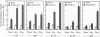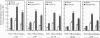Abstract
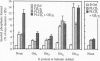
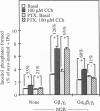
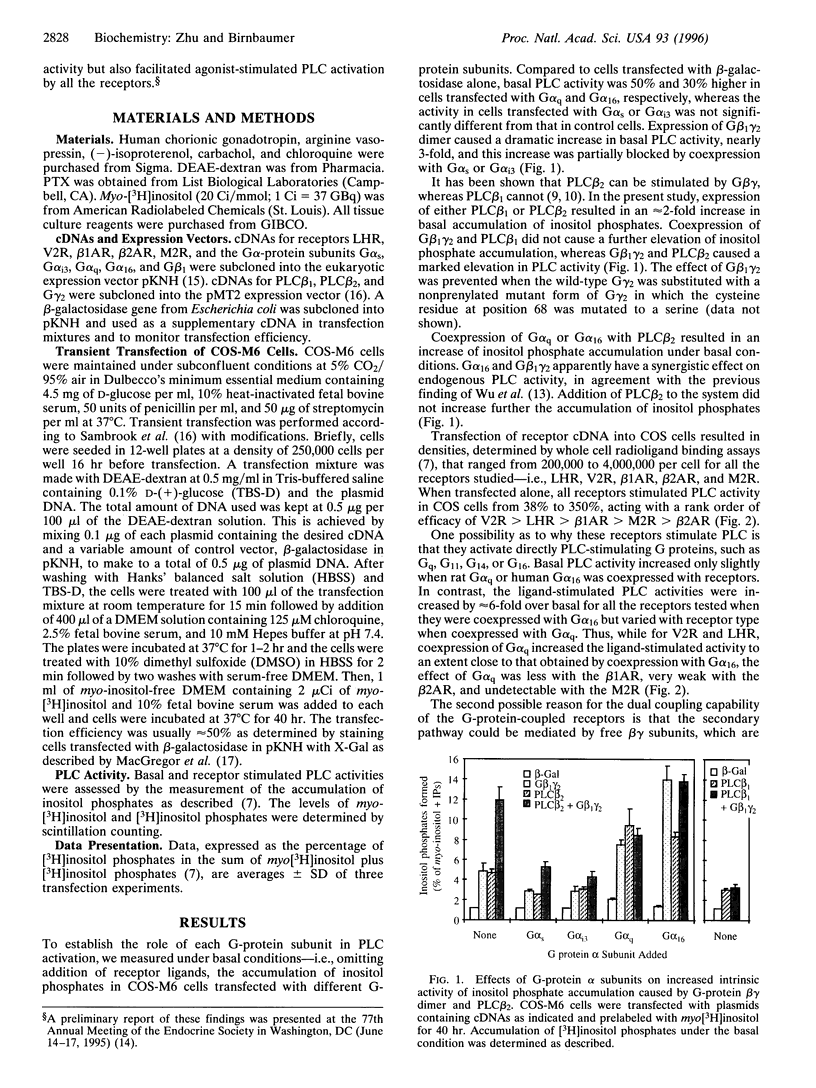
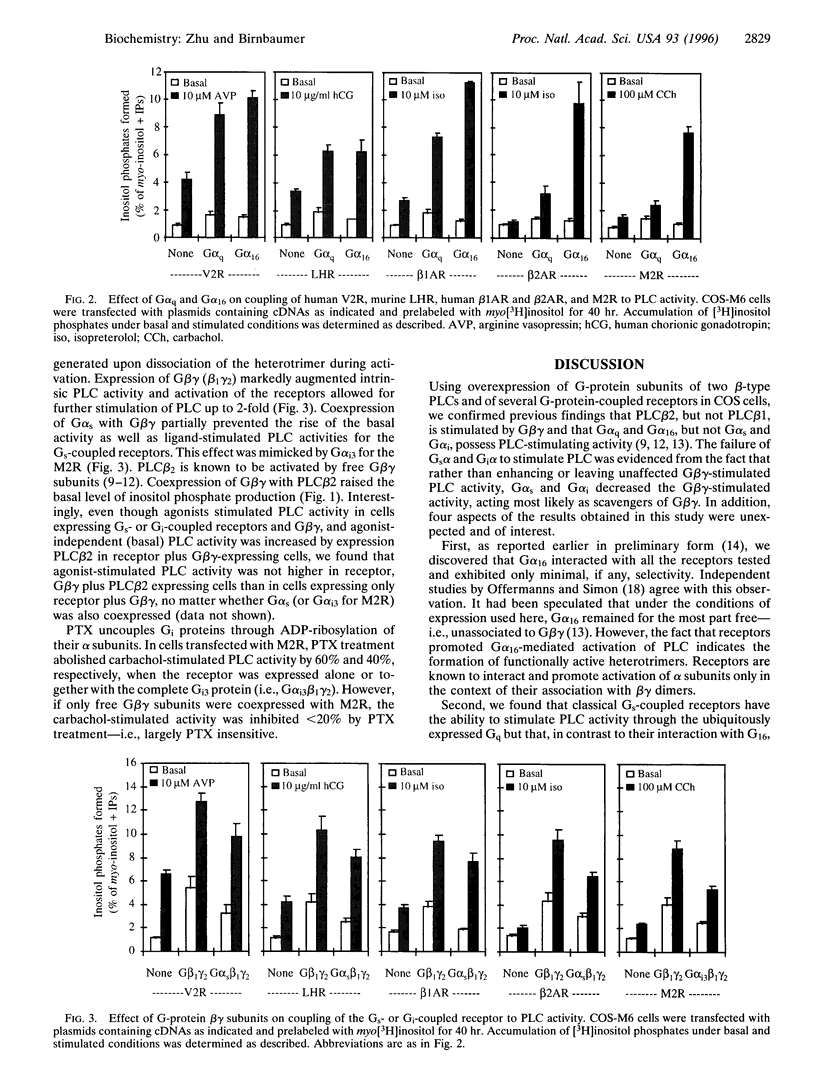
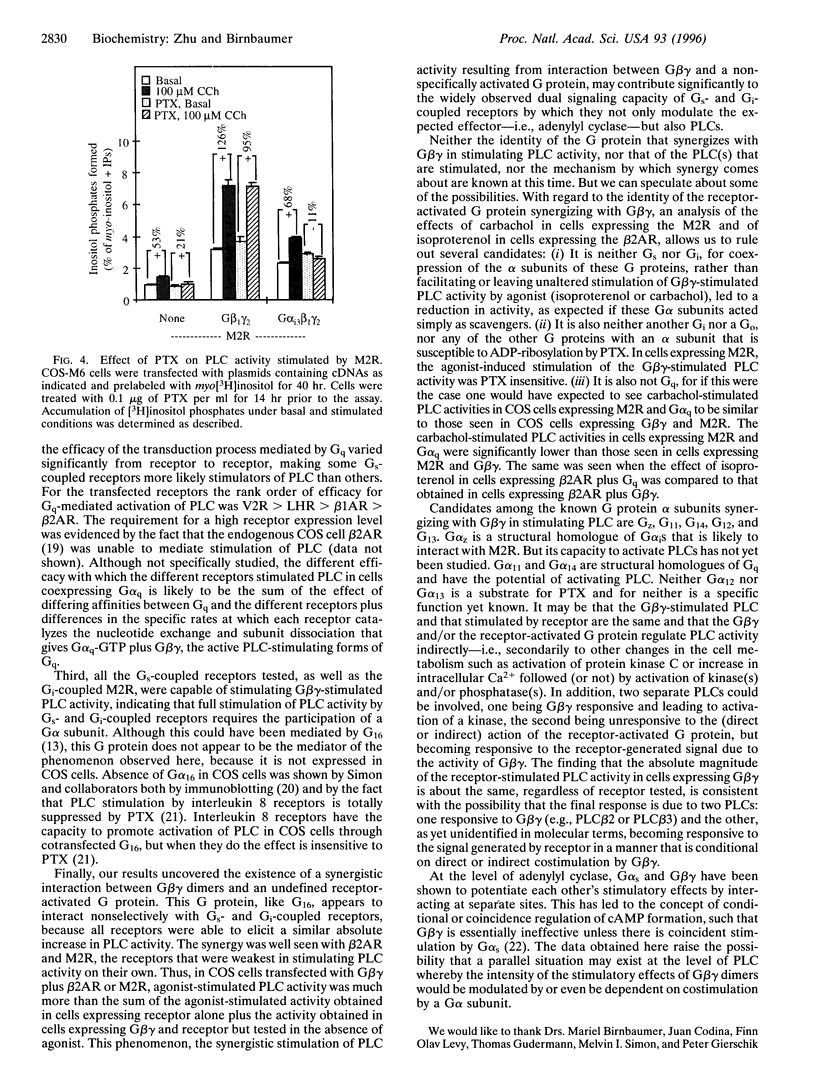
Stimulatory guanine nucleotide binding protein (Gs)-coupled receptors activated by luteinizing hormone, vasopressin, and the catecholamine isoproterenol (luteinizing hormone receptor, type 2 vasopressin receptor, and types 1 and 2 beta-adrenergic receptors) and the Gi-coupled M2 muscarinic receptor (M2R) were expressed transiently in COS cells, alone and in combination with Gbeta gamma dimers, their corresponding Galphas (Galpha(s), or Galpha(i3)) and either Galpha(q) or Galpha(16). Phospholipase C (PLC) activity, assessed by inositol phosphate production from preincorporated myo[3H]inositol, was then determined to gain insight into differential coupling preferences among receptors and G proteins. The following were observed: (i) All receptors tested were able to stimulate PLC activity in response to agonist occupation. The effect of the M2R was pertussis toxin sensitive. (ii) While, as expected, expression of Galpha(q) facilitated an agonist-induced activation of PLC that varied widely from receptor to receptor (400% with type 2 vasopressin receptor and only 30% with M2R), expression of Galpha(16) facilitated about equally well the activation of PLC by any of the tested receptors and thus showed little if any discrimination for one receptor over another. (iii) Gbeta gamma elevated basal (agonist independent) PLC activity between 2- and 4-fold, confirming the proven ability of Gbeta gamma to stimulate PLCbeta. (iv) Activation of expressed receptors by their respective ligands in cells coexpressing excess Gbeta gamma elicited agonist stimulated PLC activities, which, in the case of the M2R, was not blocked by pertussis toxin (PTX), suggesting mediation by a PTX-insensitive PLC-stimulating Galpha subunit, presumably, but not necessarily, of the Gq family. (v) The effects of Gbeta gamma and the PTX-insensitive Galpha elicited by M2R were synergistic, suggesting the possibility that one or more forms of PLC are under conditional or dual regulation of G protein subunits such that stimulation by one sensitizes to the stimulation by the other.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Carozzi A., Camps M., Gierschik P., Parker P. J. Activation of phosphatidylinositol lipid-specific phospholipase C-beta 3 by G-protein beta gamma subunits. FEBS Lett. 1993 Jan 11;315(3):340–342. doi: 10.1016/0014-5793(93)81190-b. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Kobilka B. K., Daniel K. W., Nolan R. D., Lapetina E. Y., Caron M. G., Lefkowitz R. J., Regan J. W. Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990 Jan 5;265(1):63–69. [PubMed] [Google Scholar]
- Gudermann T., Birnbaumer M., Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992 Mar 5;267(7):4479–4488. [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Mogg A. E., Burke J. F., Caskey C. T. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987 May;13(3):253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Simon M. I. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995 Jun 23;270(25):15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- Park D., Jhon D. Y., Lee C. W., Lee K. H., Rhee S. G. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem. 1993 Mar 5;268(7):4573–4576. [PubMed] [Google Scholar]
- Rosenthal W., Seibold A., Antaramian A., Lonergan M., Arthus M. F., Hendy G. N., Birnbaumer M., Bichet D. G. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992 Sep 17;359(6392):233–235. doi: 10.1038/359233a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Wu D., Katz A., Lee C. H., Simon M. I. Activation of phospholipase C by alpha 1-adrenergic receptors is mediated by the alpha subunits of Gq family. J Biol Chem. 1992 Dec 25;267(36):25798–25802. [PubMed] [Google Scholar]
- Wu D., Katz A., Simon M. I. Activation of phospholipase C beta 2 by the alpha and beta gamma subunits of trimeric GTP-binding protein. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5297–5301. doi: 10.1073/pnas.90.11.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., LaRosa G. J., Simon M. I. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993 Jul 2;261(5117):101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- Zhu X., Gilbert S., Birnbaumer M., Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994 Sep;46(3):460–469. [PubMed] [Google Scholar]