Abstract
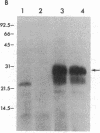
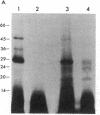
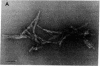
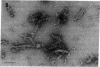
Scrapie of sheep and goats as well as Creutzfeldt-Jakob disease (CJD) of humans are neurologic disorders caused by slow infectious pathogens. The novel molecular properties of the pathogen causing scrapie have prompted introduction of the term "prion" to denote a small proteinaceous infectious particle that resists inactivation by nucleic acid-modifying procedures. Antiserum to the major hamster scrapie prion protein (PrP 27-30) was found to cross-react with murine CJD proteins. The CJD proteins had molecular weights similar to those observed for scrapie prion proteins as determined by NaDodSO4 gel electrophoresis. In addition, the CJD proteins were resistant to digestion by proteinase K and appear to polymerize into rod-shaped particles. The purification procedure developed for scrapie prions was found to be useful in purifying the CJD agent. Purification of the two infectious pathogens by virtually identical procedures provided further evidence for similarities in their molecular structures. We conclude that the molecular and biologic properties of the CJD agent are sufficiently similar to those of the scrapie prion protein that CJD should be classified as a prion disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T., Haig D. A., Clarke M. C. The exceptionally small size of the scrapie agent. Biochem Biophys Res Commun. 1966 Feb 3;22(3):278–284. doi: 10.1016/0006-291x(66)90478-5. [DOI] [PubMed] [Google Scholar]
- BECK E., DANIEL P. M., PARRY H. B. DEGENERATION OF THE CEREBELLAR AND HYPOTHALAMONEUROHYPOPHYSIAL SYSTEMS IN SHEEP WITH SCRAPIE; AND ITS RELATIONSHIP TO HUMAN SYSTEM DEGENERATIONS. Brain. 1964 Mar;87:153–176. doi: 10.1093/brain/87.1.153. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Hart M. N., Cancilla P. A. Additional evidence of spiroplasma in Creutzfeldt-Jakob disease. Lancet. 1981 Mar 21;1(8221):660–660. doi: 10.1016/s0140-6736(81)91571-3. [DOI] [PubMed] [Google Scholar]
- Bastian F. O. Spiroplasma-like inclusions in Creutzfeldt-Jakob disease. Arch Pathol Lab Med. 1979 Dec;103(13):665–669. [PubMed] [Google Scholar]
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Bockman J. M., Kingsbury D. T., McKinley M. P., Bendheim P. E., Prusiner S. B. Creutzfeldt-Jakob disease prion proteins in human brains. N Engl J Med. 1985 Jan 10;312(2):73–78. doi: 10.1056/NEJM198501103120202. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Molecular characteristics of the major scrapie prion protein. Biochemistry. 1984 Dec 4;23(25):5898–5906. doi: 10.1021/bi00320a002. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Diener T. O., McKinley M. P., Prusiner S. B. Viroids and prions. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5220–5224. doi: 10.1073/pnas.79.17.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Kennedy R. C., Hadlow W. J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967 Feb;117(1):15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- Field E. J., Narang H. K. An electron-microscopic study of scrapie in the rat: further observations on "inclusion bodies" and virus-like particles. J Neurol Sci. 1972 Nov;17(3):347–364. doi: 10.1016/0022-510x(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Field E. J., Raine C. S., Joyce G. Scrapie in the rat: an electron-microscopic study. II. Glial inclusions. Acta Neuropathol. 1967 Nov 29;9(4):305–315. doi: 10.1007/BF01371190. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C., Gibbs C. J., Jr, Asher D. M., Brown P., Diwan A., Hoffman P., Nemo G., Rohwer R., White L. Precautions in medical care of, and in handling materials from, patients with transmissible virus dementia (Creutzfeldt-Jakob disease). N Engl J Med. 1977 Dec 8;297(23):1253–1258. doi: 10.1056/NEJM197712082972304. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C., Latarjet R. Unusual resistance to ionizing radiation of the viruses of kuru, Creutzfeldt-Jakob disease, and scrapie. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6268–6270. doi: 10.1073/pnas.75.12.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C. Transmission of scrapie to the cynomolgus monkey (Macaca fascicularis). Nature. 1972 Mar 10;236(5341):73–74. doi: 10.1038/236073a0. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Prusiner S. B., Kennedy R. C., Race R. E. Brain tissue from persons dying of Creutzfeldt-Jakob disease causes scrapie-like encephalopathy in goats. Ann Neurol. 1980 Dec;8(6):628–632. doi: 10.1002/ana.410080615. [DOI] [PubMed] [Google Scholar]
- Hartsough G. R., Burger D. Encephalopathy of mink. I. Epizootiologic and clinical observations. J Infect Dis. 1965 Oct;115(4):387–392. doi: 10.1093/infdis/115.4.387. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
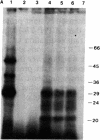
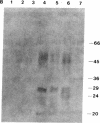
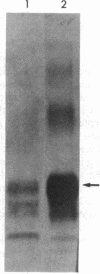
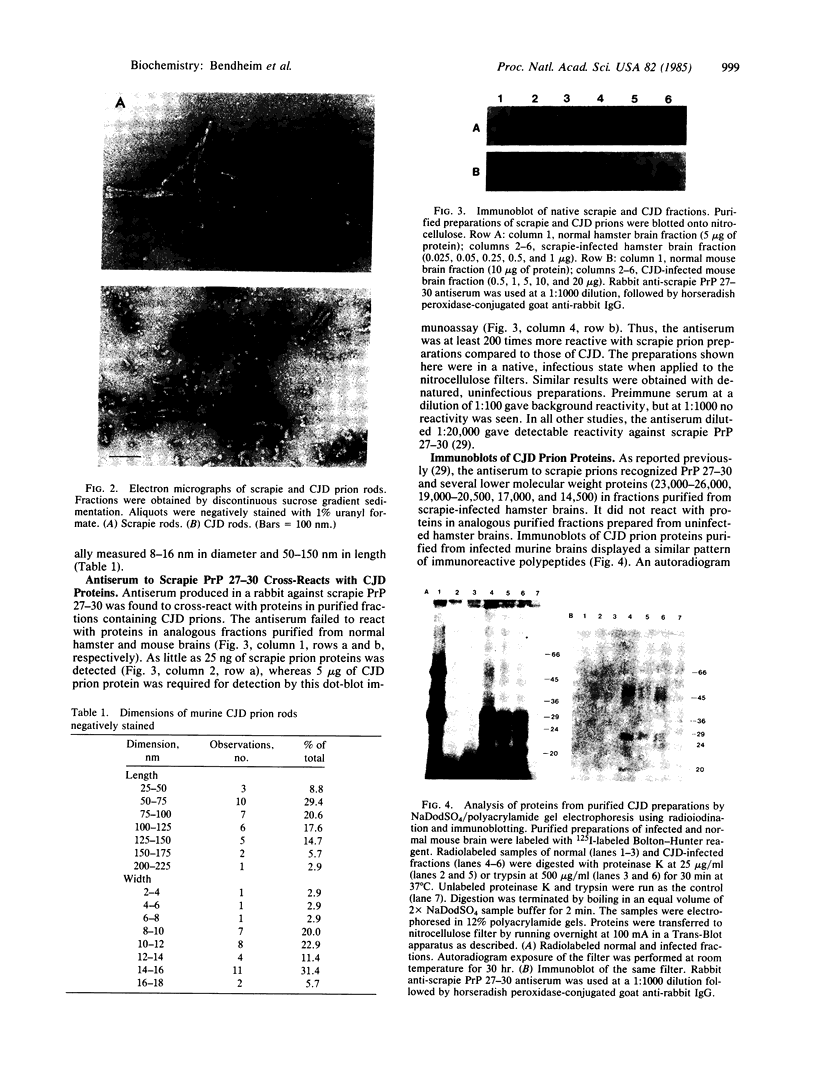
- Kingsbury D. T., Kasper K. C., Stites D. P., Watson J. D., Hogan R. N., Prusiner S. B. Genetic control of scrapie and Creutzfeldt-Jakob disease in mice. J Immunol. 1983 Jul;131(1):491–496. [PubMed] [Google Scholar]
- Kingsbury D. T., Smeltzer D. A., Amyx H. L., Gibbs C. J., Jr, Gajdusek D. C. Evidence for an unconventional virus in mouse-adapted Creutzfeldt-Jakob disease. Infect Immun. 1982 Sep;37(3):1050–1053. doi: 10.1128/iai.37.3.1050-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Smeltzer D. A., Gibbs C. J., Jr, Gajdusek D. C. Evidence for normal cell-mediated immunity in scrapie-infected mice. Infect Immun. 1981 Jun;32(3):1176–1180. doi: 10.1128/iai.32.3.1176-1180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Kimberlin R. H. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Raff M. C., Simpson E., Nehlsen S. H. Scrapie in immunologically deficient mice. Nature. 1971 Oct 1;233(5318):336–336. doi: 10.1038/233336a0. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Narang H. K. An electron microscopic study of natural scrapie sheep brain: further observations on virus-like particles and paramyxovirus-like tubules. Acta Neuropathol. 1974;28(4):317–329. doi: 10.1007/BF00685286. [DOI] [PubMed] [Google Scholar]
- Narang H. K. An electron microscopic study of the scrapie mouse and rat: further observations on virus-like particles with ruthenium red and lanthanum nitrate as a possible trace and negative stain. Neurobiology. 1974;4(6):349–363. [PubMed] [Google Scholar]
- Narang H. K. Ruthenium red and lanthanum nitrate a possible tracer and negative stain for scrapie "particles"? Acta Neuropathol. 1974;29(1):37–43. doi: 10.1007/BF00684389. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., McKinley M. P., Cochran S. P., Bowman K. A., Kasper K. C. Thiocyanate and hydroxyl ions inactivate the scrapie agent. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4606–4610. doi: 10.1073/pnas.78.7.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Hadlow W. J., Eklund C. M., Race R. E., Cochran S. P. Sedimentation characteristics of the scrapie agent from murine spleen and brain. Biochemistry. 1978 Nov 14;17(23):4987–4992. doi: 10.1021/bi00616a020. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Field E. J. Orientated tubules in axoplasm of cerebellar myelinated nerve fibres in the rat. A study of normal and scrapie animals. Acta Neuropathol. 1967 Nov 6;9(3):298–304. doi: 10.1007/BF00687939. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Koga M., Sato Y., Mori R. Properties of the transmissible agent derived from chronic spongiform encephalopathy. Ann Neurol. 1980 Apr;7(4):390–391. doi: 10.1002/ana.410070424. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Nagara H., Hikita K., Sato Y. Amyloid plaques in the brains of mice with Creutzfeldt-Jakob disease. Ann Neurol. 1984 Mar;15(3):278–280. doi: 10.1002/ana.410150313. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. S., Inderlied C. B., Kingsbury D. T. Conditions for the chemical and physical inactivation of the K. Fu. strain of the agent of Creutzfeldt-Jakob disease. Am J Public Health. 1983 Jun;73(6):661–665. doi: 10.2105/ajph.73.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. S., Young S. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis. 1982 Oct;18(4):465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]