant endpoint with the biomarker evaluated at higher levels to lower levels.
Adjusted hazard ratio modeled with platinum sensitivity and performance status factors.
pERK was dichotomized into cases with 0+ intensity (8 patients) versus cases with 1+ or 2+ intensities (11 patients).
Data suggest an association by permutation test.
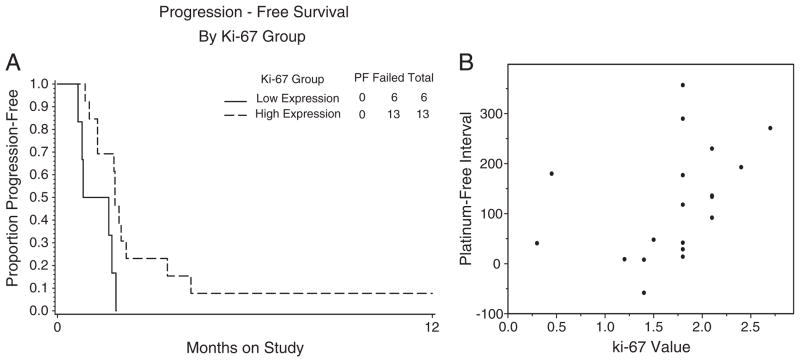
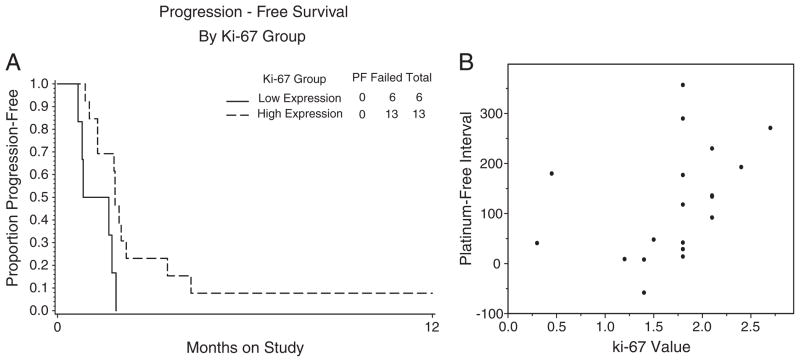
Fig. 2.
Pre-treatment Ki-67 levels. A permutation test suggested that higher levels of pre-treatment Ki-67 (H-Value) were associated with longer progression free survival (A). There was a suggested association between Ki-67 H-value and prior platinum-free interval (Spearman correlation=0.51; B).
Sequence variants in EGFR were detected using DNA isolated from FFPE tumor tissue. Ten polymorphisms and two putative mutations were observed: One patient had a mutation in exon 20 (2323T>C, C775R) and 1 patient had a mutation in exon 21 (2599A>G, K867E). The median PFS and OS time for all 25 eligible and evaluable women was 1.8 and 10.5 months, respectively. The patient with a mutation in exon 20 had PFS and survival time equal to 1.84 and 26.2 months, respectively. The patient with a mutation in exons 21 had PFS and survival time equal to 0.79 and 1.15 months, respectively. None of the mutations or polymorphisms observed was correlated with clinical outcomes (tumor response, PFS for at least 6 months, performance status, age, nor platinum sensitivity). One suggested association between a polymorphism in EGFR exon 20 (2361G>A, Q787Q) with Ki-67 was noted.
Conclusions
Preclinical data suggests that targeting the ERBB pathway could have therapeutic value in the management of EOC. Unfortunately the clinical activity of single agents has been modest at best. The activity of trastuzumab has been reported in only 1 clinical trial with a response rate of 7.3% among patients whose tumors were shown to over express Her-2\neu and a median PFS of 2 months [28].
The activity of selective EGFR inhibitors has been reported by various authors reporting response rates of 3.7%–6% with erlotinib, gefitinib and cetuximab, respectively [29–31]. Time to progression has been reported to be approximately 2–3 months. All of the aforementioned trials demonstrate that agents targeting 1 receptor of the ERBB family have minimal activity in EOC. These studies also show that strong overexpression of EGFR or HER-2/neu, as well as deleterious mutations in EGFR, are relatively uncommon events in EOC.
Although preclinical studies suggest that dual inhibition of EGFR and ERBB2 results in more effective anti-tumor activity, this has not been substantiated in the clinic. Similar to our findings, pertuzumab (a recombinant humanized monoclonal antibody that inhibits HER dimerization) was reported to have minimal activity in recurrent EOC as demonstrated by a response of 4.3% with median PFS and OS of 6.6 and 52.7 weeks, respectively [32].
Although the results of targeting the ERBB pathway have been disappointing, emerging data suggest that combining ERBB inhibitors with other agents may still play a role. For example, in a phase II randomized trial, the combination of gemcitabine plus pertuzumab significantly improved the response rate and PFS compared to single agent gemcitabine [33].
In our trial, lapatinib, when used as a single agent, has insufficient activity to warrant further investigation in an unselected patient population with EOC. Our study confirms that targeting the ERBB pathway yields limited benefit in the management of recurrent EOC. These findings may be explained, at least in part, due to the low expression of EGFR and HER2\neu. The activity of lapatinib, or similar agents, may be higher in patients enriched with the appropriate target. Interestingly, our data suggests an association between H-value of Ki-67 and prior platinum-free interval, as well as PFS and a polymorphism in EGFR exon 20 (2361G>A, Q787Q). The causal pathway between Ki-67, platinum sensitivity, PFS, and OS (if they exist) remains to be elucidated in future studies. However, plausible hypothesis are that tumors with high proliferation rate tend to respond better to cytotoxic treatments and therefore survival may have been affected by subsequent chemotherapy in patients with high Ki-67. In addition, the statistical power to detect clinically significant associations is low due to small sample sizes. Therefore, the negative translational research results from this study should not be interpreted as conclusive. Our group does not plan to conduct further trials of this agent in this patient population. However, related compounds may be investigated in the future where these questions could be posed again.
Acknowledgments
The authors would like to thank Sandra Dascomb for the management of clinical data, the GOG Tissue Bank for their assistance with the banking and distribution of specimens, Beverly Kratzel and Meg Colahan for the administration of the protocol, and Kim Blaser for publications management.
Footnotes
This work was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517), CA 99908 (to K.K.L.) and CA 140323 (to A.K.G.). A.K.G. is the Chancellors Distinguished Chair in Biomedical Sciences Endowed Professor. The following Gynecologic Oncology Group member institution participated in this study: Abington Memorial Hospital, University of Washington, Rush-Presbyterian-St. Luke's Medical Center, The Cleveland Clinic Foundation, Women's Cancer Center, University of Oklahoma, and Community Clinical Oncology Program.
Conflict of interest statement
The co-authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Harries M, Gore M. Part II: chemotherapy for epithelial ovarian cancer-treatment of recurrent disease. Lancet Oncol. 2002;3:537–45. doi: 10.1016/s1470-2045(02)00847-1. [DOI] [PubMed] [Google Scholar]
- 3.Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer. 2000;88:566–74. doi: 10.1002/1097-0215(20001115)88:4<566::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromgberg K, Stetler-Ste-venson WG. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst. 2001;93:1375–84. doi: 10.1093/jnci/93.18.1375. [DOI] [PubMed] [Google Scholar]
- 5.Campiglio M, Ali S, Knyazev PG, Ullrich A. Characteristics of EGFR family-mediated HRG signals in human ovarian cancer. J Cell Biochem. 1999;73:522–32. [PubMed] [Google Scholar]
- 6.Bast RC, Jr, Pusztai L, Kerns BJ, MacDonald JA, Jordan P, Daly L, et al. Coexpression of the HER-2 gene product, p185HER-2, and epidermal growth factor receptor, p170EGF-R, on epithelial ovarian cancers and normal tissues. Hybridoma. 1998;17:313–21. doi: 10.1089/hyb.1998.17.313. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, et al. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer. 1996;73:301–6. doi: 10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berchuck A, Kamel A, Whitaker R, Kerns B, Ott G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–91. [PubMed] [Google Scholar]
- 9.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 10.Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731–8. doi: 10.1038/sj.onc.1202319. [DOI] [PubMed] [Google Scholar]
- 11.Pack SD, Alper OM, Stromberg K, Augustus M, Ozdemirli M, Miermont AK, et al. Simultaneous suppression of epidermal growth factor receptor and c-erbB-2 reverses aneuploidy and malignant phenotype of a human ovarian carcinoma cell line. Cancer Res. 2004;64:789–94. doi: 10.1158/0008-5472.can-03-1982. [DOI] [PubMed] [Google Scholar]
- 12.Wilken JA, Webster KT, Maihle NJ. Trastuzumab sensitizes ovarian cancer cells to EGFR-targeted therapeutics. J Ovarian Res. 2010;27:3–7. doi: 10.1186/1757-2215-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takai N, Jain A, Kawamata N, Popoviciu LM, Said JW, Whittaker S, et al. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer. 2005;104:2701–8. doi: 10.1002/cncr.21533. [DOI] [PubMed] [Google Scholar]
- 14.Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 15.Rusnak DW, Affleck K, Cockerill SG, Stubberfield C, Harris R, Page M, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61:7196–203. [PubMed] [Google Scholar]
- 16.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, et al. Single-agent lapatinib for HER2-overerexpression advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–31. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:325–33. doi: 10.1016/j.ygyno.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Schilder R, Sill M, Chen X, Darcy KM, Decesare SL, Lewandowski G, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group study. Clin Cancer Res. 2005;11:5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 21.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assn. 1958;53:457–81. [Google Scholar]
- 23.Kendall MG. The treatment of ties in rank problems. Biometrika. 1945;33:239–51. doi: 10.1093/biomet/33.3.239. [DOI] [PubMed] [Google Scholar]
- 24.Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 25.Fisher RA. Statistical methods for research workers. Edinburgh: Oliver and Boyd; 1934, 1970. [Google Scholar]
- 26.Mehta CR, Patel NR. A network algorithm for performing Fisher's exact test in r×c contingency tables. J Am Stat Assoc. 1983;78:427–34. [Google Scholar]
- 27.Cox DR. Regression models and life tables. J Royal Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 28.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 29.Gordon AN, Finkler N, Edwards RP, Garcia AA, Crozier M, Irwin DH, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–92. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 30.Posadas EM, Liel MS, Kwitkowski V, Minasian L, Godwin AK, Hussain MM, et al. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109:1323–30. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schilder RJ, Pathak HB, Lokshin AE, Holloway RW, Alvarez RD, Aghajanian C, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol. 2009;113:21–7. doi: 10.1016/j.ygyno.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol. 2006;24:4324–32. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 33.Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–23. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]