Abstract
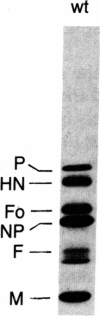
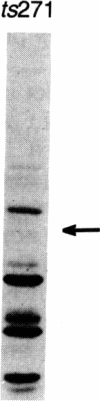
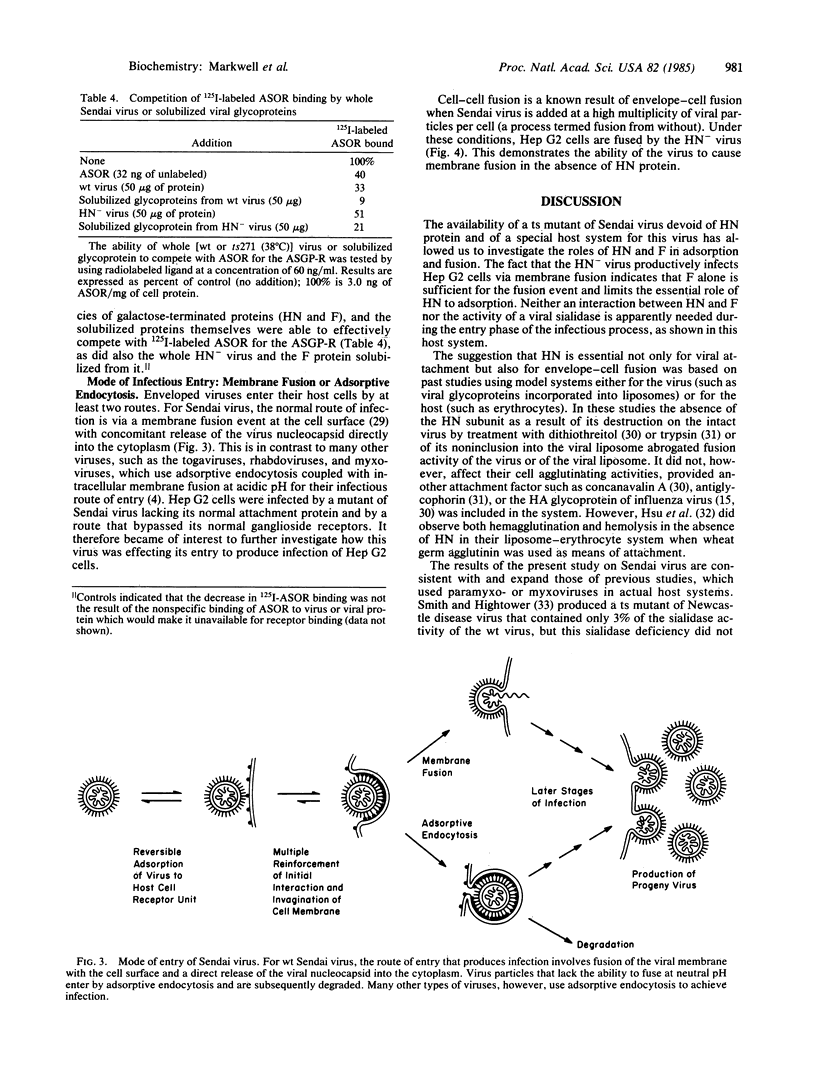
During the first stage of infection, the paramyxovirus Sendai virus attaches to host cells by recognizing specific receptors on the cell surface. Productive virus-cell interactions result in membrane fusion between the viral envelope and the cell surface membrane. It has recently been shown that the ganglioside GD1a and its more complex homologs GT1b and GQ1b are cell surface receptors for Sendai virus. We report in this paper that the temperature-sensitive mutant ts271 of the Enders strain of Sendai virus lacks the viral attachment protein HN and the biological activities of hemagglutination and sialidase activity associated with it when the virus is grown at 38 degrees C. This HN- virus was unable to infect or agglutinate conventional host cells that contained receptor gangliosides and were readily infected by the parental wild-type virus. The HN- virus did, however, attach to and infect Hep G2 cells, a line of hepatoma cells that retains the asialoglycoprotein receptor (ASGP-R) upon continuous culture. This receptor is a mammalian lectin that recognizes galactose- or N-acetylgalactosamine-terminated proteins. In accordance with the known properties of this receptor, infection by the HN- virus was abolished by treatment of Hep G2 cells with sialidase, by the presence of Ca2+ chelators, and by competition with N-acetylgalactosamine, asialoorosomucoid, and antibody to the receptor. F, the only glycoprotein on the HN- virus, was shown to compete with the galactose-terminated protein asialoorosomucoid for the ASGP-R. The ability of the HN- virus to cause cell-cell fusion of Hep G2 cells indicated that attachment of this virus to the ASGP-R still permitted viral entry by its usual mode--i.e., membrane fusion at the cell surface. These results open up the possibility that enveloped viruses, which contain glycosylated proteins or lipids, may make use of naturally occurring lectins in addition to their normal receptors as a means of attachment to host cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Sefton B. M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978 Nov;15(3):985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- Frisch A., Neufeld E. F. A rapid and sensitive assay for neuraminidase: application to cultured fibroblasts. Anal Biochem. 1979 May;95(1):222–227. doi: 10.1016/0003-2697(79)90209-4. [DOI] [PubMed] [Google Scholar]
- Heath T. D., Martin F. J., Macher B. A. Association of ganglioside-protein conjugates into cell and Sendai virus. Requirement for the HN subunit in viral fusion. Exp Cell Res. 1983 Nov;149(1):163–175. doi: 10.1016/0014-4827(83)90389-0. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Rott R., Wahn K., Klenk H. D., Kohama T. The function of the neuraminidase in membrane fusion induced by myxoviruses. Virology. 1980 Dec;107(2):313–319. doi: 10.1016/0042-6822(80)90299-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda T., Kawasaki K., Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fredman P., Svennerholm L. Receptor ganglioside content of three hosts for Sendai virus. MDBK, HeLa, and MDCK cells. Biochim Biophys Acta. 1984 Aug 8;775(1):7–16. doi: 10.1016/0005-2736(84)90228-1. [DOI] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Regulatory events in the synthesis of Sendai virus polypeptides and their assembly into virions. Virology. 1976 Aug;73(1):79–88. doi: 10.1016/0042-6822(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Portner A., Marx P. A., Kingsbury D. W. Isolation and characterization of Sendai virus temperature-sensitive mutants. J Virol. 1974 Feb;13(2):298–304. doi: 10.1128/jvi.13.2.298-304.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Marx P. S., Kingsbury D. W. A temperature-sensitive mutant of Sendai virus with an altered hemagglutinin-neuraminidase polypeptide: consequences for virus assembly and cytopathology. Virology. 1975 Sep;67(1):179–187. doi: 10.1016/0042-6822(75)90415-8. [DOI] [PubMed] [Google Scholar]
- Portner A. The HN glycoprotein of Sendai virus: analysis of site(s) involved in hemagglutinating and neuraminidase activities. Virology. 1981 Dec;115(2):375–384. doi: 10.1016/0042-6822(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Knowles B. B., Lodish H. F. Characterization of the asialoglycoprotein receptor in a continuous hepatoma line. J Biol Chem. 1981 Sep 10;256(17):8878–8881. [PubMed] [Google Scholar]
- Schwartz A. L., Marshak-Rothstein A., Rup D., Lodish H. F. Identification and quantification of the rat hepatocyte asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Rup D. Biosynthesis of the human asialoglycoprotein receptor. J Biol Chem. 1983 Sep 25;258(18):11249–11255. [PubMed] [Google Scholar]
- Schwartz A. L., Rup D., Lodish H. F. Difficulties in the quantification of asialoglycoprotein receptors on the rat hepatocyte. J Biol Chem. 1980 Oct 10;255(19):9033–9036. [PubMed] [Google Scholar]
- Schwartz A. L. The hepatic asialoglycoprotein receptor. CRC Crit Rev Biochem. 1984;16(3):207–233. doi: 10.3109/10409238409108716. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Becht H., Rott R. Isolation and purification of surface antigens from disrupted paramyxoviruses. Med Microbiol Immunol. 1973 Sep 26;159(1):1–12. doi: 10.1007/BF02122645. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Revertant analysis of a temperature-sensitive mutant of Newcastle disease virus with defective glycoproteins: implication of the fusion glycoprotein in cell killing and isolation of a neuraminidase-deficient hemagglutinating virus. J Virol. 1982 May;42(2):659–668. doi: 10.1128/jvi.42.2.659-668.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Hepatic binding protein: the protective role of its sialic acid residues. Science. 1977 Aug 12;197(4304):667–668. doi: 10.1126/science.877581. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Morioka T., Matsumoto M. Action of ortho- and paramyxovirus neuraminidase on gangliosides. Hydrolysis of ganglioside GM1 by Sendai virus neuraminidase. Biochim Biophys Acta. 1980 Sep 8;619(3):632–639. [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Hosaka Y., Fukami Y., Fukai K. Immunoelectron microscopic study on interactions of noninfectious sendai virus and murine cells. J Virol. 1981 Jul;39(1):273–281. doi: 10.1128/jvi.39.1.273-281.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J., Gerhard W. Delineation of four antigenic sites on a paramyxovirus glycoprotein via which monoclonal antibodies mediate distinct antiviral activities. J Immunol. 1982 Jun;128(6):2670–2675. [PubMed] [Google Scholar]
- Yoshima H., Nakanishi M., Okada Y., Kobata A. Carbohydrate structures of HVJ (Sendai virus) glycoproteins. J Biol Chem. 1981 Jun 10;256(11):5355–5361. [PubMed] [Google Scholar]
- Yoshimura A., Kuroda K., Kawasaki K., Yamashina S., Maeda T., Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]