Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal and prevalent cancers in humans. The molecular mechanisms leading to the development of HCC are extremely complicated and consist of prominent genetic, genomic, and epigenetic alterations. This review summarizes the current knowledge of the role of epigenetic aberrations, including changes in DNA methylation, histone modifications, and expression of microRNAs in the pathogenesis of HCC. It also emphasizes that identification of the underlying epigenetic alterations that drive cell transformation and promote development and progression of HCC is crucially important for understanding mechanisms of hepatocarcinogenesis, its detection, therapeutic intervention, and prevention.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent life-threatening human cancers that is not only increasing in worldwide incidence in the past decade [1–4], but is also a leading cause of cancer-related deaths worldwide [3–6]. HCC is an aggressive and enigmatic disease, which represents approximately 85% of liver cancers [5,6]. The most prominent etiological factors associated with HCC consist of chronic viral hepatitis B and C infections [4,7–9], nonalcoholic fatty liver disease [10–12], and toxin and alcohol exposure [6,9]. The development and progression of HCC is a multistep and long-term process characterized by the progressive sequential evolution of morphologically distinct preneoplastic lesions (formed as a result of chronic liver injury, necro-inflamation and regeneration, small cell dysplasia, low-grade and high-grade dysplastic nodules) that culminates in the formation of HCC [5,13]. However, the molecular and cellular mechanisms of HCC pathogenesis are still poorly understood [5,6].
Traditionally, the development of HCC in humans has been viewed as a progressive multistep process of transforming of normal cells into malignant driven primarily by the stepwise accumulation of genetic alterations in tumor-suppressor genes and oncogenes [14–16], with mutations in β-catenin and P53 genes being the major genetic alterations [14,15]. However, over the past decade there has been a surge in data indicating the importance of epigenetic processes, which has largely changed the view of HCC as a genetic disease only [17–19]. Presently, HCC is recognized as both a genetic and epigenetic disease, and genetic and epigenetic components cooperate at all stages of liver carcinogenesis [16,20]. While the sequential accumulation of various genetic changes in hepatocarcinogenesis has been extensively studied, the contribution of epigenetic alterations to HCC development and progression has remained relatively unexplored until recently [17–19].
2. Epigenetic alterations in HCC
The unifying molecular feature of HCC is a profoundly reshaped epigenome that is characterized by global genomic hypomethylation, gene-specific DNA hyper- or hypo-methylation, abnormal expression of DNA methyltransferases and histone modifying enzymes, altered histone modification patterns, and aberrant expression of microRNAs [6,13].
2.1. Global DNA hypomethylation in human HCC
DNA hypomethylation signifies one of the two major DNA methylation states and refers to a condition in which there is a decrease in the number of methylated cytosine bases from the “normal” methylation level. DNA hypomethylation arises mainly from the loss of methylation at normally heavily methylated areas of genome. The molecular events that may lead to hypomethylation of DNA are elusive and it is likely that multiple pathways may be involved. Hypomethylation of DNA can be achieved either passively or actively. Passive loss of methylated cytosines in the genome may be a consequence of either (i) limited availability of the universal methyl donor S-adenosyl-L-methionine (SAM), (ii) compromised integrity of DNA, or (iii) altered expression and/or activity of DNA methyltransferases (DNMTs) [21].
The methyl groups needed for DNA methylation and other cellular methylation reactions are acquired from SAM, which is synthesized from L-methionine and adenosine in an ATP-dependent reaction catalyzed by methionine adenosyltransferase 1 (MAT1) in a one-carbon pathway in the cytosol of every cell. The liver is the primary tissue for SAM biosynthesis and degradation [22]. This indispensably connects faithful DNA methylation to the proper functioning of this metabolic pathway. It is well established that either genetic polymorphism in genes involved in the methyl-group metabolism, or deficiencies in methyl group donors caused by endogenous and exogenous factors, may lead to loss of cytosine methylation in DNA [23,24].
The integrity of the genome is another critical factor that may affect the DNA methylation status. Specifically, the results of several studies have demonstrated that the presence of unrepaired lesions in DNA, e.g., 8-oxodeoxyguanosine, 5-hydroxymethyl-cytosine, apurinic/apirimidinic sites, and strand breaks, diminishes the ability of DNA methyl transferases (DNMTs) to methylate DNA [25,26]. Additionally, a large body of evidence clearly demonstrates that improper function of DNMTs may compromise the DNA methylation status [27]. Importantly, a number of well-established hepatocarcinogens may perturb the described above processes and trigger loss of DNA methylation in the liver [19,28,29].
Active loss of methylated cytosines, via an active replication-independent DNA demethylation process, was controversial and inconclusive for many years [30,31]. However, recent studies have provided compelling experimental evidence that active loss of DNA methylation is associated with the function of DNA repair machinery [32–35].
The loss of DNA methylation was the first epigenetic abnormality and one of the most common molecular alterations identified in human cancers [36], including HCC [37,38]. Global DNA demethylation in HCC primarily affects stable, methylated areas of the genome composed predominantly of repetitive DNA sequences, such as long interspersed nucleotide elements 1 (LINE1), retroviral intracesternal A particles (IAP), Alu elements, and body of genes [39]. Indeed, the results of several comprehensive studies have demonstrated extensive hypomethylation of LINE-1, ALU, and SAT2 repetitive elements in HCC [40–42]. More importantly, it has been demonstrated that the serum LINE-1 hypomethylation may be an independent prognostic marker in patients with HCC [43].
There are several molecular consequences of global DNA demethylation that may contribute to the progression of liver carcinogenesis via multiple mechanisms. Specifically, genomic hypomethylation may cause a significant elevation in mutation rates, aberrant activation of “normally” silenced tumor-promoting genes, loss of imprinting, and activation and transposition of repetitive DNA elements leading to chromosomal and genomic instability [44].
Despite the large body of evidence indicating that cancer-associated DNA demethylation is an important early event in the development of HCC, it is still less clear if the loss of DNA methylation is a cause or a consequence of the malignant transformation [45]. The notion that DNA hypomethylation plays a causative role in liver cancer is based on the results of nutritional and genetic studies using “lipogenic methyl-deficient diets” [46–48] or genetically-engineered ApcMin/+;Dnmt1chip/c mice [49], respectively. In contrast, there is also evidence that cancer-linked DNA hypomethylation may be a passive inconsequential side effect of carcinogenesis [45]. The latter is evidenced by the facts that not all liver tumors exhibit DNA hypomethylation [50] and not all carcinogenic processes are accompanied by the loss of DNA methylation [51].
2.2. Cancer-linked gene-specific DNA hypermethylation in human HCC
DNA hypermethylation is the state where the methylation of “normally” undermethylated DNA domains, those that predominantly consist of CpG islands [52], increases. CpG islands are defined as the genomic regions that contain the highest G + C content, have a high frequency of CpG dinucleotides, are at least 400–500 bp long, and are located in intragenic, intergenic, or 5′ ends of genes [53–55]. However, only CpG islands that span the 5′ regions of promoters and first exons are mainly unmethylated. For instance, it is established that less than 3% of CpG islands in gene promoters are methylated [55].
The focus of studies aimed to explore the role of DNA methylation in HCC has been on global epigenomic alterations in HCC, abnormal gains of DNA methylation (hypermethylation) of typically unmethylated CpG island-containing promoters, transcriptional repression, and loss of gene function. Specifically, gene-specific DNA hypermethylation has gathered most attention as a critical event in liver carcinogenesis. Several epigenetically inactivated genes, as evidenced by association between diminished mRNA levels with highly methylated promoters, have been identified in HCC. Among genes frequently methylated in HCC are tumor suppressors, including RASSF1A [56], p16INK4A [57,58], p15INK4B [59], RB1 [60], SOCS1 [61,62], SOCS3 [63], SYC [64], GSTP1 [65], NQO1 [66], PROX1 [67,68], NORE1B [69], RIZ1 [70], RELN [71], FBLN1 [72], and PAX5 [73]. These genes are involved in the regulation of vital biological processes, including cell-cycle control, apoptosis, cell proliferation, and xenobiotic metabolism.
In addition, there is growing evidence of the importance of non-CpG island-containing promoter coding region hypermethylation in gene inactivation. For instance, hypermethylation of the p53 promoter region and the MAT1A coding region is associated with inhibition of gene expression in human HCC [74,75]. The fact that the aberrant gene-specific hypermethylation of the aforementioned genes occurs not only in HCC, but also in premalignant pathological conditions, including chronic viral hepatitis B and C and liver cirrhosis, suggests the importance of gene-specific hypermethylation event in pathogenesis and progression of HCC.
2.3. Cancer-linked gene-specific DNA hypomethylation in human HCC
Until recently, the majority of the studies in the field of cancer research, including liver cancer, have focused on alterations in DNA hypomethylation, mainly hypomethylation of repetitive sequences, and epigenetically-driven gene silencing, as the main mechanisms favoring the development of HCC. However, mounting evidence indicates that the hypomethylation of “normally” methylated genes is significant in the pathogenesis of HCC [76]. Currently, a number of hypomethylated tumor-promoting genes, including uPA [77], HPA [78], SNCG [79], TFF3 [80], MAT2A [81], HKII [82], CD147 [83], and VIM [84] have been identified in primary human HCC.
Importantly, gene-specific DNA methylation changes, both hyper- and hypomethylation, in HCC are associated with well-established hallmarks of cancer, including the acquisition of persistent proliferative signaling, resistance to cell death, evasion of growth suppression, replicative immortality, inflammatory response, deregulation of energy metabolism, induction of angiogenesis, and activation of invasion [85]. However, while gene-specific promoter DNA hypermethylation changes are associated predominantly with deregulation of pathways important for the initiation of HCC, such as cell-cycle control, apoptosis, and cell proliferation, gene-specific promoter DNA hypomethylation changes are related to biological processes critical for tumor progression, including cell growth, cell communication, adhesion and mobility, signal transduction, and drug resistance.
The existence of two opposing hyper- and hypomethylation events in the same functional pathways complement or enhance each other in the disruption of cellular homeostasis favoring progression of HCC. For instance, hypermethylation and transcriptional inactivation of the E-cadherin (CDH1) gene [86] and hypomethylation-induced up-regulation of the Vimentin (VIM) gene [84] in HCC may exaggerate invasion and escalate further progression of HCC.
2.4. Alterations of DNA methyltransferases in human HCC
Abnormal patterns of DNA methylation in HCC is closely related to the disruption in the functioning of DNA methylation machinery. Several reports distinctly established the major role of altered gene expression of DNA methyltransferase DNMT1, a main enzyme involved in the maintenance of genomic methylation patterns, the de novo DNA methyltransferases DNMT3A and DNMT3B, and methyl-binding proteins in the development and progression of HCC [27,87–89]. This is evidenced by a progressive marked up-regulation of DNMT1, DNMT3A, and DNMT3B in premalignant non-cancerous liver tissues and in full-fledged HCC [27] and by the fact that over-expression of these DNMTs significantly correlated with CpG-island hypermethylation of tumor-related genes [90]. Additionally, it has been demonstrated that over-expression of DNMT3B4, a splice variant of DNMT3B, causes DNA hypomethylation in pericentromeric satellite regions [44].
2.5. Dysregulation of histone modifications in human HCC
DNA methylation changes in HCC are not isolated events; they occur in an environment of large-scale disruptions of the cellular epigenome that also include alterations in histone modification patterns [91]. Similarly to alterations in DNA methylation, changes in histone modifications in HCC occur genome-wide and on gene-specific scales. At least eight different classes of post-translational modifications, including methylation, acetylation, phosphorylation, ubiquitynation, sumoylation, biotinylation, and ADP-ribosylation have been identified on the core histones H2A, H2B, H3, H4, and the H1 family of linker histones [92]. With respect to HCC, acetylation and methylation of histone lysine residues are the best studied histone modifications so far. Typically, histone acetylation is associated with an active transcription, whereas methylation may be associated with either active or repressive states, depending on the modified site [91,92].
Currently, it is well-documented that transcriptional silencing of cancer-related genes in HCC is associated with DNA methylation and/or histone modifications at their promoters. For instance, a number of transcriptionally repressed genes in human HCC are associated either with hypoacetylation of lysine residues at histone H3 and H4 [66,93]. Likewise, transcriptionally silenced RIZ1, p16INK4A and RASSF1A tumor-suppressor genes in human HCC are characterized by an increased level of repressive histone H3 lysine 9 and histone H3 lysine 27 methylation marks at their promoters [70,94,95] In addition to aberrations in histone modifications at promoters of individual genes, HCC also displays genome-wide changes in histone modifications, particularly a loss of trimethylation of histone H4 lysine 20 and increase of histone H3 lysine 27 trimethylation and histone H3 phosphorylation [96,97].
2.6. Alterations of histone modifying enzymes in human HCC
It is well-documented that HCC is characterized by a prominent dysregulation of several histone-modifying enzymes, including histone deacetylase (HDAC) class I isoforms HDAC1, HDAC2, and HDAC3, SIRT1, a class III HDAC, and histone methyltransferases (HMTs) SMYD3, RIZ1, and EZH2 [98–102]. Histone deacetylases are responsible for removal of an acetyl group from lysine residues of target proteins and display multi-faceted roles in coordinating the interaction of intracellular signaling pathways through chromatin remodeling. Several recent studies have demonstrated an elevated expression of HDAC1, HDAC2, and HDAC3 [97,99] and SIRT1 in human HCC [103,104] that correlated with clinicopathological features and recurrence of HCC.
Histone methyltransferases SMYD3, RIZ1, and EZH2 are responsible for methylation of lysine residues 4, 9 and 27 at histone H3, respectively [99,100]. The up-regulation of the SMYD3 HMT promotes proliferation of HCC via increasing H3 lysine 4 methylation, a mark of transcriptional activation, and thus subsequent activation of downstream genes, including NKX2-8 gene [99], which is frequently up-regulated in human HCC [105]. In contrast, over-expression of the EZH2 in HCC may facilitate the progression of HCC through increasing trimethylation of H3 lysine 27 and enhancing heterochromatin formation at promoters of transcriptionally silenced genes [100]. Indeed, a recent report by Cheng et al. [106] has demonstrated that EZH2 inserts its oncogenic activity in HCC through EZH2-mediated epigenetic silencing of the growth-suppressive Wnt antagonists, which causes a constitutive activation of Wnt/β-catenin signaling and proliferation of HCC cells. Additionally, EZH2 recruits DNA methyltransferases to the EZH2-containing Polycomb complex leading to de novo methylation of cancer-related genes [107,108].
In contrast, down-regulation of RIZ1 HMT that is frequently found in HCC [70,109] may promote progression of hepatocarcinogenesis via reduction of the level of histone H3 lysine 9 trimethylation, followed by chromatin decondensation, and genomic instability.
Additionally, prominent epigenetic abnormalities found in HCC may be induced by alterations in intracellular metabolism. For example, the status of DNA and histone methylation in HCC may be compromised by deficiency of intracellular SAM caused by cancer-related transcriptional silencing of the liver-specific MAT1A gene [110,111]. This gene encodes methionine adenosyltransferase, which is an essential enzyme for the biosynthesis SAM [112]. Likewise, expression of several other genes involved in methionine metabolism, e.g., BHMT and CBS, is reduced in HCC [110. This corresponds to a recently emerged hypothesis that alteration of cellular epigenome in cancer is associated with cancer-linked metabolic disturbances, specifically with aberrant levels of variety of small molecules derived from intermediary intracellular metabolism, including glucose, glutathione, flavin adenine dinucleotide and nicotine adenine dinucleotide, folate, acetyl coenzyme A, and α-ketoglutarate [113,114].
2.7. MicroRNAome alterations in human HCC
Extensive studies in the past decade have indicated the existence and importance of another epigenetic mechanism of regulation of gene function by means of small non-coding microRNAs (miRNAs). Currently, miRNAs are recognized as one of the major regulatory gatekeepers of protein-coding genes in the human genome [115]. MiRNAs are small 16–29 nucleotide-long non-coding RNAs that primarily function as negative gene regulators at the post-transcriptional level [116]. MiRNAs are generated by RNA polymerase II or RNA polymerase III as long primary transcripts, primary miRNAs. Following transcription, primary miRNAs form a stem-loop structure, which is recognized and processed by the RNase III-type enzyme Drosha creating precursor miRNAs. These precursor miRNAs are transported from the nucleus to the cytoplasm by Exportin-5. In the cytoplasm, the pre-miRNAs are further processed by Dicer, an RNase III enzyme, generating miRNA:miRNA hybrids. After unwinding, one strand of the duplex is degraded, and another strand becomes a mature miRNA. MiRNAs can induce mRNA cleavage if their complementary to the 3′-untranslated region of mRNA targets are perfect or miRNAs can induce translational repression if complementarity is imperfect [115].
Currently there are more than 700 mammalian miRNAs that can potentially target up to one-third of protein-coding genes involved in development, cell differentiation, metabolic regulation, signal-transduction, cell proliferation, and apoptosis. As the deregulation of these very same biological processes is a hallmark of cancer [85], it has been suggested that changes in miRNA expression might have significance in cancer [117–119], including HCC [120,121]. In recent years, a number of comprehensive studies have documented an aberrant expression, both up-regulation and down-regulation, of miRNAs that have been associated with virtually every aspect of HCC biology, including tumor progression, e.g., up-regulated miR-21, miR-17-92, miR-155, miR-191, and miR-221/miR-222 and down-regulated miR-122 [122–126], invasion and metastasis, e.g., up-regulated miR-21 and miR-151 and down-regulated miR-200 family [122,127,128], and acquisition of resistance of malignant cells to various chemotherapeutic agents, e.g., up-regulated miR-21 and down-regulated miR-122 and miR-199a-3p [129–131].
Growing evidence indicates a direct and interdependent link between epigenetic and miRNA expression alterations illustrating the complexity of epigenetic abnormalities in HCC. For instance, high expression of miR-191 in HCC is associated with hypomethylation of the mir-191 gene [132], whereas down-regulation of miR-1, miR-124, miR-125b, and miR-203 is attributed to DNA hypermethylation [133–135]. On the other hand, altered expression of miR-29, miR-152, and miR-200a causes substantial alterations in DNA methylation and histone modifications [136–138].
3. Molecular diagnosis of HCC: epigenetic biomarkers
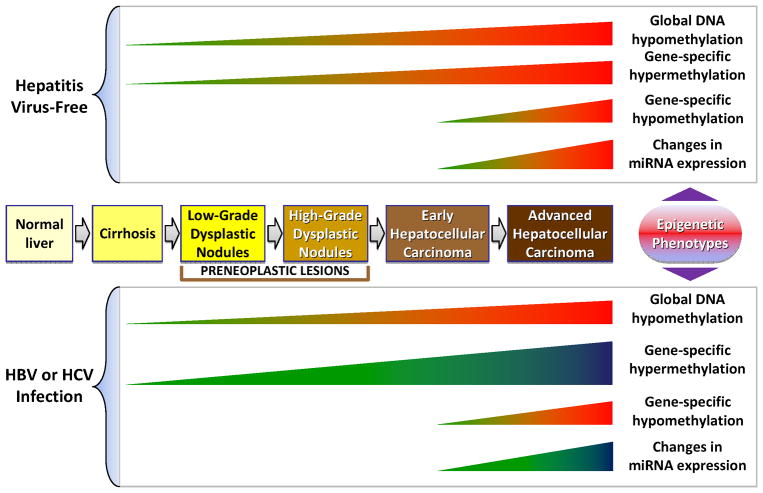
Understanding the molecular mechanisms involved in neoplastic hepatocyte transformation, promotion, and progression of hepatocarcinogenesis are crucial for the diagnosis, prognosis, and determination of treatment strategies of HCC. The post-genomic era molecular tools, e.g., transcriptomic and genome-wide association studies, have been used succesfully for the identification of new diagnostic and prognostic biomarkers of HCC [139–142]. Mapping the patterns of DNA methylation has also been proposed recently as a valuble molecular diagnostic tool for HCC. Here we show the proposed chronology of epigenetic events in the progression of human HCC (Figure 1).
Figure 1.
The chronology of DNA methylation and miRNA alterations in human multistage hepatocarcinogenesis.
Many studies provide evidence that cancer-linked DNA methylation alterations may be used as early indicators of liver carcinogenesis, as well as prognostic markers of cancer progression and response to chemotherapy [143–149]. Specifically, using a DNA methylation profiling approach it is possible to differentially diagnose HCC from preneoplastic lesions, e.g. low-grade and high-grade dysplastic nodules and cirrhosis [143,146]. This is evidenced by different pattern and magnitude of gene-specific DNA methylation changes in preneoplastic livers and full-fledged HCC. Recently, Nagashio et al. [144] was able to predict HCC with 95.6% sensitivity and 100% specificity using a quantification of DNA methylation level approach in preneoplastic liver tissue. These authors also demonstrated that the methylation status of gene-specific DNA regions significantly correlates with the outcome of patients with HCC. More importantly, the results of several comprehensive studies have demonstrated clearly that aberrant gene-specific DNA methylation patterns discriminate HCC, with an etiology associated with viral hepatitis B and C infection and alcohol intake [146–151]. Additionally, several reports have indicated that gene-specific methylation, e.g, RIZ1 gene, in surgically resected non-tumorous tissue significantly associated with reoccurrence of HCC [152,153].
The steadiness and specificity of cancer-associated DNA hypo- or hypermethylation changes offer substantial advantages over other molecular markers for cancer diagnostics. Firstly, DNA hypermethylation is a positive signal not observed in normal cells, and therefore it is independent of contamination with normal cells. Secondly, because DNA methylation changes are stable, the same aberrant DNA methylation patterns specific for the primary tumor can be identified in plasma and other body fluids. Indeed, several studies have shown that promoter hypermethylation of cancer-related genes, including p16INK4A, RASSF1A, GSTP, and RUNX3 genes [154–159], as well as LINE-1 hypomethylation [43] were detected in the plasma of the majority of patients diagnosed with HCC. Such observations indicate that cancer-linked aberrant DNA methylation may be a potential valuable noninvasive diagnostic and prognostic biomarker for HCC.
Little evidence has been presented to clarify whether or not miRNA alterations may distinguish HCC from preneoplastic lesions; however, similar to DNA methylation changes, emerging evidence indicates that hepatic miRNA profile may predict the recurrence of HCC after resection [160] and circulatory blood miRNA levels may be used as a potential biomarker for noninvasive diagnosis of HCC [161–163].
4. Animal models of HCC
In humans, most of the research on HCC is conducted on patients who have already developed the disease. This limits the scope of the investigation to tumor biology and does not allow extensive study into the mechanisms of disease progression, which is critical for early diagnostic and successful treatment. On the contrary, relevant animal models of liver carcinogenesis provide a unique opportunity to understand the underlying molecular mechanisms involved in pathogenesis of HCC and substantially complement many shortcomings of humans-only studies [164–166]. The application of animal models to study process of hepatocarcinogenesis is further supported by a recent evidence [167] showing that despite major differences in etiology of mouse and human HCC (human HCC arises predominantly within an environment of chronic inflammation, hepatocellular degeneration, necrosis and regeneration, fibrosis and cirrhosis [168]) there are great similarities in the molecular landscape between human and spontaneous HCC in B6C3F1 mice.
The most commonly used animal models of HCC consist of those that are induced by different genotoxic chemical carcinogens, e.g., diethylnitrosamine, aflatoxin B1, and 2-acetylaminofluorene, non-genotoxic chemical agents, e.g., peroxisome proliferators, transgenic mouse models of hepatitis B and C viral infection, and constitutive and conditional transgenic mice with over-expression of oncogenes, e.g., c-myc and β-catenin, or growth factors, e.g., TGFα, TGFβ, EGF, and PDGF-C [164–166]. One of the most extensively studied models of rodent HCC that is relevant to humans is endogenous liver carcinogenesis induced by dietary methyl deficiency [169]. This model is unique because dietary omission of sources of methyl groups, rather than xenobiotic addition, leads to tumor formation. In addition, the sequence of pathological and molecular events is remarkably similar to the development of human HCC that is associated with viral hepatitis B and C infections, alcohol exposure, and metabolic liver diseases, all of which are currently considered major risk factors of HCC worldwide.
5. Perspectives
It is clear that epigenetic alterations are critical determinants of human hepatocellular cancer. The progressive accumulation of epigenetic changes during development of HCC gives a unique opportunity to use them as biomarkers in cancer detection. However, not all aberrations may be equally important for the tumorigenic process [170]. Specifically, it is highly unlikely that all epigenetic aberrations play a significant role in hepatocarcinogenesis. For example, some epigenetic changes may drive other events that contribute to the formation of a transformed phenotype, while others may be passenger events that accompany the transformation process. In this respect, the identification of alterations that drive cell transformation and promote hepatocarcinogenesis is crucially important for understanding mechanisms of HCC progression and prevention. Additionally, the presence of epigenetic abnormalities in premalignant and non-tumorous livers unequivocally indicates the involvement of an “epigenetic field cancerization” effect in the pathogenesis of HCC [90,143,152,153,171–174]. This emphasizes the role and the usefulness of epigenetic biomarkers for early detection and prediction of HCC development. More importantly, the potential reversibility of epigenetic alterations opens a novel mechanism-based approach to molecular-targeted HCC treatment and prevention.
Acknowledgments
This work was supported, in part, by grants from NIH (R01 ES015241 and P42 ES005948).
Footnotes
The views expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
References
- 1.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999-through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. in press. [DOI] [PubMed] [Google Scholar]
- 3.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 4.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 5.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. doi: 10.1038/sj.onc.1209559. [DOI] [PubMed] [Google Scholar]
- 8.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25:3834–3847. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15:14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 10.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetzel TM, Graubard BI, Zeuzem S, El-Serag H, Davila JA, McGlynn KA. Metabolic syndrome increases risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res. 2011 doi: 10.1111/j.1872-034X.2011.00872.x. in press. [DOI] [PubMed] [Google Scholar]
- 13.Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 2005;25:16–27. doi: 10.1111/j.1478-3231.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 14.Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- 15.Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Semin Liver Dis. 2011;31:173–187. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- 16.Nishida N, Goel A. Genetic and epigenetic signatures in human hepatocellular carcinoma: a systematic review. Curr Genomics. 2011;12:130–137. doi: 10.2174/138920211795564359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713–2722. doi: 10.1172/JCI31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischoff I, Tannapfe A. DNA methylation in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1741–1748. doi: 10.3748/wjg.14.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herceg Z, Paliwal A. Epigenetic mechanisms in hepatocellular carcinoma: how environmental factors influence the epigenome. Mutat Res. 2011;727:55–61. doi: 10.1016/j.mrrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Harath NI, Leggett BA, MacDonald GA. Review of genetic and epigenetic alterations in hepatocarcinogenesis. J Gastroenterol Hepatol. 2006;21:15–21. doi: 10.1111/j.1440-1746.2005.04043.x. [DOI] [PubMed] [Google Scholar]
- 21.Pogribny IP, Beland FA. DNA hypomethylation in the origin and pathogenesis of human diseases. Cell Mol Life Sci. 2009;66:2249–2261. doi: 10.1007/s00018-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 23.Zeisel SH. Genetic polymorphisms in methyl-group metabolism and epigenetic: lessons from humans and mouse models. Brain Res. 2008;1237:5–11. doi: 10.1016/j.brainres.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulrey CL, Liu L, Andrews LG, Tollefsbol TO. The impact of metabolism on DNA methylation. Hum Mol Genet. 2005;14:R139–R147. doi: 10.1093/hmg/ddi100. [DOI] [PubMed] [Google Scholar]
- 25.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 26.Chia N, Wang L, Senut MC, Brenner C, Ruden DM. Environmental regulation of 5-hydroxymethyl-cytosine by oxidative stress. Epigenetics. 2011;6:853–856. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, Hirohashi S. Increased protein expression of DNA methyltransferase (DNMT1) is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105:527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Li S, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004;25:1779–1786. doi: 10.1093/carcin/bgh161. [DOI] [PubMed] [Google Scholar]
- 29.Mandrekar P. Epigenetic regulation in alcohol liver disease. World J Gastroenterol. 2011;28:2456–2464. doi: 10.3748/wjg.v17.i20.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooi SK, O’Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. J Cell Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle. 2009;8:1526–1531. doi: 10.4161/cc.8.10.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YF, Li BZ, Li Z, Wang Y, Tang Q, Ding J, Jiz Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-hydroxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1333. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, Abramowitz LK, Bartolomei MS, Rambow F, Bassi MR, Bruno T, Fanciulli M, Renner C, Klein-Szanto AJ, Matsumoto Y, Kobi D, Davidson I, Alberti C, Larue L, Bellacosa A. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 37.Lin CH, Hsieh SY, Sheen IS, Lee WC, Chen TC, Shyu WC, Liaw YF. Genome-wide hypomethylation in hepatocellular carcinogenesis. Cancer Res. 2001;61:4238–4443. [PubMed] [Google Scholar]
- 38.Guerrero-Preston R, Santella RM, Blanco A, Desai M, Berdasco M, Fraga M. Global DNA hypomethylation in liver cancer cases and controls: a phase I preclinical biomarker development study. Epigenetics. 2007;2:223–226. doi: 10.4161/epi.2.4.5214. [DOI] [PubMed] [Google Scholar]
- 39.De Smet C, Loriot A. DNA hypomethylation in cancer: Epigenetic scars of a neoplastic journey. Epigenetics. 2010;5:206–213. doi: 10.4161/epi.5.3.11447. [DOI] [PubMed] [Google Scholar]
- 40.Takai D, Yagi Y, Habib N, Sugimura T, Ushijima T. Hypomethylation of LINE1 retrotransposon in human hepatocellular carcinomas, but not in surrounding liver cirrhosis. Jpn J Clin Oncol. 2000;30:306–309. doi: 10.1093/jjco/hyd079. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, White-Cross JA, Shen L, Issa JP, Rashid A. Hypomethylation of long interspersed nuclear element-1 in hepatocellular carcinomas. Mod Pathol. 2009;22:442–449. doi: 10.1038/modpathol.2008.203. [DOI] [PubMed] [Google Scholar]
- 42.Lee HS, Kim BH, Cho NY, Yoo EJ, Choi M, Shin SH, Jang JJ, Suh KS, Kim YS, Kang GH. Prognostic implications of and relationship between CpG island hypermethylation and repetitive DNA hypomethylation in hepatocellular carcinoma. Clin Cancer Res. 2009;15:812–820. doi: 10.1158/1078-0432.CCR-08-0266. [DOI] [PubMed] [Google Scholar]
- 43.Tangkijvanich P, Hourpai N, Rattanatanyong P, Wisedopas N, Mahachai V, Mutirangura A. Serum LINE-1 hypomethylation as a potential prognostic marker for hepatocellular carcinoma. Clin Chim Acta. 2007;379:127–133. doi: 10.1016/j.cca.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Saito Y, Kanai Y, Sakamoto M, Saito H, Ishii H, Hirohashi S. Overexpression of a splice variant of DNA methyltransferase 3b, DNMT3b4, associated with DNA hypomethylation on pericentromeric satellite regions during human hepatocarcinogenesis. Proc Natl Acad Sci USA. 2002;99:10060–10065. doi: 10.1073/pnas.152121799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild L, Flanagan JM. Genome-wide hypomethylation in cancer may be a passive consequence of transformation. Biochim Biophys Acta. 2010;1806:50–57. doi: 10.1016/j.bbcan.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res. 1992;52:2071s–2077s. [PubMed] [Google Scholar]
- 47.Christman JK. Dietary effects on DNA methylation: do they account for hepatocarcinogenic properties of lipotrope diets? Adv Exp Med Biol. 1995;369:141–154. doi: 10.1007/978-1-4615-1957-7_13. [DOI] [PubMed] [Google Scholar]
- 48.Pogribny IP, James SJ, Jernigan S, Pogribna M. Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat Res. 2004;548:53–59. doi: 10.1016/j.mrfmmm.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y, Jackson-Grusby L, Linhart H, Meissner A, Eden A, Lin H, Jaenisch R. Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13580–13585. doi: 10.1073/pnas.0506612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tränkenschuh W, Puls F, Christgen M, Albat C, Heim A, Poczkaj J, Fleming P, Kreipe H, Lehmann U. Frequent and distinct aberrations of DNA methylation patterns in fibrolamellar carcinoma of the liver. PLoS One. 2010;5:e13688. doi: 10.1371/journal.pone.0013688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagnyukova TV, Tryndyak VP, Montgomery B, Churchwell MI, Karpf AR, James SR, Muskhelishvili L, Beland FA. Genetic and epigenetic changes in rat preneoplastic liver tissue induced by 2-acetylaminofluorene. Carcinogenesis. 2008;29:638–646. doi: 10.1093/carcin/bgm303. [DOI] [PubMed] [Google Scholar]
- 52.Rollins RA, Haghighi F, Edwards JR, Das R, Zhang MQ, Ju J, Bestor TH. Large-scale structure of genomic methylation patterns. Genome Res. 2006;16:157–163. doi: 10.1101/gr.4362006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJM, Haussler D, Marra MA, Hirst M, Wang T, Costello JF. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci USA. 2002;99:3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Illingworth RS, Bird AP. CpG islands – “a rough guide”. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Schagdarsurengin U, Wilkens L, Steinemann D, Flemming P, Kreipe HH, Pfeifer GP, Schlegelberger B, Dammann R. Frequent epigenetic inactivation of the RASSF1A gene in hepatocellular carcinoma. Oncogene. 2003;22:1866–1871. doi: 10.1038/sj.onc.1206338. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Hui AM, Sun L, Hasegawa K, Torzilli G, Minagawa M, Takayama T, Makuuchi M. p16INK4A hypermethylation is associated with hepatitis virus infection, age, and gender in hepatocellular carcinoma. Clin Cancer Res. 2004;10:7484–7489. doi: 10.1158/1078-0432.CCR-04-1715. [DOI] [PubMed] [Google Scholar]
- 58.Harder J, Opitz OG, Brabender J, Olschewski M, Blum HE, Nomoto S, Usadel H. Quantitative promoter methylation analysis of hepatocellular carcinoma, cirrhotic and normal liver. Int J Cancer. 2008;122:2800–2804. doi: 10.1002/ijc.23433. [DOI] [PubMed] [Google Scholar]
- 59.Roncalli M, Bianchi P, Bruni B, Laghi L, Destro A, Di Gioia S, Gennari L, Tommasini M, Malesci A, Coggi G. Methylation framework of cell cycle gene inhibitors in cirrhosis and associated with hepatocellular carcinoma. Hepatology. 2002;36:427–432. doi: 10.1053/jhep.2002.34852. [DOI] [PubMed] [Google Scholar]
- 60.Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, Matsuda M, Fujii H, Scoazec JY, Ohgaki H. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334–341. doi: 10.1002/ijc.11254. [DOI] [PubMed] [Google Scholar]
- 61.Okochi O, Hibi K, Sakai M, Inoue S, Takeda S, Kaneko T, Nakao A. Methylation-mediated silencing of SOCS-1 gene in hepatocellular carcinoma derived from cirrhosis. Clin Cancer Res. 2003;9:5295–5298. [PubMed] [Google Scholar]
- 62.Miyoshi H, Fujie H, Moriya K, Shintani Y, Tsutsumi T, Makuuchi M, Kimura S, Koike K. Methylation status of suppressor of cytokine signaling-1 gene in hepatocellular carcinoma. J Gastroenterol. 2004;39:563–569. doi: 10.1007/s00535-003-1343-0. [DOI] [PubMed] [Google Scholar]
- 63.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signaling in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 64.Yuan Y, Wang J, Li J, Wang L, Li M, Yang Z, Zhang C, Dai JL. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin Cancer Res. 2006;12:6687–6695. doi: 10.1158/1078-0432.CCR-06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong S, Tang MW, Yeo W, Liu C, Lo YM, Johnson PJ. Silencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomas. Clin Cancer Res. 2002;8:1087–1092. [PubMed] [Google Scholar]
- 66.Tada M, Yokosuka O, Fukai K, Chiba T, Imazeki F, Tokuhisa T, Saisho H. Hypermethylation of NAD(P)H: quinone oxidoreductase 1 (NQO1) gene in human hepatocellular carcinoma. J Hepatol. 2005;42:511–519. doi: 10.1016/j.jhep.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 67.Shimoda M, Takahashi M, Yoshimoto T, Kono T, Ikai I, Kubo H. A homeobox protein, Prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res. 2006;12:6005–6011. doi: 10.1158/1078-0432.CCR-06-0712. [DOI] [PubMed] [Google Scholar]
- 68.Laerm A, Helmbold P, Goldberg M, Dammann R, Holzhausen HJ, Ballhausen WG. Prospero-related homeobox 1 (PROX1) is frequently inactivated by genomic deletions and epigenetic silencing in carcinomas of the biliary system. J Hepatol. 2007;46:89–97. doi: 10.1016/j.jhep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 69.Macheiner D, Heller G, Kappel S, Bichler C, Stättner S, Ziegler B, Kandioler D, Wrba F, Schulte-Hermann R, Zöchbauer-Müller S, Grasl-Kraupp B. NORE1B, a candidate tumor suppressor, is epigenetically silenced in human hepatocellular carcinoma. J Hepatol. 2006;45:81–89. doi: 10.1016/j.jhep.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Zhang C, Li H, Liu W, Zhang Q, Zhang T, Zhang X, Han B, Zhou G. Epigenetic inactivation of the tumor suppressor gene RIZ1 in hepatocellular carcinoma involves both DNA methylation and histone modifications. J Hepatol. 2010;53:889–895. doi: 10.1016/j.jhep.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Olamura Y, Nomoto S, Kanda M, Hayashi M, Nishikawa Y, Fuji T, Sugimoto H, Takeda S, Nakao A. reduced expression of reelin (RELN) gene is associated with high recurrence rate of hepatocellular carcinoma. Ann Surg Oncol. 2011;18:572–579. doi: 10.1245/s10434-010-1273-z. [DOI] [PubMed] [Google Scholar]
- 72.Kanda M, Nomoto S, Okamura Y, Hayashi M, Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S, Nakao A. Promoter hypermethylation of fibulin 1 gene is associated with tumor progression in hepatocellular carcinoma. Mol Carcinog. 2011;50:571–579. doi: 10.1002/mc.20735. [DOI] [PubMed] [Google Scholar]
- 73.Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G, Li L, Dai N, SIJ, Tao Q, Sung JJ, Yu J. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843–853. doi: 10.1002/hep.24124. [DOI] [PubMed] [Google Scholar]
- 74.Pogribny IP, James SJ. Reduction of p53 expression in human primary hepatocellular carcinoma is associated with promoter region methylation without coding region mutation. Cancer Lett. 2002;176:169–174. doi: 10.1016/s0304-3835(01)00748-0. [DOI] [PubMed] [Google Scholar]
- 75.Tomasi ML, Li TW, Mato JM, Lu SC. Inhibition of human methionine adenosyltransferase 1A transcription by coding region methylation. J Cell Physiol. 2012 doi: 10.1002/jcp.22875. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallet M, Han ZG, Szyf M. Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res. 2011;71:5891–5893. doi: 10.1158/0008-5472.CAN-10-3823. [DOI] [PubMed] [Google Scholar]
- 77.Chan CF, Yau TO, Jin DY, Wong CM, Fan ST, Ng IO. Evaluation of nuclear factor-kappaB, urokinase-type plasminogen activator, and HBx and their clinicopathological significance in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4140–4149. doi: 10.1158/1078-0432.CCR-03-0574. [DOI] [PubMed] [Google Scholar]
- 78.Xiao Y, Kleeff J, Shi X, Büchler MW, Friess H. Heparanase expression in hepatocellular carcinoma and the cirrhotic liver. Hepatol Res. 2003;26:192–198. doi: 10.1016/s1386-6346(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 79.Zhao W, Liu H, Liu W, Wu Y, Chen W, Jiang B, Zhou Y, Xue R, Luo C, Wang L, Jiang JD, Liu J. Abnormal activation of the synuclein-gamma gene in hepatocellular carcinomas by epigenetic alteration. Int J Oncol. 2006;28:1081–1088. [PubMed] [Google Scholar]
- 80.Okada H, Kimura MT, Tan D, Fujiwara K, Igarashi J, Makuuchi M, Hui AM, Tsurumaru M, Nagase H. Frequent trefoil factor 2 (TFF3) overexpression and promoter hypomethylation in mouse and human hepatocellular carcinomas. Int J Oncol. 2005;26:369–377. [PMC free article] [PubMed] [Google Scholar]
- 81.Yang H, Huang ZZ, Zeng Z, Chen C, Selby RR, Lu SC. Role of promoter methylation in increased methionine adenosyltransferase 2A expression in human liver cancer. Am J Physiol Gastrointest Liver Physiol. 2001;280:G184–G190. doi: 10.1152/ajpgi.2001.280.2.G184. [DOI] [PubMed] [Google Scholar]
- 82.Goel A, Mathupala SP, Pedersen PL. Glucose metabolism in cancer. Evidence that demethylation events play a role in activating type II hexokinase gene expression. J Biol Chem. 2003;278:15333–15240. doi: 10.1074/jbc.M300608200. [DOI] [PubMed] [Google Scholar]
- 83.Kong LM, Liao CG, Chen L, Yang HS, Zhang SH, Zhang Z, Bian HJ, Xing JL, Chen ZN. Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J Cell Mol Med. 2011;15:1415–1428. doi: 10.1111/j.1582-4934.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitamura Y, Shirahata A, Sakuraba K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, Hibi K. Aberrant methylation of the Vimentin gene in hepatocellular carcinoma. Anticancer Res. 2011;31:1289–1291. [PubMed] [Google Scholar]
- 85.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of E-cadherin/catenin complex in hepatocellular carcinomas. World J Gastroenterol. 2008;14:5665–5673. doi: 10.3748/wjg.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saito Y, Kanai Y, Sakamoto M, Saito H, Ishii H, Hirohashi S. Expression of mRNA for DNA methyltransferases and methyl-CpG-binding proteins and DNA methylation status on CpG islands and pericentromeric satellite regions during human hepatocarcinogenesis. Hepatology. 2001;33:561–568. doi: 10.1053/jhep.2001.22507. [DOI] [PubMed] [Google Scholar]
- 88.Oh K, Kim H, Park HJ, Shim YH, Choi J, Park C, Park YN. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J Mol Med. 2007;20:65–73. [PubMed] [Google Scholar]
- 89.Fan H, Zhao ZJ, Cheng J, Su XW, Wu QX, Shan YF. Overexpression of DNA methyltransferase 1 and its biological significance in primary hepatocellular carcinoma. World J Gastroenterol. 2009;15:2020–2026. doi: 10.3748/wjg.15.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanai Y. Genome-wide DNA hypomethylation profiles in precancerous conditions and cancers. Cancer Sci. 2010;101:36–45. doi: 10.1111/j.1349-7006.2009.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin B, Robertson KD. DNA methylation: superior or subordinate in epigenetic hierarchy? Genes Cancer. 2011;2:607–617. doi: 10.1177/1947601910393957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Chiba T, Yokosuka O, Arai M, Tada M, Fukai K, Imazeki F, Kato M, Seki N, Saisho H. Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. J Hepatol. 2004;41:436–445. doi: 10.1016/j.jhep.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 94.Kondo Y, Shen L, Suzuki S, Kurokawa T, Masuko K, Tanaka Y, Kato H, Mizuno Y, Yokoe M, Sugauchi F, Hirashima N, Orito E, Osada H, Ueda R, Guo Y, Chen X, Issa JP, Sekido Y. Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res. 2007;37:974–983. doi: 10.1111/j.1872-034X.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 95.Ya JY, Zhang L, Zhang X, He ZY, Ma Y, Hui LJ, Wang X, Hu YP. H3K27 trimethylation is an early epigenetic event of p16INK4a silencing for regaining tumorigenesis in fusion reprogrammed hepatoma cells. J Biol Chem. 2010;285:18828–18837. doi: 10.1074/jbc.M109.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sistayanarain A, Tsuneyama K, Zheng H, Takahashi H, Nomoto K, Cheng C, Murai Y, Tanaka A, Takano Y. Expression of Aurora-B kinase and phosphorylated histone H3 in hepatocellular carcinoma. Anticancer Res. 2006;26:3585–3593. [PubMed] [Google Scholar]
- 97.Cai MY, Hou JH, Rao HL, Luo RZ, Li M, Pei XQ, Lin MC, Guan XY, Kung HF, Zeng YX, Xie D. High expression of H3K27me3 in human hepatocellular carcinomas correlates closely with vascular invasion and predicts worse prognosis in patients. Mol Med. 2011;17:12–20. doi: 10.2119/molmed.2010.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang GL, Liu L, Buyse IM, Simon D, Huang S. Decreased RIZ1 expression but not RIZ2 in hepatoma and suppression of hepatoma and suppression of hepatoma tumorigenicity by RIZ1. Int J Cancer. 1999;83:543–546. doi: 10.1002/(sici)1097-0215(19991112)83:4<541::aid-ijc17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 99.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 100.Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, Wakiyama S, Fujita S, Shinouzu K, Mori M. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J cancer. 2005;92:1754–1758. doi: 10.1038/sj.bjc.6602531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quint K, Agaimy A, Fazio P, Montalbano R, Steindorf C, Jung R, Hellerbrandt C, Hartmann A, Sitter H, Neureiter D, Ocker M. Clinical significance of histone deacetylases 1, 2, 3, and 7: HDAC2 is an independent predictor of survival in HCC. Virchows Arch. 2011;459:129–139. doi: 10.1007/s00428-011-1103-0. [DOI] [PubMed] [Google Scholar]
- 102.Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, Yu J, Ng HK, Ling MT, Huang AL, Cai XF, Ko BC. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71:4138–4149. doi: 10.1158/0008-5472.CAN-10-4274. [DOI] [PubMed] [Google Scholar]
- 103.Wu LM, Yang Z, Zhou L, Zhang F, Xie HY, Feng XW, Wu J, Zheng SS. Identification of histone deacetylase 3 as a biomarker for tumor recurrence following liver transplantation in HBV-associated hepatocellular carcinoma. PLoS One. 2010;5:e14460. doi: 10.1371/journal.pone.0014460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Choi HN, Bae JS, Jamiyandorj U, Noh SJ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011;26:503–510. doi: 10.3892/or.2011.1301. [DOI] [PubMed] [Google Scholar]
- 105.Apergis GA, Crawford N, Ghosh D, Steppan CM, Vorachek WR, Wen P, Locker J. A novel nk-2-related transcription factor associated with human fetal liver and hepatocellular carcinoma. J Biol Chem. 1998;273:2917–2925. doi: 10.1074/jbc.273.5.2917. [DOI] [PubMed] [Google Scholar]
- 106.Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H, Ching AK, Cheung KF, Wong HK, Tong JH, Jin H, Choy KW, Yu J, To KF, Wong N, Huang TH, Sung JJ. EZH2-mediated concordant repression of Wnt antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011;71:4028–4039. doi: 10.1158/0008-5472.CAN-10-3342. [DOI] [PubMed] [Google Scholar]
- 107.Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 108.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 109.Kim KC, Geng L, Huang S. Inactivation of a histone methyltransferase by mutations in human cancers. Cancer Res. 2003;63:7619–7623. [PubMed] [Google Scholar]
- 110.Avila MA, Berasain C, Torres L, Martin-Duce A, Corrales FJ, Yang H, Prieto J, Lu SC, Caballería J, Rodés J, Mato JM. Reduce mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 111.Tomasi ML, Li TW, Li M, Mato JM, Lu SC. Inhibition of human methionine adenosyltransferase 1A transcription by coding region methylation. J Cell Physiol. 2011 doi: 10.1002/jcp.22875. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corrales FJ, Pérez-Mato I, Sánchez Del Pino MM, Ruiz F, Castro C, Garcia-Trevijano ER, Latasa U, Martínez-Chantar ML, Martínez-Cruz A, Avila MA, Mato JM. Regulation of mammalian liver methionine adenosyltransferase. J Nutr. 2002;132:2377S–2381S. doi: 10.1093/jn/132.8.2377S. [DOI] [PubMed] [Google Scholar]
- 113.Burgio G, Onorati MC, Corona DF. Chromatin remodeling regulation by small molecules and metabolites. Biochim Biophys Acta. 2010;1799:671–680. doi: 10.1016/j.bbagrm.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Teperino R, Schoonjans K, Aurwex J. Histone methyl transferases and demethylases; can they link metabolism and transcription. Cell Metab. 2010;12:321–327. doi: 10.1016/j.cmet.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 118.Ventura A, Jacks ST. MicroRNAs in cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Leva G, Croce CM. Roles of small miRNAs in tumor formation. Trends Mol Med. 2010;16:257–267. doi: 10.1016/j.molmed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gramantieri L, Fornari F, Callegari E, Sabbioni S, Lanza G, Croce CM, Bolondi L, Negrini M. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mott JL. MicroRNas involved in tumor suppressor and oncogene pathways: implications for hepatobiliary neoplasia. Hepatology. 2009;50:630–637. doi: 10.1002/hep.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Connoly E, Melegari M, Landgraf P, Tchaikovskaya T, Tennant BC, Slagle BL, Rogler LE, Zavolan M, Tuschl T, Rogler CE. Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am J Pathol. 2008;173:856–864. doi: 10.2353/ajpath.2008.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elyakim E, Sitbon E, Faerman A, Tabak S, Montia E, Belanis L, Dov A, Marcusson EG, Bennett CF, Chajut A, Cohen D, Yerushalmi N. has-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010;70:8077–8087. doi: 10.1158/0008-5472.CAN-10-1313. [DOI] [PubMed] [Google Scholar]
- 125.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, Negrini M. Mir-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 126.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luedde T. MicroRNA-151 and its hosting gene FAK (focal adhesion kinase) regulate tumor cell migration and spreading of hepatocellular carcinoma. Hepatology. 2010;52:1164–1166. doi: 10.1002/hep.23854. [DOI] [PubMed] [Google Scholar]
- 128.Wong QW, Ching AK, Chan AW, Choy KW, To KF, Lai PB, Wong N. MiR-222 overexpression confers cell migratory advantages in hepatocellular carcinoma through enhancing AKT signaling. Clin Cancer Res. 2010;16:867–875. doi: 10.1158/1078-0432.CCR-09-1840. [DOI] [PubMed] [Google Scholar]
- 129.Tomimaru Y, Eguchi H, Nagano H, Wada H, Tomokuni A, Kobayashi S, Marubashi S, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M. MicroRNA-21 induces resistance to the anti-tumor effect of interferon-α/5-fluorouracil in hepatocellular carcinoma cells. Br J Cancer. 2010;103:1617–1626. doi: 10.1038/sj.bjc.6605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, Chieco P, Negrini M, Bolondi L. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 131.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 132.He Y, Cui Y, Wang W, Gu J, Guo S, Ma K, Luo X. Hypomethylation of the has-miR-191 locus causes high expression of has-miR-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia. 2011;13:841–853. doi: 10.1593/neo.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Datta J, Kutay H, Nasser MW, Nuovo GJ, Wang B, Majumder S, Liu CG, Volinia S, Croce CM, Schmittgen TD, Choshal K, Jacob ST. Methylation mediated silencing of microRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766–776. doi: 10.1093/carcin/bgp250. [DOI] [PubMed] [Google Scholar]
- 135.Alpini G, Glasser SS, Zhang JP, Francis H, Han Y, Gong J, Stokes A, Francis T, Hughart N, Hubble L, Zhuang SM, Meng F. Regulation of placenta growth factor by microRNA-125b in hepatocellular cancer. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.04.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011 doi: 10.1038/onc.2011.193. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuan JH, Yang F, Chen BF, Lu Z, Huo XS, Zhou WP, Wang F, Sun SH. The histone deacetylase 4/Sp1/miR-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology. 2011 doi: 10.1002/hep.24606. in press. [DOI] [PubMed] [Google Scholar]
- 138.Huang J, Wang Y, Guo Y, Sun S. Down-regulated microRNA-152 induces aberrant DNA methylation in hepatitis B virus-related hepatocellular carcinoma by targeting DNA methyltransferase 1. Hepatology. 2010;52:60–70. doi: 10.1002/hep.23660. [DOI] [PubMed] [Google Scholar]
- 139.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Cupta S, Moore J, Wrobel MJ, Lerner J, Reich M, Chan JA, Glickman JN, Ikeda K, Hashimoto M, Watanabe G, Daidone MG, Roayaie S, Schwartz M, Thung S, Salvesen HB, Gabriel S, Mazzaferro V, Bruix J, Friedman SL, Kumada H, Llovet JM, Golub TR. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317–328. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, Thug SN, Bruix J, Newell P, April C, Fan JB, Roayaie S, Mazzaferro V, Schwartz ME, Llovet JM. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501–1512. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ammerpohl O, Pratschke J, Schafmayer C, Haake A, Faber W, von Kampen O, Brosch M, Sipos B, von Schönfels W, Balschun K, Röcken C, Arlt A, Schniewind B, Grauholm J, Kalthoff H, Neuhaus P, Stickel F, Schreiber S, Becker T, Siebert R, Hampe J. Distinct DNA methylation patterns in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 2011 doi: 10.1002/ijc.26136. in press. [DOI] [PubMed] [Google Scholar]
- 144.Nagashio R, Aral E, Ojima H, Kosuge T, Kondo Y, Kanai Y. Carcinogenic risk estimation based on quantification of DNA methylation levels in liver tissue at the precancerous stage. Int J Cancer. 2011;129:1170–1179. doi: 10.1002/ijc.26061. [DOI] [PubMed] [Google Scholar]
- 145.Hernandez-Vargas H, Lambert MP, Le Calvez-Kelm F, Gouysse G, McKay-Chopin S, Tavtigian SV, Scoazec JY, Herceg Z. Hepatocellular carcinoma displays distinct DNA methylation signatures with potential as clinical predictors. PLoS ONE. 2010;5:e9749. doi: 10.1371/journal.pone.0009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nishida N, Nagasaka T, Nishimura T, Ikai I, Boland CR, Goel A. Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology. 2008;47:908–918. doi: 10.1002/hep.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Archer KJ, Mas VR, Maluf DG, Fisher RA. High-throughput assessment of CpG site methylation for distinguishing between HCV-cirrhosis and HCV-associated hepatocellular carcinoma. Mol Genet Genomics. 2010;283:341–349. doi: 10.1007/s00438-010-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Um TH, Kim H, Oh BK, Kim MS, Kim KS, Jung G, Park YN. Aberrant CpG island hypermethylation in dysplastic nodules and early HCC of hepatitis B virus-related human multistep hepatocarcinogenesis. J Hepatol. 2011;54:939–947. doi: 10.1016/j.jhep.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 149.Deng YB, Nagae G, Midorikawa Y, Yagi K, Tsutsumi S, Yamamoto S, Hasegawa K, Kokudo N, Aburatani H, Kaneda A. Identification of genes preferentially methylated in hepatitis C virus-related hepatocellular carcinoma. Cancer Sci. 2010;101:1501–1510. doi: 10.1111/j.1349-7006.2010.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feng Q, Stern JE, Hawes SE, Lu H, Jiang M, Kiviat NB. DNA methylation changes in normal liver tissues and hepatocellular carcinoma with different viral infection. Exp Mol Pathol. 2010;88:287–292. doi: 10.1016/j.yexmp.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lambert MP, Paliwal A, Vaissière T, Chemin I, Zoulim F, Tommasino M, Hainaut P, Sylla B, Scoazec JY, Tost J, Herceg Z. Aberrant DNA methylation distinguishes hepatocellular carcinoma associated with HBV and HCV infection and alcohol intake. J Hepatol. 2011;54:705–715. doi: 10.1016/j.jhep.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 152.Lou C, Du Z, Yang B, Gao Y, Wang Y, Fang S. Aberrant DNA hypomethylation profile of hepatocellular carcinoma and surgically resected margin. Cancer Sci. 2009;100:996–1004. doi: 10.1111/j.1349-7006.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Formeister EJ, Tsuchiya M, Fujii H, Shpyleva S, Pogribny IP, Rusyn I. Comparative analysis of promoter methylation and gene expression endpoints between tumorous and non-tumorous tissues from HCV-positive patients with hepatocellular carcinoma. Mutat Res. 2010;692:26–33. doi: 10.1016/j.mrfmmm.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wong IH, Lo YM, Zhang J, Ng MH, Wong N, Lai PB, Lau WY, Hjelm NM, Johnson PJ. Detection of aberrant p16 methylation in the plasma and serum of liver cancer. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 155.Yeo W, Wong N, Wong WL, Lai PB, Zhong S, Johnson PJ. High frequency of promoter hypermethylation of RASSF1A in tumor and plasma of patients with hepatocellular carcinoma. Liver Int. 2005;25:266–272. doi: 10.1111/j.1478-3231.2005.01084.x. [DOI] [PubMed] [Google Scholar]
- 156.Wang J, Qin Y, Li B, Sun Z, Yang B. Detection of aberrant promoter methylation of GSTP1 in the tumor and serum of Chinese human primary hepatocellular carcinoma patients. Clin Biochem. 2006;39:344–348. doi: 10.1016/j.clinbiochem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Zhang YJ, Wu HC, Shen J, Ahsan H, Tsai WY, Yang HI, Wang LY, Chen SY, Chen CJ, Santella RM. Predicting hepatocellular carcinoma by detection of aberrant promoter methylation in serum DNA. Clin Cancer Res. 2007;13:2378–2384. doi: 10.1158/1078-0432.CCR-06-1900. [DOI] [PubMed] [Google Scholar]
- 158.Tan SH, Ida H, Lau QC, Goh BC, Chieng WS, Loh M, Ito Y. Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncol Rep. 2007;18:1225–1230. [PubMed] [Google Scholar]
- 159.Chan KC, Lai PB, Mok TS, Chan HL, Ding C, Yeung SW, Lo YM. Quantitative analysis of circulating methylated DNA as a biomarker for hepatocellular carcinoma. Clin Chem. 2008;54:1528–1536. doi: 10.1373/clinchem.2008.104653. [DOI] [PubMed] [Google Scholar]
- 160.Sato F, Hatano E, Kitamura K, Myomoto A, Fujiwara T, Takizawa S, Tsuchiya S, Tsujimoto G, Uemoto S, Shimizu K. MicroRNA profile predicts recurrence after resection in patients with hepatocellular carcinoma within the Milan criteria. PLoS ONME. 2011;(6):e16435. doi: 10.1371/journal.pone.0016435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 162.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, Lin D. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 163.Li J, Wang Y, Yu W, Chen J, Luo J. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun. 2011;406:70–73. doi: 10.1016/j.bbrc.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 164.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JF. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90:367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Did. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 167.Hoenerhoff MJ, Pandiri AR, Lahousse SA, Hong HH, Ton TV, Masinde T, Auerbach SS, Gerrish K, Bushel PR, Shockley KR, Peddada SD, Sills RC. Global gene profiling of spontaneous hepatocellular carcinoma in B6C3F1 mice: similarities in the molecular landscape with human liver cancer. Toxicol Pathol. 2011;39:678–699. doi: 10.1177/0192623311407213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cabibbo G, Craxí A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2010;14:352–255. [PubMed] [Google Scholar]
- 169.Pogribny IP, James SJ, Beland FA. Molecular alterations in hepatocarcinogenesis induced by dietary methyl deficiency. Mol Nutr Food Res. 2012 doi: 10.1002/mnfr.201100524. in press. [DOI] [PubMed] [Google Scholar]
- 170.Kalari S, Pfeifer GP. Identification of driver and passenger DNA methylation in cancer by epigenomic analysis. Adv Genet. 2010;70:277–308. doi: 10.1016/B978-0-12-380866-0.60010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40:142–150. doi: 10.5483/bmbrep.2007.40.2.142. [DOI] [PubMed] [Google Scholar]
- 172.Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori M. Molecular signatures of noncancerous liver tissue can predict the risk for late recurrence of hepatocellular carcinoma. J Gastroeterol. 2010;45:146–152. doi: 10.1007/s00535-009-0164-1. [DOI] [PubMed] [Google Scholar]
- 173.Tsuchiya M, Parker JS, Kono H, Matsuda M, Fujii H, Rusyn I. Gene expression in nontumoral liver tissue and recurrence-free survival in hepatitis C virus-positive hepatocellular carcinoma. Mol Cancer. 2010;9:74. doi: 10.1186/1476-4598-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Utsunomiya T, Shimada M. Molecular characteristic of non-cancerous liver tissue in non-B non-C hepatocellular carcinoma. Hepatol Res. 2011;41:711–721. doi: 10.1111/j.1872-034X.2011.00818.x. [DOI] [PubMed] [Google Scholar]