Abstract
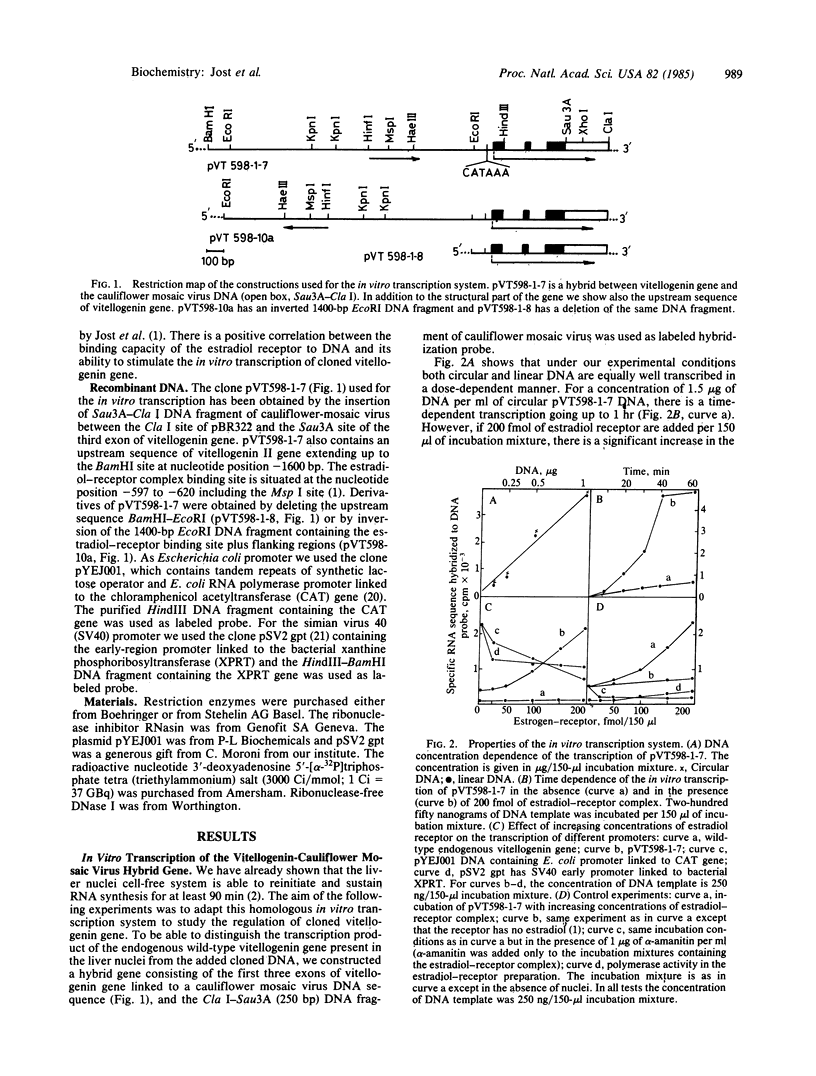
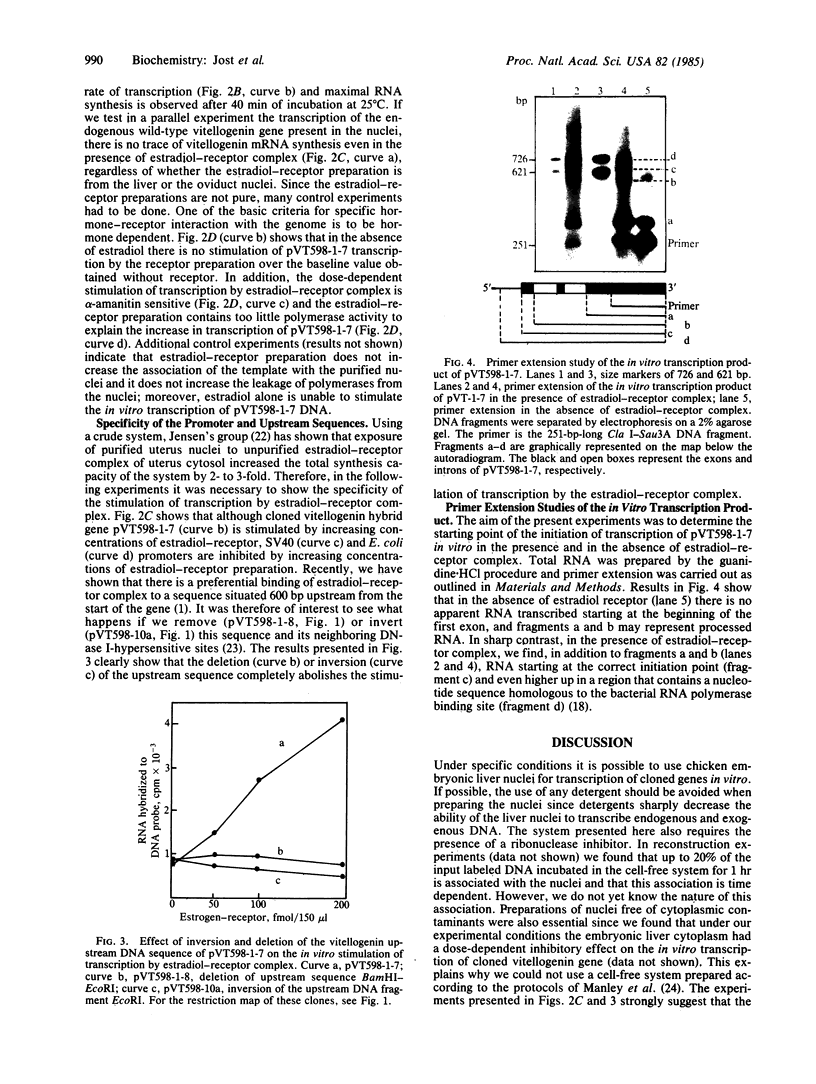
Avian vitellogenin-cauliflower mosaic virus hybrid gene is effectively transcribed in vitro in the homologous embryonic liver nuclei system. The transcription of the hybrid gene is modulated by the addition of an estradiol-receptor preparation that has been shown to bind selectively to an upstream region of cloned vitellogenin gene. Stimulation of the transcription of cloned vitellogenin hybrid gene by estradiol receptor is alpha-amanitin sensitive, hormone dependent, and promoter specific. Simian virus 40 and Escherichia coli promoters are not stimulated by the estradiol-receptor complex. The endogenous silent vitellogenin II gene (wild type) present in the nuclei is not turned on by the addition of estradiol-receptor complex. Deletion or inversion of the DNA sequence where the estradiol-receptor complex binds results in the complete suppression of the in vitro stimulation of transcription by estradiol receptor. Correct initiation of the transcription was demonstrated by primer extension studies of the newly synthesized RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebee T. J., Butterworth P. H. Transcription of isolated nuclei and nucleoli by exogenous RNA polymerase A and B. Eur J Biochem. 1975 Feb 21;51(2):537–545. doi: 10.1111/j.1432-1033.1975.tb03954.x. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Busiello E., Di Girolamo M. RNA metabolism in nuclei isolated from HeLa cells. Eur J Biochem. 1975 Jun 16;55(1):61–70. doi: 10.1111/j.1432-1033.1975.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Ernest M. J., Schutz G., Feigelson P. RNA synthesis in isolated hen oviduct nuclei. Biochemistry. 1976 Feb 24;15(4):824–829. doi: 10.1021/bi00649a015. [DOI] [PubMed] [Google Scholar]
- Geiser M., Mattaj I. W., Wilks A. F., Seldran M., Jost J. P. Structure and sequence of the promoter area and of a 5' upstream demethylation site of the estrogen-regulated chicken vitellogenin ii gene. J Biol Chem. 1983 Jul 25;258(14):9024–9030. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gross R. H., Ringler J. Ribonucleic acid synthesis in isolated Drosophila nuclei. Biochemistry. 1979 Oct 30;18(22):4923–4927. doi: 10.1021/bi00589a021. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Seldran M., Geiser M. Preferential binding of estrogen-receptor complex to a region containing the estrogen-dependent hypomethylation site preceding the chicken vitellogenin II gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):429–433. doi: 10.1073/pnas.81.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazier C. B. Ontogeny of the vitellogenic response to oestradiol and of the soluble nuclear oestrogen receptor in embryonic-chick liver. Biochem J. 1978 Jul 15;174(1):143–152. doi: 10.1042/bj1740143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. Rna synthesis in isolated nuclei processing of adenovirus serotype 2 late messenger rna precursors. J Mol Biol. 1982 Aug 25;159(4):581–599. doi: 10.1016/0022-2836(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Mohla S., DeSombre E. R., Jensen E. V. Tissue-specific stimulation of RNA synthesis by transformed estradiol-receptor complex. Biochem Biophys Res Commun. 1972 Jan 31;46(2):661–667. doi: 10.1016/s0006-291x(72)80191-8. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Sippel A. A., Hynes N. E., Groner B., Schütz G. Preferential transcription of the ovalbumin gene in isolated hen oviduct nuclei by RNA polymerase B. Proc Natl Acad Sci U S A. 1978 Feb;75(2):686–690. doi: 10.1073/pnas.75.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Ohno T., Jost J. P. In vitro RNA synthesis and expression of vitellogenin gene in isolated chicken liver nuclei. Nucleic Acids Res. 1978 Apr;5(4):1353–1370. doi: 10.1093/nar/5.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Soberon X., Marumoto Y., McMahon J., Itakura K. Biological expression of an Escherichia coli consensus sequence promoter and some mutant derivatives. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3203–3207. doi: 10.1073/pnas.80.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M. H., Feman E. R., Baglioni C. RNA synthesis in isolated HeLa cell nuclei. Biochim Biophys Acta. 1976 Jan 5;418(1):29–38. doi: 10.1016/0005-2787(76)90324-5. [DOI] [PubMed] [Google Scholar]
- Schäfer K. The RNA synthesizing capacity of nuclei isolated from cultured mouse cells. Biochem Biophys Res Commun. 1976 Jan 12;68(1):219–226. doi: 10.1016/0006-291x(76)90032-2. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Parks W. P., Scolnick E. M. In vitro transcription of Moloney leukemia virus genes in infected cell nuclei and chromatin: elongation of chromatin associated ribonucleic acid by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1977 May 3;16(9):1795–1801. doi: 10.1021/bi00628a005. [DOI] [PubMed] [Google Scholar]
- Sklar V. E., Roeder R. G. Transcription of specific genes in isolated nuclei by exogenous RNA polymerases. Cell. 1977 Mar;10(3):405–414. doi: 10.1016/0092-8674(77)90028-9. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Huang R. C. Transcription in vitro of immunoglobulin kappa light chain genes in isolated mouse myeloma nuclei and chromatin. Proc Natl Acad Sci U S A. 1976 Mar;73(3):775–779. doi: 10.1073/pnas.73.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Roop D. R., Stumph W. E., Tsai M. J., O'Malley B. W. Evidence that deoxyribonucleic acid sequences flanking the ovalbumin gene are not transcribed. Biochemistry. 1980 Apr 29;19(9):1755–1761. doi: 10.1021/bi00550a005. [DOI] [PubMed] [Google Scholar]
- Udvardy A., Seifart K. H. Transcription of specific genes in isolated nuclei from HeLa cells in vitro. Eur J Biochem. 1976 Feb 16;62(2):353–363. doi: 10.1111/j.1432-1033.1976.tb10167.x. [DOI] [PubMed] [Google Scholar]




