Abstract
Purpose:
Uncontrolled radiation exposure due to radiological terrorism, industrial accidents or military circumstances is a continuing threat for the civilian population. Age plays a major role in the susceptibility to radiation; younger children are at higher risk of developing cognitive deterioration when compared to adults. Our objective was to determine if an exposure to radiation affected the vulnerability of the juvenile hippocampus to a subsequent moderate traumatic injury.
Materials and methods:
Three-week-old (juvenile) and eight-week-old young adult C57BL/J6 male mice received whole body cesium-137 (137Cs) irradiation with 4 gray (Gy). One month later, unilateral traumatic brain injury was induced using a controlled cortical impact system. Two months post-irradiation, animals were tested for hippocampus-dependent cognitive performance in the Morris water-maze. After cognitive testing, animals were euthanized and their brains frozen for immunohistochemical assessment of activated microglia and neurogenesis in the hippocampal dentate gyrus.
Results:
All animals were able to learn the water maze task; however, treatment effects were seen when spatial memory retention was assessed. Animals that received irradiation as juveniles followed by a moderate traumatic brain injury one month later did not show spatial memory retention, i.e., were cognitively impaired. In contrast, all groups of animals that were treated as adults showed spatial memory retention in the probe trials.
Conclusion:
Although the mechanisms involved are not clear, our results suggest that irradiation enhanced a young animal's vulnerability to develop cognitive injury following a subsequent traumatic injury.
Keywords: Radiation, age, hippocampus, neurogenesis, inflammation
Introduction
Uncontrolled radiation exposure as a result of radiologic terrorism, military activity or nuclear accident presents unique challenges that are not encountered in a clinical situation. Radiation quality and dose homogeneity will likely be uncertain, and there will be a wide range of delivered doses and subsequent tissue/body effects. In addition, radiation effects will likely be exacerbated by other types of injury (trauma, burns, infection, etc.) that either occur at the time of irradiation or at some time thereafter. Additionally, biological factors such as age may further complicate not only the extent or development of injury, but also the strategies used to manage the consequences of single or combined insults. Given the growing worldwide threat of radiological/nuclear terrorism, the concept of radiation combined injury (RCI) has been identified as a high priority research area (Pellmar and Rockwell 2005, DiCarlo et al. 2008).
Animal and some human data are available regarding whole body radiation exposure alone (Pandey et al. 2010), but there is a paucity of information regarding the magnitudes and mechanisms underlying the interactions between irradiation and other forms of injury (DiCarlo et al. 2008). Most of the available data involve particularly sensitive tissues such as gut and bone marrow. However, in an exposure scenario that involves doses below the thresholds for the lethal radiation syndromes, or if adequate treatments are available to mitigate lethal effects, the responses of other critical tissues such as the central nervous system (CNS) may become problematic. In fact, studies are becoming available regarding how the CNS responds to RCI after doses of low or high LET irradiation (Rosi et al. 2012a, 2012b).
In a radiological event, particularly in an urban environment, children could account for a significant portion of the affected population (American Academy of Pediatrics Committee on Environmental Health 2003). However, relatively few studies have investigated low dose radiation effects in juveniles (Rola et al. 2004), and even less information is available concerning RCI. With regard to the CNS, age plays a major role in the susceptibility to radiation, with children showing at higher susceptibility for cognitive deteriorations when compared to adults (Abayomi 1996).
Within the hippocampus, the subgranular zone of the dentate gyrus (DG) contains neural stem/progenitor cells that throughout life provide new cells that differentiate into neurons (Biebl et al. 2000, Gage 2000). In normal animals, increased neurogenesis as a result of environmental enrichment and/or exercise, generally results in improved performance in hippocampal-dependent memory tasks (van Praag et al. 2000, Naylor et al. 2005, 2008). In addition, disruption of hippocampal neurogenesis by some insults such as ionizing irradiation, have been shown to be associated with decreased performance in hippocampal-dependent tasks (Raber et al. 2004a). Given this information we wanted to determine if neurogenesis played a role in the brain's response to RCI.
In the present study, cognitive performance, and immunohistochemical measures of neurogenesis and neuroinflammation (activated microglia) were assessed to determine if photon irradiation delivered to the whole body would predispose the brain to a heightened vulnerability after subsequent traumatic brain injury. In addition, to determine if such effects were influenced by age, we delivered our irradiation dose to animals that were either juveniles 3 weeks (wks) or young adults (8 wks) at the time of exposure. Thirty days after irradiation, animals received a moderate focal traumatic brain injury (Rosi et al. 2012a), and seven weeks after that, animals underwent cognitive training and testing and their brains immunohistochemically analyzed.
Materials and methods
Animals
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), either 21-days-old (n = 40) or eight-weeks-old (n = 40) were used in this study. Mice were housed and cared for in compliance with the United States Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and institutional IACUCs. In both the juvenile and adult cohorts, animals randomly received sham treatment (n = 10), radiation only (n = 10), trauma only (n = 10) or trauma combined with irradiation (n = 10).
Irradiation
Whole body irradiation was performed using a 137Cs irradiator (Gamma cell 3000; MDS Nordion Inc. Ottawa, ON, Canada). Animals were irradiated individually in a specially designed restrainer that fit into the irradiator. Dosimetry was performed using film exposure within the cesium irradiator and employing the same geometry used for the animal treatments. The film readings were calibrated against a range of doses obtained using a linear accelerator. The dose chosen for this study was 4.0 Gy, a dose that does not induce significant changes in the gut or bone marrow in mice. The total time of irradiation was approximately 1 min.
Traumatic brain injury
Traumatic brain injury or sham surgery was induced 4 weeks after irradiation (Figure 1). Each mouse was anesthetized with 4% isoflurane, maintained via a non-rebreathing apparatus connected to a nose cone on the stereotaxic head frame (Kopf, Tujunga, CA, USA). Ointment was applied to the eyes to protect vision, and heads were shaved with an electric clipper. The skin was prepped with betadine solution, and a midline incision was made through the scalp. A circular craniotomy, 3.5 mm in diameter was made in the left parietal skull between the bregma and lambda, 0.5 millimeter (mm) lateral to the midline. The skullcap was carefully removed without disruption of the dura. All mice, regardless of injury type, were subjected to this surgical procedure. Mice that were randomly selected for the trauma only (no irradiation) or RCI treatment groups were subjected to a controlled cortical impact (Rola et al. 2006, Rosi et al. 2012b). The lesion was produced with a pneumatic impact device using a 3-mm-diameter convex tip, mounted 20° from the vertical to account for the curvature of the mouse skull. The contact velocity was set at 4.5 metre per second (m/s) with a deformation 1.5 mm below the dura and a sustained depression of 150 millisecond (msec), producing a moderate lesion to the cortex without encroaching on the hippocampus. After the procedure, the scalp was sutured and each animal received a subcutaneous injection of warm physiologic saline (1 ml) to prevent dehydration. During surgery and subsequent recovery, body temperature was maintained with a circulating water heating pad.
Figure 1.
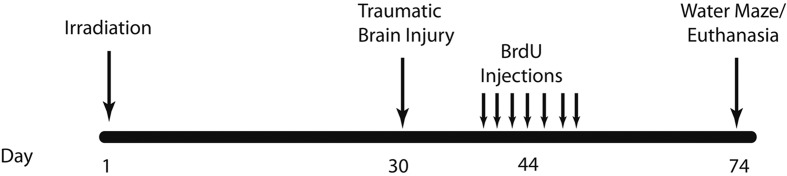
Schematic diagram showing experimental design. Three-week-old and eight-week-old C57BL/6 mice received whole body irradiation with 4 Gy 137Cs. Four weeks later animals received either focal traumatic brain injury or sham injury. Two weeks later, animals were injected daily for 7 days with BrdU (100 mg/kg). Four weeks after BrdU injections, animals underwent Morris water maze testing for 5 days.
The controlled cortical impact model of traumatic brain injury is widely preferred because it generates many of the motor and cognitive impairments seen in trauma patients (Brody et al. 2007). In the open head model, a portion of the skull is removed, and an impacting rod is driven into the dura to produce deformation of the cortex. Increasing the depth and velocity of the impact intensifies cortical cavitation as well as deficits in motor and behavioral function (Saatman et al. 2006, Yu et al. 2009). The chosen impact depth (1.5 mm) used here was based on the previous studies performed in young adult mice where a 0.50 mm deformation produced mild injury, a 1.0 mm deformation produced moderate injury, and a 2.0 mm deformation produced severe injury (Yu et al. 2009).
BrdU injection
Fourteen days following sham injury or traumatic brain injury, all mice received daily i.p. injections of BrdU (100 mg/kg) for 7 consecutive days. Four weeks after the first BrdU injection, mice underwent Morris water maze training and testing and then they were euthanized and tissues were collected for analysis of neurogenesis (Figure 1).
Morris water maze
Assessment of hippocampus-dependent cognitive performance was performed 6 weeks after irradiation, trauma or RCI using the Morris water maze test (Benice et al. 2006). A circular pool (diameter 140 cm) was filled with opaque water (24°C) and mice were trained to locate a platform (luminescence: 200 lux). To determine if treatment affected the ability to swim or learn the water maze task, mice were first trained to locate a clearly marked platform (visible platform, Days 1 and 2). Mice were subsequently trained to locate the platform when it was hidden beneath the surface of the opaque water (Days 3–5). Hidden platform training (acquisition) required the mice to learn the location of the hidden platform based on extra-maze spatial cues. For both visible and hidden platform paradigms, there were 2 daily sessions which were 2 hours (h) apart. Each session consisted of 3 trials (with 10-min inter-trial intervals). A trial ended when the mice located the platform. Mice that failed to locate the platform within 60 seconds (sec) were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they were physically on the platform for a minimum of 3 sec. During visible platform training, the platform was moved to a different quadrant of the pool for each session. For the hidden platform training, the platform location was kept constant. Mice were placed into the water facing the edge of the pool in one of 9 randomized locations. The start location was changed for each trial. Swimming patterns were recorded with the Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, The Netherlands) set at 6 samples/second.
To measure spatial memory retention, probe trials (platform removed) were conducted 1 h after the last trial on each day of hidden platform training (i.e., 3 separate probe trials). For the probe trials, mice were placed into the water in the quadrant opposite from the target quadrant. The time spent in the target quadrant, i.e., where the platform was previously located during hidden platform training, was compared to the time spent in the 3 non-target quadrants. Average velocity and distance to platform were also used as a measure of performance for the visible and hidden sessions.
Histological procedures and analysis
After the last probe trial, mice were killed by cervical dislocation and decapitated. Brains were removed quickly (within 60 sec), frozen in − 70°C isopentane and then stored at − 80°C until being sectioned on a cryostat. Brain sections were taken from the medial portion of the dorsal hippocampus (anteroposterior ˜2.92–4.0 mm from bregma). Tissues from multiple animals were blocked together and cryosectioned (Rosi et al. 2005). Each slide contained a 20 micrometer-thick sample from each of the experimental conditions: 0 Gy/sham surgery; 4 Gy/sham surgery; 0 Gy/trauma; 4 Gy/trauma. All slides were stored at − 80°C until processed for immunocytochemical analysis.
Neurogenesis
To determine the effects of single or combined treatment on the survival of newly born cells in the dentate subgranular zone (SGZ) a double labeling protocol was used to identify newly born Bromodeoxyuridine positive (BrdU+) neurons Neuronal Nuclei positive (NeuN+). For BrdU staining, sections were fixed for 8 min in 2% paraformaldehyde, and then rinsed in Tris buffered saline (TBS) (pH 7.4). Endogenous peroxidase activity was quenched by 30-min incubation in freshly prepared 3% H2O2 solution. After 2 × 5-min washes in TBS buffer the tissue was treated with 2N hydrochloric acid for 30 min at 37°C to denature DNA. The slides were immersed in 0.1 M Na2B4O7 to neutralize the acid, followed by eight rinses in TBS for 5 min each to return the pH to approximately 7.4. Non-specific antigen binding was blocked with TBS containing TSA blocking reagent (PerkinElmer life Sciences, Emeryville, CA, USA) at ambient temperature for 30 min. Newly born cells were stained using rat anti-BrdU (1:50 Accurate Chemical & Scientific Corporation, Westbury, NY, USA) incubated overnight at 4°C. The primary antibody was then detected by 2-h incubation with an anti- rat Red X (Jackson ImmunoResearch, Carlsbad, CA, USA). Neuronal staining was performed using an antibody for the neuron-specific nuclear protein NeuN (1:500; Millipore, Billerica, MA, USA). The primary antibody was then detected by 2-h incubation with an anti-mouse Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA). BrdU and NeuN positive cells were double labeled in the same section.
Total and newly activated born microglia
The numbers of total activated microglia were determined using an anticluster of differentiation 68 (CD68) antibody and number of newly born activated microglia was determined by counting cells double labeled for CD68 and BrdU. Briefly, sections were fixed for 10 min in 4% paraformaldehyde and after three washes with TBS and quenching of endogenous peroxidase activity in 1% H2O2 solution, they were incubated in TSA blocking buffer containing 3% normal rabbit serum for 30 min to block non-specific antigen binding. The sections were then incubated with rat anti-mouse CD68 antibody (1: 1000, Abcam, Cambridge, MA, USA) overnight at 4°C followed by incubation with rabbit anti-rat IgG (1:200, Vector, Burlingame, CA, USA) for 2 h at room temperature. Staining signals were further amplified with an avidin/biotin amplification system (Vector, Burlingame, CA, USA) followed by Cyanine 3 (Cy3) tyramide amplification (PerkinElmer, Waltham, MA, USA). To label newly born activated microglia (CD68+/BrdU+), sections were further washed with TBS-tween and treated for Deoxyribonucleic acid (DNA) denaturation as described above. Rat anti-BrdU primary antibody (1: 50 Accurate Chemical & Scientific Corporation, Westbury, NY, USA) was applied for overnight at 4°C and detected with anti- rat Fluorescein isothiocyanate (FITC) (Jackson, West Grove, PA, USA) secondary antibody. Only those cells for which the BrdU nucleus was unambiguously associated with the marker for activated microglia (CD68) were scored as positive for newly born activated microglia. The results were expressed as numbers of cells/mm2.
Microscopic analysis
Images were reconstructed as described previously (Rosi et al. 2012a). Briefly, mosaics were collected with a Zeiss AxioImager Apotome microscope using a 20 × objective. The parameters were kept constant across sections. Regions of interest were selected using AxioImager imaging software (Carl Zeiss, Hertfordshire, UK) and the numbers of positive cells were counted from both the upper and lower blades of the dentate gyrus of both hippocampi were counted in a blinded fashion. At least four sections per animal were analyzed, from the medial portion of the dorsal hippocampus (from 3.2–4.00 mm posterior to bregma). Cells were counted (without knowledge of treatments) under high power (× 40) using an Apotome Zeiss microscope (Carl Zeiss, Hertfordshire, UK).
Data analysis
Statistical analyses and graphs for the immunohistochemistry cell counts were generated by using Prism version 6 (GraphPad Software, Inc., San Diego, CA, USA). All results were expressed as mean ± standard error of the mean. For analysis of performance in the water maze probe trials, one-way Analysis of variance (ANOVAs) were used along with Newman Keuls post hoc test when appropriate. Statistical analyses for the behavioral data were generated using R statistical programming language. Visible and hidden water maze learning curves were analyzed using 2 mixed model repeated measures ANOVAs. Model 1 was used to assess whether each genotype learned over time. Here Radiation and Treatment were between group factors. Day was treated as a continuous variable. This model was fit separately to Juvenile-RCI and Adult-RCI data. Model 2 was used to compare performance of each group at each day. Radiation and Treatment were between group factors and Day was treated as categorical to better allow for day-specific comparisons among groups, and eliminate the assumption of a linear trend in performance over time. The Holm's correction was used to control for multiple comparisons. Separate analyses were conducted for the visible and hidden platform learning curves. For analysis of performance in the water maze probe trials, one-way ANOVAs were used along with Newman Keuls post hoc test when appropriate. Differences were considered to be statistically significant when p < 0.05.
Results
Morris water maze
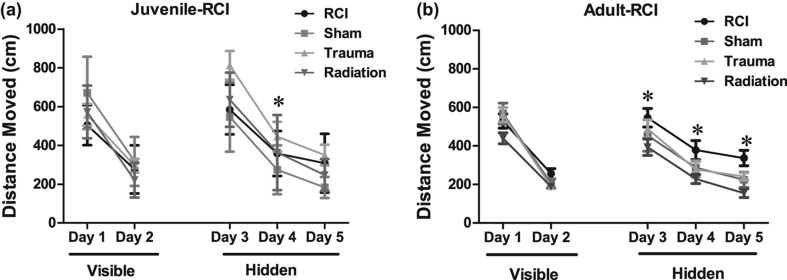
Juvenile-RCI distance moved. First, the mice were trained to locate a visible platform; distance moved was used as a performance measure. All groups improved their performance with training (distance moved, effect of day: F (1, 34) = 125.84, p < 0.001). During the platform training, there was no significant statistical evidence that irradiation (F (1, 34) = 3.56, p = 0.067) or trauma (F (1, 34) = 1.18, p = 0.283) affected performance. Mice that received radiation only swam shorter paths to the platform compared to sham animals. However, Holm's correction revealed that there were no significant differences between treatment groups on either training day. There was no significant treatment × day interactions which suggests that radiation, trauma or combined injury did not affect performance over time.
During hidden platform training, all groups also improved their performance with training (distance moved, effect of day: F (1, 72) = 21.25, p < 0.001; ANOVA model 1). There was no effect of radiation (F (1, 34) = 0.26, p = 0.609; ANOVA model 1) but there was an effect of trauma (F (1, 34) = 8.18, p = 0.007; ANOVA model 1). A subsequent post hoc analysis showed that after trauma only, significantly longer swim paths were required to find the hidden platform compared to sham-treated mice on day 3 (day 3 Sham vs. trauma, p < 0.01; Holm's correction). There was also a radiation × trauma interaction (F (1, 72) = 7.16, p < 0.05). However, Holm's correction also revealed that there were no significant differences between treatment groups. There were no other significant main effects or interactions which suggest that in animals irradiated as juveniles radiation, trauma or combined injury did not affect performance over time.
Juvenile-RCI probe trials
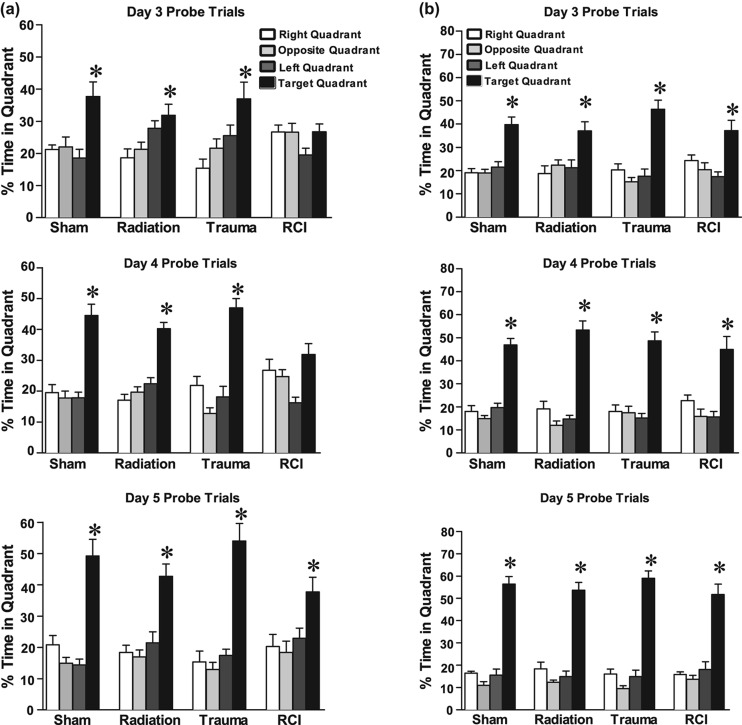
In the first probe trial of the Water maze test, sham-treated animals and mice that received radiation only or trauma only showed spatial memory retention by spending more time in the target quadrant (ANOVA quadrant within group analysis; Sham, Target versus any other Quadrant: p < 0.05; Trauma, Target versus any other Quadrant: p < 0.05; Radiation, Target versus Right and Opposite Quadrant: p < 0.05; Target versus Left Quadrant: p > 0.05 (Figure 2a).
Figure 2.
Cumulative distance to the target platform during visible and hidden training sessions. (a) During the visible platform training (day 1 and 2), all experimental groups swam similar distances to the platform. All groups showed daily improvements in their abilities to locate during the hidden platform training (day 3–5). However, trauma only (*p < 0.05; day 4, Holms) swam longer escape distances compared to sham mice. (b) All groups showed daily improvements in their abilities to locate during the hidden platform training (day 3–5). Radiation only (*p < 0.05; day 3–5, Holms) swam shorter escape distances compared to sham mice. Each datum point represents the mean of 8–10 mice; error bars are standard error of the mean (SEM).
In the probe trial following an additional day of hidden platform training (Probe 2), sham-treated animals and mice that received irradiation only or trauma only showed spatial memory retention (Sham, Target versus any other Quadrant: p < 0.05; Trauma, Target versus any other Quadrant: p < 0.05; Radiation, Target versus any other Quadrant: p < 0.05; RCI, Target versus left Quadrant: p < 0.05). However, a significant memory impairment was observed in the juvenile-RCI group Target versus any other Quadrant; p > 0.05; Figure 2a). Following a third day of hidden platform training (Probe 3), all of the groups including RCI showed memory retention and spent more time searching in the target quadrant than in any other quadrant (Sham, Target versus any other Quadrant: p < 0.05; Trauma, Target versus any other Quadrant: p < 0.05; Radiation, Target versus any other Quadrant: p < 0.05; RCI, Target versus any other Quadrant: p < 0.05; Figure 2a).
Adult-RCI cognition
Adult-RCI distance moved. During the visible platform training, all groups improved their performance with training (distance moved: effect of day: F (1, 36) = 189.88, p < 0.001; ANOVA model 1). During the platform training, there was no significant statistical evidence that radiation (F (1, 36) = 2.25, p = 0.14; ANOVA model 1) or trauma (F (1, 36) = 1.52, p = 0.22; ANOVA model 1) effected performance. There was a day × trauma interaction (F (1, 36) = 5.22, p < 0.05; ANOVA model 1). However, Holm's correction revealed that there were no significant differences on either training day. There was no other significant treatment × day interactions suggesting that neither radiation nor combined injury affected performance over time.
During hidden platform training, all groups also improved their performance with training (distance moved: effect of day: F (1, 76) = 63.32, p < 0.001). There was effect of radiation (F (1, 36) = 9.06; p < 0.05) but no effect of trauma (F (1, 36) = 0.144, p = 0.70) (Figure 3b). A subsequent post-hoc test showed that mice that received radiation only, swam significantly shorter paths to find the hidden platform compared to sham-treated mice on day 3–5 (day 3–5 Sham vs. radiation p < 0.05; Holm's correction model 2; Figure 3b). There was a radiation × trauma interaction (F (1, 36) = 6.41; p < 0.05). However, Holm's correction revealed that there were no significant differences in distance moved on either training day. There was no other significant treatment × day interactions suggesting that neither trauma nor radiation or combined injury affected performance over time.
Figure 3.
Spatial memory retention in mice during the Morris water maze probe trial following the first day of hidden platform training. (a) Juvenile-RCI mice showed an impairment of hippocampal-dependent spatial memory during the day 3 and day 4 probe trials. (b) All of the adult mice, showed memory retention in the water maze by spending most of their time in target quadrant which contained hidden platform. For all four treatments, when time spent in the target quadrant was compared to all other quadrants there was a significant (p < 0.05) preference. Each bar represents the mean of 8–10 mice; error bars are standard error of the mean (SEM).
Adult-RCI probe trial
Following hidden platform training, all four groups showed spatial memory retention in the probe trial and searched significantly longer in the target quadrant than in any other quadrant (Sham, Target versus any other Quadrant: p < 0.05; Trauma, Target versus any other Quadrant, p < 0.05; Radiation, Target versus any other Quadrant: p < 0.05; RCI, Target versus any other Quadrant: p < 0.05; Figure 2b). In summary, when adult animals were irradiated followed 30 days later by traumatic brain injury, there were no cognitive impairments relating to spatial information.
Juvenile-RCI neurogenesis
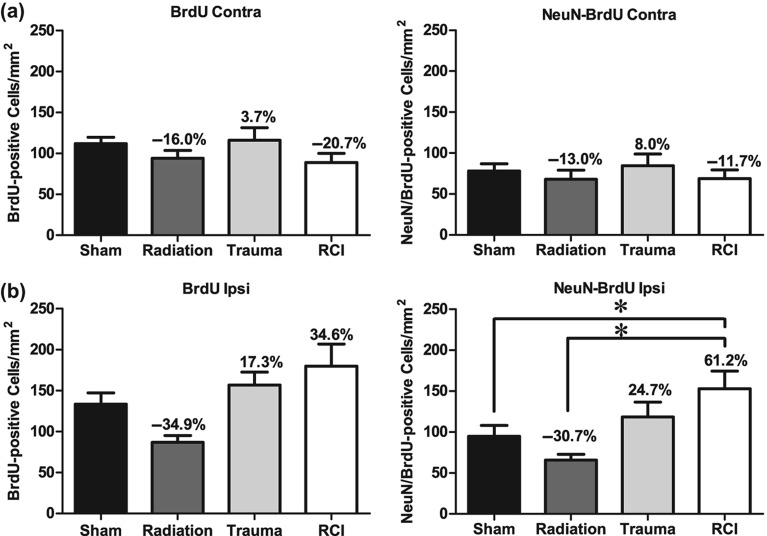
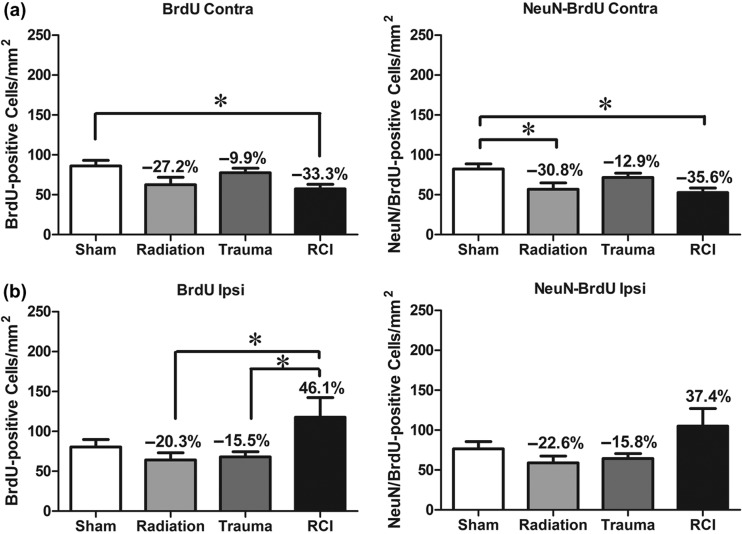
The fate of newly born cells was determined by quantifying cells that were co-labeled with BrdU, and either NeuN for neurons or CD68 for activated microglia. The presence of BrdU only represents the long-term survival of newly generated cells, independent of phenotype. There were no significant differences in the numbers of BrdU+ cells in the contralateral hemispheres across the various treatment groups (F (3, 30) = 1.47, p = 0.242; Figure 4a). In the ipsilateral hemisphere, there was a significant injury effect on the number of BrdU+ cells (F (3, 30) = 4.99, p = 0.006; Figure 4b). There was an average of 133.7 ± 13.5 cells/mm2 in sham animals with a minor increase following trauma (156.8 ± 16.07 cells/mm2) and RCI (179.9 ± 27.0 cells/mm2). There also was a minor but insignificant decrease after radiation (86.9 ± 8.3 cells/mm2) compared to sham (Figure 4b).
Figure 4.
Total number of BrdU+ cells and BrdU+/NeuN+ cells per mm2 in the dentate subgranular zone of the Juvenile-RCI cohort. (a) In the contralateral hemisphere, there was a no significant group difference for BrdU+ cells (p = 0.242) or BrdU+/NeuN+ (p = 0.697). Percentage change compared with sham-treated animals. (b) In the ipsilateral hemisphere, there was a significant group difference for BrdU+ cells (p = 0.006) and BrdU+/NeuN+ (p = 0.004). RCI significantly increased the numbers of BrdU+ cells and BrdU+/NeuN+ compared to sham-treated and radiation only (p < 0.05). Percentage change compared with sham-treated animals. Each bar represents the mean of 9–10 mice; error bars are standard error of the mean (SEM).
With regard to newly born neurons (BrdU+/NeuN+), there were no significant differences in the numbers of neurons/mm2 in the contralateral hemispheres across the 4 treatment groups (F (3, 30) = 0.482, p = 0.697; Figure 4a). However, in the ipsilateral hemisphere there was a significant injury effect on the number of BrdU+/NeuN+ cells (ANOVA: F (3, 30) = 5.297, p = 0.01). There was an average of 94.9 ± 13.0 BrdU+/NeuN+ cells/mm2 in sham-treated animals with a minor reduction following radiation only (63.7 ± 7.0 neurons/mm2) (Figure 4b). There was a minor but insignificant increase after trauma only (118.4 ± 18.2 neurons/mm2). However, RCI significantly increased in the number of newly born neurons (153.1 ± 21.5 neurons/mm2) compared to sham-treated (94.97 ± 13.05 BrdU+/NeuN+ cells /mm2; p < 0.05) and radiation only (65.75 ± 7.06 BrdU+/NeuN+ cells /mm2; p < 0.001; Figure 4b).
Juvenile-RCI total activated and newly born activated microglia
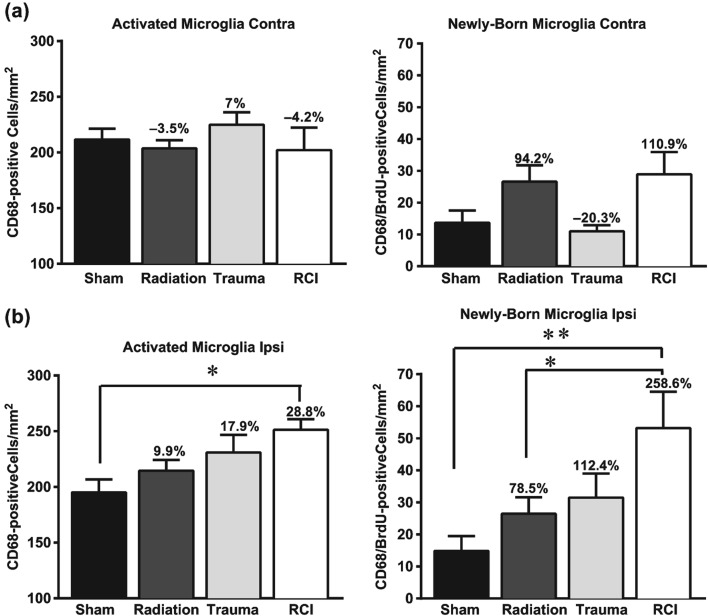
In the contralateral hemisphere, there were no significant injury effects on the numbers of total activated microglia (ANOVA: F (3, 30) = 0.622, p = 0.606; Figure 5a). However, there was a significant injury effect on the total number of activated microglia in the ipsilateral hemisphere (ANOVA: F (3, 30) = 4.14, p = 0.01). Sham-treated mice averaged 195.1 ± 11.6 activated microglia cells/mm2. There were minor but insignificant increases in total numbers of activated microglia after radiation only and trauma only in the ipsilateral hemisphere. After RCI, however, there was a significant increase in the number of activated cells (251.3 ± 9.5 cells/ mm2; p < 0.05; Figure 5b) compared to sham-treated animals or mice.
Figure 5.
Total number of activated microglia (CD68+) and newly born microglia (BrdU+/CD68+) per mm2 in the dentate subgranular zone of the Juvenile-RCI cohort. (a) In the contralateral hemisphere, there was a no significant group difference in the total numbers of activated microglia (p = 0.606). There was a significant group difference for newly born microglia (p = 0.027). There were minor but insignificant increases in total numbers of activated microglia after radiation only and RCI. (b) In the ipsilateral hemisphere, there was a significant group difference for CD68+ cells (p = 0.01) and BrdU+/CD68+ (p = 0.009). RCI significantly increased in the number of activated microglia compared to sham-treated animals. Percentage change compared with sham-treated animals. Each bar represents the mean of 8–10 mice; error bars are standard error of the mean (SEM).
In the contralateral hemisphere, cell numbers averaged newly born microglia (BrdU+/CD68+) 13.6 ± 3.8 cells/mm2 in sham-treated mice and 10.9 ± 1.9 cells/mm2 in mice that received trauma only. There were minor but insignificant increases in the numbers of newly born microglia after radiation only and RCI (Figure 5a). In terms of the ipsilateral hemisphere, there was a significant injury effect on the number of newly born activated microglia (F (3, 29) = 4.62, p = 0.009). There was an average of 14.8 ± 4.6 cells/mm2 in sham-treated mice and a small but insignificant increase after radiation only (26.4 ± 4.6 cells/ mm2) or trauma only (31.4 ± 7.5 cells/mm2; Figure 5b). After RCI, there was a significant increase in the numbers of newly born activated microglia (53.1 ± 11.3 cells/mm2) compared to sham-treated (14.8 ± 4.6 cells/mm2; p < 0.001; Figure 5b) and radiation only (26.4 ± 5.1 cells/mm2; p < 0.05; Figure 5b).
Adult-RCI neurogenesis
In the contralateral hemisphere there was a significant injury effect on the number of BrdU+ cells (F (3, 35) = 3.349, p = 0.029). Compared to sham-treated mice (82.1 ± 6.6 BrdU+ cells/mm2), there was a significant decrease in the average number of BrdU+ cells in RCI mice (52.8 ± 5.6 cells/mm2; p < 0.05; Figure 6a). In the ipsilateral hemisphere there was also a significant injury effect on the number of difference on BrdU+ cells (F (3, 35) = 3.43, p = 0.027; Figure 6b). Sham-treated mice averaged of 80.5 ± 9.0 BrdU+ cells/mm2. RCI (117.9 ± 24.5 cells/mm2) significantly increased in the number of newly born neurons compared to radiation only (64.1 ± 9.1 cells/mm2; p < 0.05) and trauma only (68.0 ± 6.4 cells/mm2; p < 0.05; Figure 6b). With regard to newly born neurons in the contralateral hemisphere, there was a significant injury effect on the number of BrdU+/NeuN+ cells (F (3,35) = 3.97, p < 0.05). There was an average of 82.1 ± 6.6 newly born neurons/mm2 in sham-treated controls, and there was a significant decrease following radiation only (56.7 ± 8.1 cells/mm2; p < 0.05; Figure 6a) and RCI (52.88 ± 5.6 cells/mm2; p < 0.05; Figure 6a). In the ipsilateral hemisphere, there were no significant differences in the numbers of newly born neurons in the ipsilateral hemisphere across the various treatment groups (Figure 6b).
Figure 6.
Total number of BrdU+ cells and BrdU+/NeuN+ cells in the dentate subgranular zone of the Adult-RCI cohort. (a) In the contralateral hemisphere, there was a significant group difference for BrdU+ cells (p = 0.029) and BrdU+/NeuN+ (p = 0.015). Radiation significantly decreased the numbers BrdU+ cells and BrdU+/NeuN+ neurons in radiation only and RCI mice (p < 0.05). Percentage change compared with sham-treated animals. (b) In the ipsilateral hemisphere, there was a significant group difference for BrdU+ cells (p = 0.027). RCI significantly increased the numbers of BrdU+ cells compared to radiation and trauma only (p < 0.05). In terms of newly born neurons, there was a trend toward a group difference (p = 0.0508). Percentage change compared with sham-treated animals. Each bar represents the mean of 9–10 mice; error bars are standard error of the mean (SEM).
Adult-RCI total activated and newly born microglia
There were no significant differences in the numbers of activated microglia (CD68 only) in either hemisphere across the treatment groups. In the contralateral hemisphere, newly born activated microglia (BrdU+/CD68+) averaged (42.19 ± 4.9 cells/mm2). There were minor but insignificant increases in the total numbers of activated microglia after radiation only, trauma only and RCI (not shown). In the ipsilateral hemisphere of sham-treated mice, there was an average 49.3 ± 7.7 and 47.4 ± 5.6 newly activated microglia/mm2 in radiated only mice. There were minor but insignificant increases after trauma only and RCI (not shown).
Discussion
Our data show that when animals are exposed to irradiation as juveniles followed by a moderate traumatic brain injury one month later (Juvenile-RCI) they have an impairment of hippocampal dependent spatial memory. In contrast, animals exposed to irradiation as adults followed by traumatic brain injury one month later (Adult-RCI) do not show any impairment. Cognitive impairment in the Juvenile-RCI animals was associated with an increase in the numbers of total activated microglia as well as newly born activated microglia in the ipsilateral hemisphere of the DG. In the adult animals, there were no differences in numbers of activated microglia between any of the treatment groups. Further analyses revealed that there was increased neurogenesis in the ipsilateral DG in the Juvenile-RCI, but not in the Adult-RCI group when compared to their respective sham-treated controls. Our data suggest that whole body exposure to radiation affects the vulnerability of the brain to a subsequent traumatic injury but only when the animals were irradiated as juveniles.
Cognitive function after single and combined injury
The cognitive training and testing paradigm used here employed three probe trials, one of which was performed at the end of each day of multiple hidden training sessions (Rosi et al. 2012b). Juvenile sham-treated controls and animals that received radiation only and trauma only showed spatial memory retention during all of the probe trials, that is they were not cognitively impaired. In contrast, juvenile-RCI only showed spatial memory retention during the third probe trial. These data suggest that irradiation of the young animal brain enhances vulnerability to develop cognitive injury following a subsequent traumatic injury in young adulthood. In contrast, all groups of animals that were treated as adults showed memory retention and spent more time in the target quadrant than in any other quadrant. This suggests that the adult brain is less vulnerable to the harmful effects of a second insult.
The underlying mechanisms associated with cognitive impairment after single or combined injures are likely to be multifactorial. Previous studies from our lab have shown that cells in the neurogenic zone of the hippocampus are extremely sensitive to low/moderate does of irradiation. Significant losses of neuronal precursor cells occur within a few hours after relatively low radiation doses (Mizumatsu et al. 2003), and the surviving precursor cells have a reduced ability to differentiate into mature neurons, an effect that is dose-dependent (Mizumatsu et al. 2003). The reduction in precursor proliferation is still observed months after exposure and is associated with hippocampal-dependent cognitive impairment (Raber 2010). Given our own data, along with those from others, we felt it was possible that changes in neurogenesis might play an important role in the behavioral consequence of RCI.
In the current study, irradiation alone of juveniles resulted in only a 30% decrease in DG neurogenesis, and the animals were not cognitively impaired. Similarly, there were no cognitive deficits or any decrease in neurogenesis in animals that received trauma alone. The only animals that showed a significant change in neurogenesis (61% increase, Figure 4b), were those animals that received RCI, and they were cognitively impaired. These findings are in contrast to previous work by us and others showing that reduced neurogenesis was associated with cognitive impairment after irradiation only. This surprising result, showing increased neurogenesis in cognitively impaired animals, raises some doubt as the importance of neurogenesis per se in the context of RCI, and suggests that other mechanistic factors are involved, and should be investigated.
A number of studies suggest that inflammation may contribute to cognitive function following CNS irradiation or trauma (Mizumatsu et al. 2003, Monje and Palmer 2003, Rola et al. 2004, d’Avila et al. 2012). One of the hallmarks of inflammation is the activation of microglia (Mannix and Whalen 2012). In an uninjured brain, microglia monitor the microenvironment to ensure that homeostasis is maintained (Stoll and Jander 1999, Loane and Byrnes 2010). After injury, activated microglia play an important role in the phagocytosis of dead cells, and sustained microglial activation contributes to the chronic inflammatory state in the brain (Joo et al. 2012). Depending on their functional phenotype, microglia, can be either detrimental or supportive for neurogenesis in the injured brain (Ekdahl et al. 2009, Loane and Byrnes 2010) and more work is required to determine if and to what extent this dicotomy may play a role in single or combined injury.
In the present study, we quantified the numbers of total activated microglia (CD68+ only) and the numbers of newly born activated microglia (BrdU+/CD68+). In the adult mice that received radiation and delayed trauma, there were no significant changes in the numbers of activated microglia or newly born activated microglia across the treatment groups. In addition, none of these animals displayed any cognitive deficits. This suggests that at least from the context of activated microglia, radiation or trauma used in our experiment did not elicit a significant inflammatory response within the adult cohort.
In the juvenile mice, trauma only and radiation only increased the numbers of activated microglia and newly born activated microglia relative to sham treatment, but the changes were not significant. However, after combined injury, there was a significant increase in the numbers of activated microglia and numbers of newly born activated microglia in the ipsilateral hemisphere (Figure 5a, 5b). In addition, performance in the water maze was significantly impaired in RCI mice (Figure 2a). This suggests that in this population of animals, the newly born microglial cells could play a negative role in the current injury paradigm and that hippocampal dependent spatial memory retention was affected by the elevated inflammation.
Previous studies from our lab have reported an increase in the number of activated microglia and changes of neurogenesis following radiation injury as well as trauma (Raber et al. 2004a, Rola et al. 2004, 2006). Moreover, these cellular changes are often accompanied by deficits in hippocampal dependent memory retention (Raber et al. 2004b, Rola et al. 2004, 2006). Consistent with previous studies, in the present study there was an increase in newly born activated microglia and impaired neurogenesis in animals that received radiation only as juveniles. However, those animals showed spatial memory retention. Juvenile-RCI and trauma only, increased both numbers of total activated microglia and neurogenesis. However, there was a 100% increase in the numbers of newly born activated microglial in juvenile-RCI compared to trauma. In addition, juvenile-RCI mice were cognitively impaired, whereas mice that received trauma only showed spatial memory retention suggesting a negative role for these endogenous inflammatory cells after RCI. We recognize the complexities associated with inflammation and microglia, and it was not the objective of this study to do an in depth investigation of inflammatory mediators as a function of single or combined injury. We focused strictly on the numbers of activated microglia to determine if the changes in those cells were associated with neurogenesis and hippocampal-dependent behavioral function. Further studies are warranted to delineate what factors associated with inflammation are associated with newly born activated microglia and if they have specific effects on cognitive performance.
In conclusion, our data suggest that whole body exposure to radiation in juveniles, affected the vulnerability of the brain to a subsequent traumatic injury, and this relationship may change as a function of age. Although the conceptual basis of this study was guided by an interest in combined injury in a nuclear or radiologic terrorism scenario, the data are also relevant to individuals exposed to radiation in a therapeutic setting. An increasing number of patients treated for childhood malignances survive into adulthood. Further studies are in progress to delineate the specific molecular mechanisms involved in age-dependent variability in cognitive alterations in a combined radiation-traumatic brain injury situation.
Acknowledgments
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The research described was supported by National Institutes of Health (NIH) grant R33 AI08053 and Award Number K12GM081266 from the National Institute of General Medical Sciences/National Institutes of Health.
References
- 1.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Environmental Health. Radiation disasters and children. Pediatrics. 2003;111((6 Pt 1)):1455–1466. [PubMed] [Google Scholar]
- 3.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self- renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 5.Brody DL, MacDonald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, Kim E, Schwetye KE, Holtzman DM, Bayly PV. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d’Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, Massa S, Liu J, Swanson RA. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, Ramakrishnan N. Medical countermeasures for radiation combined injury: Radiation with burn, blast, trauma and/or sepsis. report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169:712–721. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH. Mammalian neural stem cells. Science. 2000;287((5457)):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 10.Joo KM, Jin J, Kang BG, Lee SJ, Kim KH, Yang H, Lee YA, Cho YJ, Im YS, Lee DS, et al. Trans-differentiation of neural stem cells: A therapeutic mechanism against the radiation induced brain damage. PLoS One. 2012;7:25936. doi: 10.1371/journal.pone.0025936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannix RC, Whalen MJ. Traumatic brain injury, microglia, and Beta amyloid. Int J Alzheimers Dis. 2012;2012:608732. doi: 10.1155/2012/608732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 14.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 15.Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005;93:2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- 16.Naylor AS, Bull C, Nilsson MK, Zhu C, Bjork-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey BN, Kumar A, Tiwari P, Mishra KP. Radiobiological basis in management of accidental radiation exposure. Int J Radiat Biol. 2010;86:613–635. doi: 10.3109/09553001003746059. [DOI] [PubMed] [Google Scholar]
- 18.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 19.Raber J. Unintended effects of cranial irradiation on cognitive function. Toxicol Pathol. 2010;38:198–202. doi: 10.1177/0192623309352003. [DOI] [PubMed] [Google Scholar]
- 20.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004a;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 21.Raber J, Rola R, LeFevour A, Morhardt DR, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-Induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004b;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 22.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosi S, Ferguson R, Fishman K, Allen A, Raber J, Fike JR. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS One. 2012a;7:31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, Fike JR. Trauma-induced alterations in cognition and Arc expression are reduced by previous exposure to 56Fe irradiation. Hippocampus. 2012b;22:544–554. doi: 10.1002/hipo.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 28.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 29.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, Sanberg PR, Borlongan CV. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–163. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]