Abstract
Purpose: Respiratory motion and partial-volume effects are the two main sources of image degradation in whole-body PET imaging. Simultaneous PET-MR allows measurement of respiratory motion using MRI while collecting PET events. Improved PET images may be obtained by modeling respiratory motion and point spread function (PSF) within the PET iterative reconstruction process. In this study, the authors assessed the relative impact of PSF modeling and MR-based respiratory motion correction in phantoms and patient studies using a whole-body PET-MR scanner.
Methods: An asymmetric exponential PSF model accounting for radially varying and axial detector blurring effects was obtained from point source acquisitions performed in the PET-MR scanner. A dedicated MRI acquisition protocol using single-slice steady state free-precession MR acquisitions interleaved with pencil-beam navigator echoes was developed to track respiratory motion during PET-MR studies. An iterative ordinary Poisson fully 3D OSEM PET reconstruction algorithm modeling all the physical effects of the acquisition (attenuation, scatters, random events, detectors efficiencies, PSF), as well as MR-based nonrigid respiratory deformations of tissues (in both emission and attenuation maps) was developed. Phantom and 18F-FDG PET-MR patient studies were performed to evaluate the proposed quantitative PET-MR methods.
Results: The phantom experiment results showed that PSF modeling significantly improved contrast recovery while limiting noise propagation in the reconstruction process. In patients with soft-tissue static lesions, PSF modeling improved lesion contrast by 19.7%–109%, enhancing the detectability and assessment of small tumor foci. In a patient study with small moving hepatic lesions, the proposed reconstruction technique improved lesion contrast by 54.4%–98.1% and reduced apparent lesion size by 21.8%–34.2%. Improvements were particularly important for the smallest lesion undergoing large motion at the lung-liver interface. Heterogeneous tumor structures delineation was substantially improved. Enhancements offered by PSF modeling were more important when correcting for motion at the same time.
Conclusions: The results suggest that the proposed quantitative PET-MR methods can significantly enhance the performance of tumor diagnosis and staging as compared to conventional methods. This approach may enable utilization of the full potential of the scanner in oncologic studies of both the lower abdomen, with moving lesions, as well as other parts of the body unaffected by motion.
Keywords: motion correction, PSF modeling, oncologic PET-MRI
INTRODUCTION
Respiratory motion is the main cause of image degradation in abdominal PET imaging. While the intrinsic resolution of PET scanners is in the range of ∼4–5 mm full-width at half maximum (FWHM), the effective spatial resolution of PET images is reduced to 6–10 mm in abdominal imaging studies due to motion.1 The lower abdomen, where diaphragmatic excursions range from 4 to 40 mm,2 is affected by respiratory motion the most. This translates into dramatically hampered lesion detectability3, 4 and large errors in measured lesion uptake.5 In addition to blurring, motion creates inconsistencies between emission and attenuation data which generate confounding artifacts at the lung-liver interface6, 7 and introduce further biases in measured radiotracer concentration.8
Respiratory motion blurring may be alleviated using gating.9 To do so, the patient's respiratory cycle is recorded and divided into a fixed number of frames (gates). The events detected within each gate are reconstructed into separate image volumes in which the motion is assumed to be negligible. However, because of the reduced number of counts (typically 1/8 of the total number of detected events), each gated volume has dramatically reduced signal-to-noise ratio.
Motion correction methods may be used to overcome the limitations of gating. The motion correction can be performed postreconstruction by transforming each gated volume to a reference gate and summing all the registered gates. The motion transformation can be obtained using nonrigid registration of the gated PET volumes themselves,10 or using motion information from another modality such as CT (Refs. 11 and 12) or MRI.13, 14, 15, 16, 17, 18 Another approach is to incorporate organ motion directly into the PET reconstruction to reconstruct an image without motion blurring in a single reconstruction process. For maximum-likelihood image reconstruction algorithms, the latter approach has been shown to provide images with superior quantitative accuracy and better bias-variance tradeoff as compared to the postreconstruction approach.19, 20
Once the patient's respiratory motion is compensated for, the Partial Volume Effects (PVEs), resulting from the scanner's limited Point Spread Function (PSF), becomes a major source of image spatial resolution degradation. PVEs cause signal to spread from a target tissue into neighboring tissues (“spill-out”) and from those neighboring tissues into the target tissue (“spill-in”), which significantly hampers the quantitative accuracy of the PET images. For lesions smaller than the reconstructed spatial resolution, PVEs may result in more than 50% underestimation of the true radiotracer concentration.21, 22 PVEs are caused by several physical effects including positron range, photon noncollinearity, parallax, and intercrystal scattering. Iterative PET reconstruction algorithms allow modeling these components in the system matrix to significantly enhance image spatial resolution and contrast recovery. PSF models can be derived from Monte Carlo simulations,23 analytical calculation,24 and physical measurements with point or line sources25, 26, 27, 28 or positron beams in combined PET-MR scanners.29 The obtained PSF model can then be incorporated into the PET system matrix as a blurring kernel acting either in the image25, 28 or data (i.e., sinogram) space.23, 26, 27 The latter approach is usually preferred since the main distorting effects occur in the projection space.
Simultaneous PET-MR imaging is a novel, powerful hybrid modality capable of combining the molecular and physiologic information from PET together with the high-resolution anatomical information provided by MRI. PET-MR is increasingly used in the clinic and shows promising results in oncologic applications.30 Because of the superior soft-tissue contrast of MRI compared to CT and the absence of ionizing radiation, it is believed that PET-MR has the potential to outperform PET-CT in certain applications. Moreover, the simultaneous acquisition of PET and MR data enables the use of MRI-derived information, e.g., MR-based motion information, to help improve PET images. Taking advantage of that synergy, MR-based PET motion correction has become an area of active research in recent years. Our group has previously reported on motion correction using deformable phantom and animal data acquired on a prototype MR-compatible brain PET scanner.13, 14, 15 In a recent study, Würslin and colleagues16 reported a MR-based motion correction method in in vivo abdominal studies. Significant improvements in lesion contrast were reported but the motion correction was applied postreconstruction, leading to possibly suboptimal performance. Also, none of the aforementioned studies addressed the limitations caused by PVEs.
In this work, we assessed the relative role of MR-based motion correction and PSF modeling in whole-body PET-MR imaging using the Siemens Biograph mMR scanner. First, we developed robust MRI acquisition and processing methods allowing the measurement of respiratory motion during PET acquisition. To reduce PVEs, we measured the PSF of the scanner and obtained a model of radially varying detector blurring effects in both radial and axial directions. Then, we developed an iterative OP (ordinary Poisson) fully 3D OSEM PET reconstruction algorithm, modeling all the physical effects of the acquisition (attenuation, scatter, random events, detector efficiencies, PSF), and incorporating the MR-based nonrigid respiratory deformation of tissues (in both emission and attenuation maps). In this manner, all resolution-degrading effects were incorporated within one unified maximum likelihood iterative reconstruction framework, allowing significant enhancement of PET image quality and quantitative accuracy. The impact of the proposed quantitative PET-MR methods was evaluated using phantom and 18F-FDG patient studies with static and moving lesions. To our knowledge, this is the first report of modeling PSF and patient motion for the simultaneous whole-body PET-MR scanner.
MATERIALS AND METHODS
Siemens Biograph mMR scanner
All acquisitions were performed on a whole-body simultaneous PET-MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). The MR system is composed of a 3T magnet (length, 163 cm; bore, 60 cm), a whole-body gradient coil system and a radiofrequency (RF) body coil. The PET camera is installed between the gradient and the RF coil and operates in fully 3D mode. The PET system is composed of eight rings of 56 detector blocks made of 8 × 8 lutetium oxyorthosilicate crystals (4 × 4 × 20 mm). The PET imaging field of view (FOV) is 59.4 and 25.8 cm in transaxial and axial directions, respectively. The PET transaxial spatial resolution varies across the FOV from 4.3 to 5.0 mm FWHM at 1 and 10 cm off the center of FOV, respectively.31
MR-based respiratory motion measurement
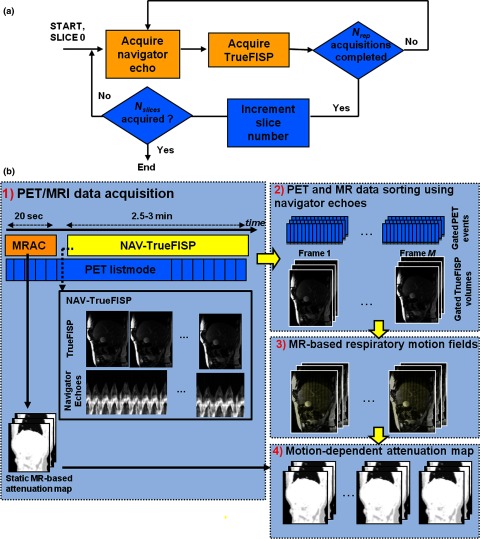
In previous studies, we used tagged MRI to measure motion for MR-based PET motion correction.13, 14, 15 However, MR tagging is impractical for respiratory motion estimation in human studies because of tag lines fading, especially in the liver due to its relatively short T1 (∼800 ms at 3T) compared to typical human respiratory cycle (∼5 s). To address these limitations, we developed a dedicated navigated TrueFISP MRI sequence (“NAV-TrueFISP”) to measure respiratory motion. The NAV-TrueFISP sequence is composed of single-slice steady-state free precession MRI acquisitions (TrueFISP) interleaved by pencil-beam navigator echoes [Fig. 1a]. The navigators are used to track the lung-liver interface during the respiratory cycle and are collected prior to each slice acquisition to monitor the respiratory phase of that slice. The obtained internal motion surrogates allow accurate monitoring of the respiratory cycle (binning of both PET and MRI data into respiratory phases) and handling of respiratory cycle irregularities. To cover a maximum of respiratory positions for each slice, each TrueFISP acquisition is repeated Nrep times. After a given slice has been acquired Nrep times, a different slice is acquired with the same procedure, and so on until the whole volume of Nslices has been covered.
Figure 1.
PET and MR data acquisition and processing chain. (a) NAV-TrueFISP sequence. (b) Data acquisition and processing.
The PET and MRI data acquisition and processing chain is illustrated in Fig. 1b. First, an attenuation map is acquired using the vendor's supplied MR-based attenuation correction sequence (“MRAC”). The PET list-mode acquisition starts simultaneously with the MRAC protocol. The NAV-TrueFISP acquisition protocol starts right after MRAC completion to track the respiratory motion of tissues. MRI slices and simultaneously acquired PET events are binned into M respiratory phases based on the navigator amplitude. The gated MR volumes are then used to compute respiratory motion fields. A dynamic attenuation map is obtained by transforming the static attenuation map into each frame using the estimated motion fields. Finally, the iterative reconstruction algorithm (denoted “MC-PSF-OP-OSEM”), which incorporates motion correction, PSF modeling, and dynamic attenuation correction factors, is used to reconstruct the collected PET list-mode data.
Respiratory motion estimation
Respiratory motion fields were estimated using B-spline nonrigid registration of gated TrueFISP volumes with minimization of sum of squared differences for enforcing data consistency and constraint term for motion field regularization.32 Noting f(t, x), a MR volume at a given respiratory phase t, the registration algorithm searches for an optimal 3D cubic B-spline motion field between a pair of volumes f(t, x) and f(t′, x) such that
| (1) |
where x is the voxel position, N is the total number of voxels in one volume, γ is a regularization parameter, and R(·) is a regularizer. The regularization term penalizes the differences between adjacent B-spline coefficients and imposes local invertibility. A bi-level multiresolution strategy was used to increase the robustness and speed of the registration algorithm.33 Temporally adjacent volumes were successively registered and B-spline interpolation was used to estimate the motion between each frame and the reference frame. End-exhalation (EE), the most reproducible respiratory phase, was used as the reference phase.
Biograph mMR scanner PSF modeling
Despite the fully 3D list-mode (span 1) capability of the Biograph mMR, correction sinograms, such as the detectors normalization file, are stored after axial compression (span 11) which makes it necessary to develop a reconstruction and resolution model matching with this format. In span 11, the mMR PET sinograms contain 344 radial bins and 252 azimuthal angle bins with 837 projection planes. In what follows, a sinogram is noted as a four-dimensional object S(ρ, φ, z, θ) where variable ρ denotes the radial sinogram bins, φ the azimuthal angle bins, z the distance at which the sinogram intersects the scanner's axis, and θ the axial tilt of the sinogram.
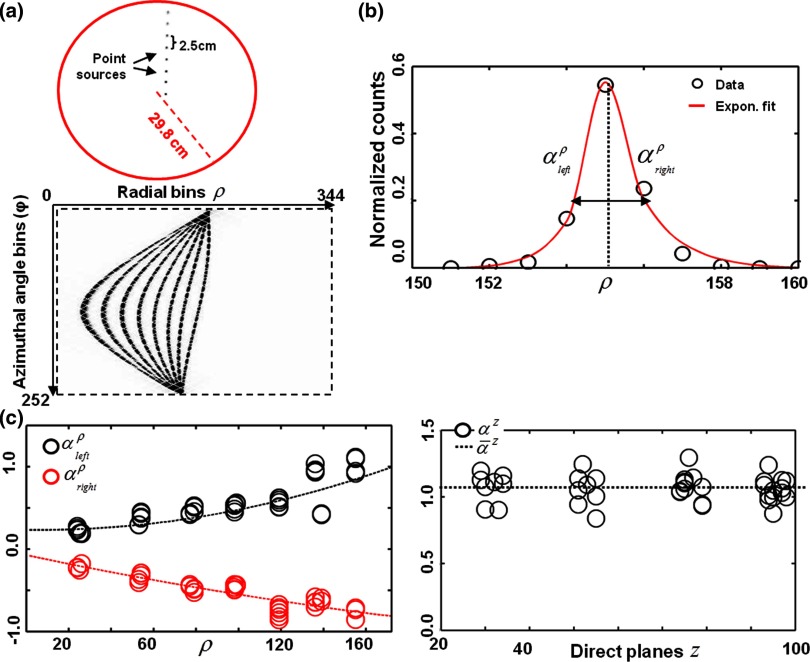
A set of eight submillimeter 18F point sources covering the FOV over a radial distance of 24.8 cm with uniform 2.5 cm spacing was used to measure the scanner's PSF [Fig. 2a, top row]. The eight point sources were acquired at four different axial positions. The bed stayed outside of the FOV for all measurements to avoid scattering. List-mode data containing about 20 × 106 counts per axial position were collected. List-mode data were rebinned into span 11 sinograms and corrected for detectors sensitivity [Fig. 2a, bottom row]. For each point source, axial and radial sinogram profiles through the point source peak value were extracted. The PSF was assumed to be independent of azimuthal angle and axial tilt and was measured at a fixed azimuthal angle in the direct planes only (θ = 0°). All extracted point source profiles were fitted to the separable exponential model
| (2) |
where ρ0 and z0 are, respectively, the radial and axial locations of each point source and A• is a normalization factor. An asymmetric kernel, with slope parameters and , was chosen to model the radial component while symmetric response was assumed in the axial direction. An example of a radial fit is shown in Fig. 2b. Quadratic functions were fitted to the measured and to obtain kernels at any radial location [Fig. 2c] assuming a symmetric system response in each half of the FOV. In the axial direction, a shift-invariant axial blurring parameter was obtained by averaging the measured axial width parameters. The obtained radially varying PSF kernels were incorporated into the PET reconstruction as detailed in Sec. 2E.
Figure 2.
PSF modeling in the Siemens Biograph mMR. (a) Point source measurement setup and obtained point source sinogram data. (b) Measured radial PSF with fitted asymmetric exponential kernel. (c) PSF kernel parameters along radial and axial directions. A quadratic function (dashed line) was fitted to the measured radial parameters to obtain a PSF kernel at each radial bin. A shift-invariant PSF was chosen in the axial direction.
PET reconstruction
An iterative OP fully 3D OSEM reconstruction algorithm, incorporating all the physical effects (attenuation, scatter, random events, detectors efficiencies, PSF) and MR-based nonrigid respiratory deformation of tissues (in both emission and attenuation maps), was specifically developed for the mMR scanner.
The coincidences detected during the acquisition follow a Poisson distribution with mean34
| (3) |
where y* = [y1, …, yM]T contains the column vectors sinograms yt of size I (number of sinogram bins) containing the events rebinned into M motion frames using the MR navigators, f* = [f, …, f]T contains the column vectors f of size J (number of voxels) containing the voxels’ radiotracer concentration at end-exhalation that are to be estimated, and contain, respectively, the estimated scattered and random coincidences that contribute to the expected data in each motion frame, and P* = diag[P1, …, PM] contains the motion-dependent system matrices P• of size I × J. To simplify, the scatter and random events distributions were assumed to be independent of respiratory motion so that and where and are the scatters and random events distribution estimates throughout the entire acquisition, regardless of motion.
The motion-dependent system matrix Pt, whose elements contain the probability of a photon emitted in voxel j and motion-frame t being detected in LOR i, is decomposed as
| (4) |
where Mt is the MR-based nonrigid warping operator that registers a given phase t to the reference phase, G is the forward-projection operator, At is a diagonal matrix containing the LOR attenuation correction factors for each frame t, B models the two-dimensional PSF blurring effects in the projection space, and N is the diagonal matrix of the detector normalization factors. In all of our reconstructions, At included the attenuation effects of scanner's hardware and the object. The attenuation effects of the MRI surface coils were neglected in this study.
The MC-PSF-OP-OSEM algorithm updating loop is then given by
| (5) |
where Dt is the relative duration of frame t and 1I is a column vector of size I with all ones. Angular subsets were used to accelerate the convergence of the algorithm. Scattered coincidences’ distributions were estimated using a three-dimensional version of the single scatter simulation algorithm.35 Random coincidences’ distributions were obtained using a smoothed delayed coincidence window. Since each motion frame contributes independently from other frames to each subset in the updating loop, the reconstruction was parallelized using multithreading.
PET-MR studies
Phantom and patient studies were performed to evaluate the proposed approach. To remove any bias from motion, the impact of PSF modeling was assessed with a resolution phantom and a small cohort of patients with nonmoving soft-tissue lesions. The impact of motion correction and PSF modeling was investigated in one patient study with hepatic lesions. All patient studies were approved by the Institutional Review Board at the Massachusetts General Hospital and all subjects signed a written informed consent form.
ACR phantom
An ACR phantom (Data Spectrum Corporation, Hillsborough, NC) filled with 148 MBq of 18F was placed at the center of the scanner and scanned for 20 min in the mMR. The phantom included four hot cylinders of various diameters embedded in a warm background (activity to background ratio = 4:1). Five independent 4 min noise realizations were obtained from the acquired list-mode data. Due to the absence of MR signal from the plastic structures of the phantom, a separate CT scan, acquired afterwards on a Biograph 64 PET-CT scanner (Siemens Healthcare, Erlangen, Germany), was used to generate the attenuation map.
Soft-tissue sarcoma patients
One 61-year-old male and one 60-year-old female (subjects 1 and 2) with soft-tissue lesions in arm and the thigh, respectively, were selected to participate in the study. Subject 2 was scanned twice, before and after surgical intervention. For each study, list-mode PET data were acquired for 10 min, 1 h after injection of 307 and 363 MBq of 18F-FDG, for subjects 1 and 2, respectively. Conventional T1 weighted MRI was also performed (TE = 23 ms, TR = 769 ms, FA = 120°, FOV 450 × 450 mm, and resolution of 0.88 × 0.88 × 3 mm3). Attenuation maps (four tissue classes: air, lung, fat, nonfat soft-tissue) were obtained using the dedicated MR-based attenuation protocol (MRAC) provided by the manufacturer (∼20 s acquisition).
Liver patient study
A 79 year old male subject (subject 3) with known liver lesions (n = 4) was enrolled to participate to the study. Subject 3 was scanned on the mMR ∼2 h after a routine PET-CT examination, for which 592 MBq of 18F-FDG was injected. The subject was first asked to hold his breath at end-exhalation during the 20-s MRAC acquisition. Following MRAC protocol acquisition, Nslices = 30 sagittal slices covering the lower abdomen were acquired using the NAV-TrueFISP sequence [see Fig. 1a] with the following acquisition parameters: TE = 1.67 ms, TR = 3.20 ms, FA = 51°, FOV 399 × 199 mm [feet-head (FH) × anterior-posterior (AP)], spatial resolution of 2 × 2 × 8 mm3 [FH × AP × right-left (RL)]. Each slice acquisition was repeated Nrep = 30 times to ensure proper coverage of the breathing cycle. The pencil-beam navigator was positioned in the dome of the liver and oriented in the FH direction. The total NAV-TrueFISP acquisition time was less than 3 min. PET list-mode events were simultaneously collected for 3.5 min. Only the PET events acquired simultaneously with the NAV-TrueFISP acquisition were corrected for respiratory motion. Postcontrast coronal T1-weighted 3D FLASH MRI (TE = 1.2 ms, TR = 3.51 ms, FA = 12°, FOV 344 × 400 mm [LR × FH], spatial resolution of 1.4 × 1.4 × 2.5 mm3 [RL × FH × AP]) was acquired 30 min after an injection of 0.2 mmol l−1 per kg of MR contrast agent (Gadolinium) during multiple breath-holds.
Image reconstruction and figures of merit
Based on the reconstruction framework previously described, four different reconstruction algorithms were implemented:
OP-OSEM: conventional sinogram-based reconstruction with attenuation and detector efficiencies correction.
PSF-OP-OSEM: OP-OSEM with PSF modeling.
MC-OP-OSEM: OP-OSEM with MR-based respiratory motion correction.
MC-PSF-OP-OSEM: OP-OSEM with both motion correction and PSF modeling.
The reconstruction algorithms were implemented in C++ on our cluster equipped with four 6-core Intel Xeon X5690 CPUs with 3.46 GHz clock speed and 64 GB of memory. The ACR phantom and sarcoma patient datasets were reconstructed using OP-OSEM and PSF-OP-OSEM. Subject 3's liver scan was reconstructed using all four reconstruction methods with M = 7 respiratory phases. Additionally, a respiratory gated volume—denoted “OP-OSEM Gated”—was reconstructed using the PET counts detected at EE (∼1/7th of the total number of acquired counts), based on the navigator-derived respiratory phase information obtained during the NAV-TrueFISP acquisition. The gated volume, albeit with dramatically reduced statistics, was treated as the “motion-free” reference standard of this study. To avoid the propagation of emission/attenuation artifacts in the motion corrected PET volumes, the spatial agreement match between the MRAC attenuation map and the reference EE TrueFISP MR volume was checked prior to reconstruction. All phantom and patient reconstructions were performed on an isotropic 2 × 2 × 2 mm3 voxel grid.
The performance of PSF modeling was analyzed by measuring cylinders’ contrast recovery and image noise using the reconstructed ACR phantom images. For each noise realization n, the contrast recovery coefficient in each cylinder was computed using
| (6) |
where Sn and Bn are, respectively, the average activity concentration in the cylinder and in a nearby large homogenous background region, and c is the true cylinder contrast (4:1). The mean CRC over all noise realizations () for each cylinder was used as the metric for quantitative assessment. Statistical background noise was computed using
| (7) |
where is the average background activity concentration over all noise realizations and NR is the number of noise realizations.
In the patient studies, image quality was quantitatively assessed by computing the target-to-background ratio (TBR) of each lesion. TBR was computed as the ratio between the maximal uptake in a region of interest including the lesion and the mean uptake in a volume of interest in an adjacent homogeneous region. In subject 3's study, lesion apparent size was computed as the FWHM obtained after fitting a Gaussian to a profile crossing the tumor peak value and oriented in the direction of maximal motion (head-to-feet).
RESULTS
ACR phantom study
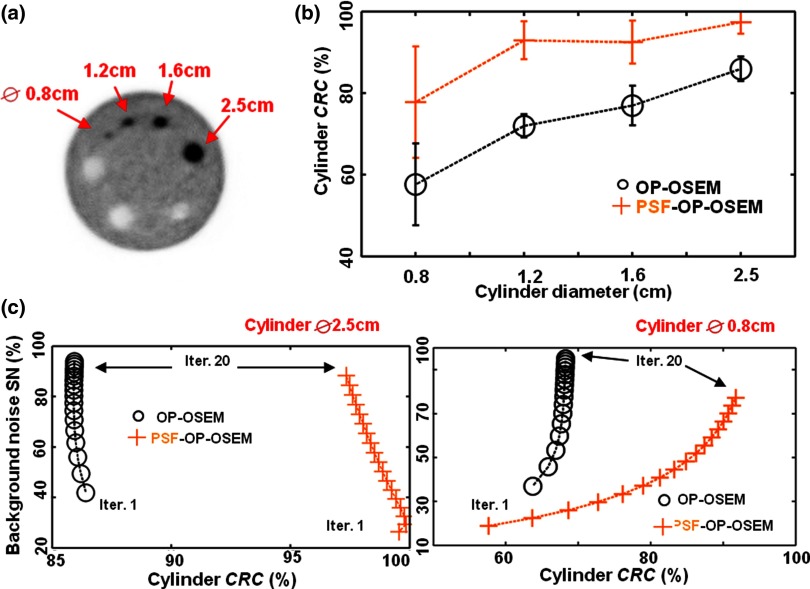
The results of the ACR phantom study are shown in Fig. 3. Figure 3b shows the cylinder contrast recovery coefficient as a function of the cylinder diameter for OP-OSEM and PSF-OP-OSEM at a fixed number of iterations (20 iterations, 21 subsets). Superior contrast recovery is achieved using PSF-OP-OSEM for all cylinder sizes. Figure 3c illustrates the trade-off between background noise and contrast recovery for the smallest and largest cylinder (Ø 0.8 and 2.5 cm). As expected, slower convergence is observed for smaller cylinders. Convergence is also slower for PSF-OP-OSEM as compared to OP-OSEM. At a fixed level of noise, PSF-OP-OSEM significantly improves contrast recovery (13.3%–35.0%) and reaches levels that cannot be achieved with OP-OSEM.
Figure 3.
ACR phantom reconstructions using OP-OSEM and PSF-OP-OSEM. (a) ACR phantom reconstructed PET image. (b) Cylinders contrast recovery plotted against cylinder size. (c) Contrast recovery coefficients in two cylinders plotted against background statistical noise for each image reconstruction iteration.
Sarcoma patient studies
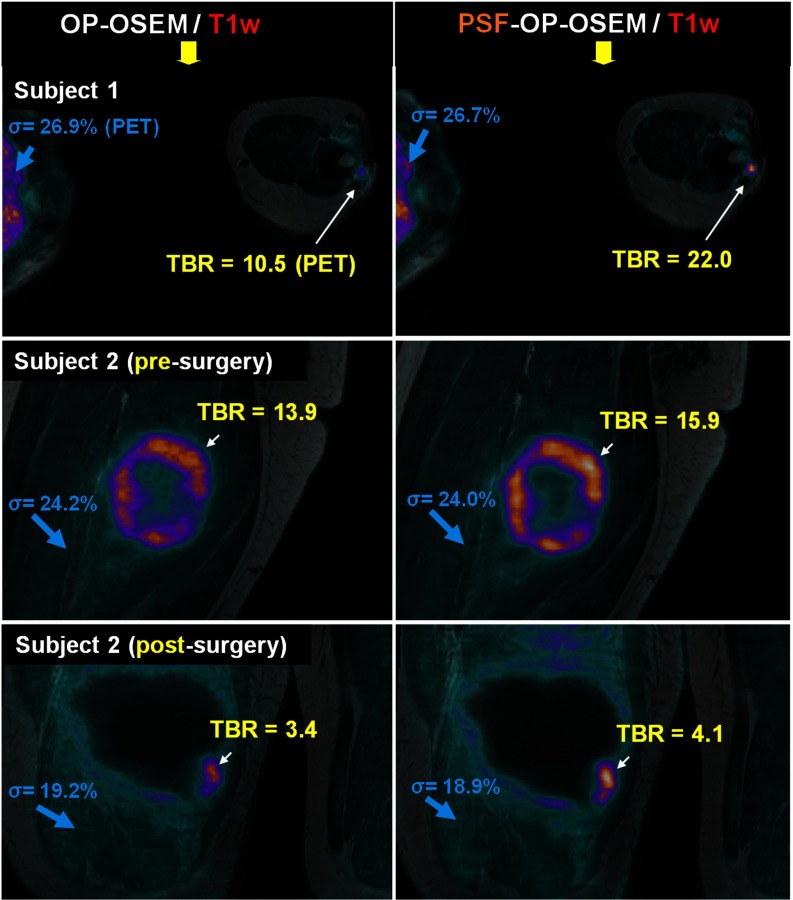
Sarcoma patient datasets were reconstructed using OP-OSEM and PSF-OP-OSEM. Figure 4 shows the reconstructed PET images in transaxial (subject 1) and coronal orientations (subject 2) fused with the coregistered T1-weighted MR images. The iteration number was selected for each reconstruction algorithm so that the displayed images depict similar level of background noise. In all studies, PSF-OP-OSEM substantially enhanced lesion contrast as compared to OP-OSEM, with improvements in the range of 19.7%–109%. Lesion contrast improvement was particularly important for the small focal lesion in subject 1's left arm, due to its small size and location toward the edges of the FOV. PSF-OP-OSEM yielded sharper lesion boundaries and more uniform uptake in the metabolically active part of the tumor, as illustrated in subject 2's presurgery reconstructions.
Figure 4.
Soft-tissue sarcoma patient studies reconstructed with OP-OSEM and PSF-OP-OSEM fused with the corresponding T1-weighted MR image. The chosen slices intercept subject 1's arm (top row) and subject 2's thigh, before (middle row) and after surgical intervention (last row).
Liver patient study
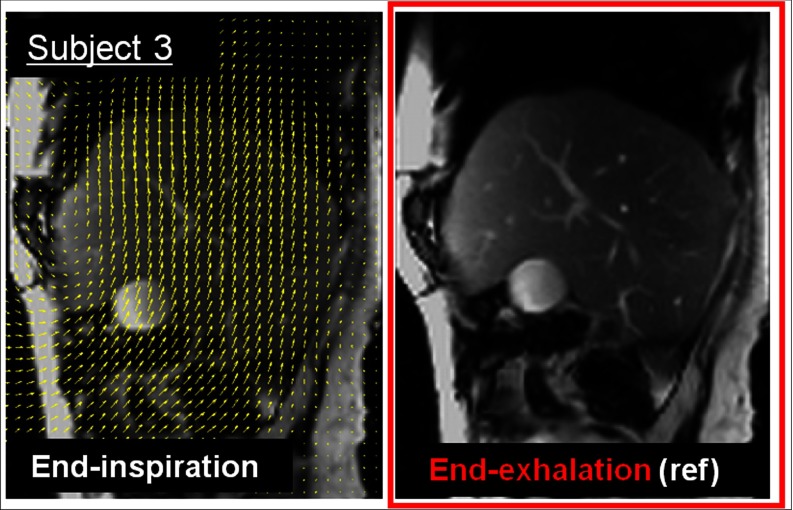
The acquired liver PET-MR dataset was processed according to the MR-based motion-correction methodology described above. Figure 5 shows the same sagittal TrueFISP slice acquired in subject 3 at end-exhalation and end-inspiration phases along with superimposed motion field (yellow arrows). The arrows show the complex movement of abdominal tissues (e.g., liver) which move upward as air is expelled from the lungs during exhalation. TrueFISP's excellent soft-tissue contrast allows many anatomic structures (e.g., liver vascular tree) to act as landmarks within the homogenous liver tissue to facilitate the task of the nonrigid registration.
Figure 5.
Respiratory motion estimation in subject 3 with hepatic lesions using NAV-TrueFISP MRI data. The chosen sagittal TrueFISP slice intercepting subject 3's liver is shown at end-inspiration and end-exhalation motion phases. The respiratory motion field estimated between the two respiratory phases is superimposed on the end-inspiration image (arrows).
The computation times for one iteration of each reconstruction algorithm are summarized in Table 1. MC-OP-OSEM execution time is close to OP-OSEM′ as the contribution from each motion frame to each image update was calculated in parallel using multithreading [see Eq. 5]. The combination of PSF and motion correction (MC-PSF-OP-OSEM) substantially increases reconstruction times as compared to OP-OSEM. Execution times of the current multithreaded CPU implementation could be dramatically reduced using GPUs.36
Table 1.
Computation times for one iteration of OP-OSEM, MC-OP-OSEM, PSF-OP-OSEM, and MC-PSF-OP-OSEM reconstruction algorithms for Biograph mMR.
| Reconstruction algorithm | Computation time (min) |
|---|---|
| OP-OSEM | 42 |
| PSF-OP-OSEM | 43 |
| MC-OP-OSEM | 45 |
| MC-PSF-OP-OSEM | 62 |
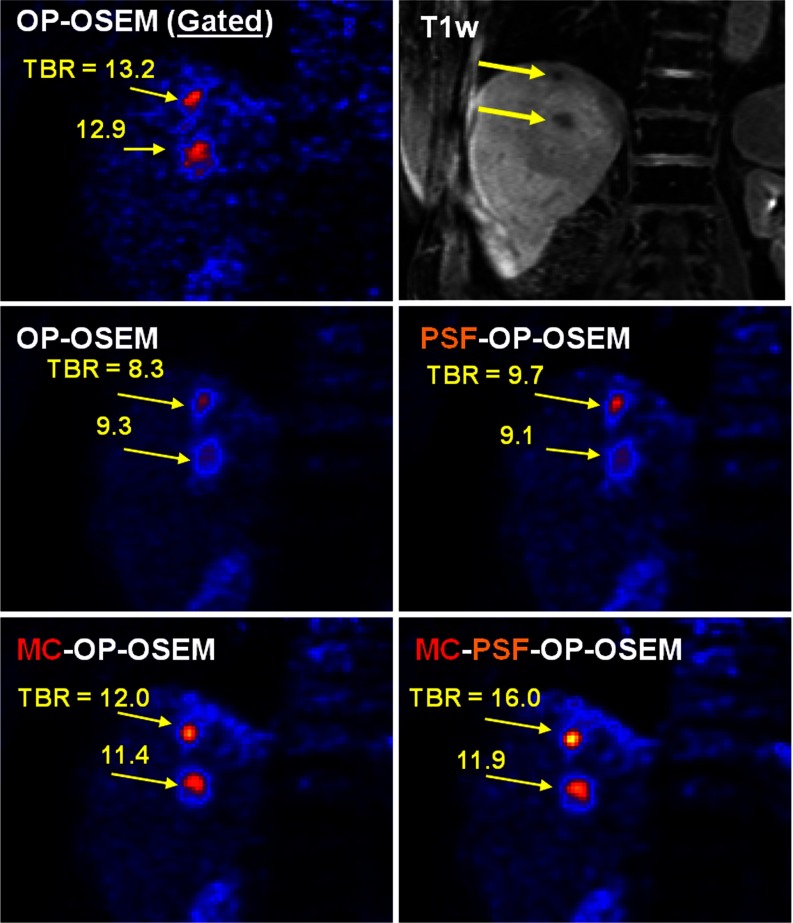
Figure 6 shows the same coronal PET slice from subject 3 reconstructed using all four reconstruction methods together with the corresponding postcontrast T1-weighted MRI slice (top row, right column). A 4-mm FWHM Gaussian smoothing filter was applied for display. The chosen slice intersects lesions 1 and 2 located near the dome of the liver. The characteristic “comet-like” lesions shape in the OP-OSEM images confirms significant respiratory motion blurring in the craniocaudal direction. Gating removed motion blurring but also significantly increased image noise. The proposed MR-based motion correction technique considerably reduced motion blurring and significantly improved lesion contrast as compared to without motion correction. When both PSF modeling and motion correction were included, further improvement was observed on lesions’ contrasts, which is particularly evident in lesion 1 in the uppermost region of the liver.
Figure 6.
Same PET and MRI coronal slice from subject 3 obtained using different reconstruction methods. Lesions 1 and 2, located in the dome of the liver, can be identified on that slice. The proposed methods significantly improved the contrast of the two lesions as compared to OP-OSEM.
Semiquantitative measurements (TBR and FWHM) were performed on three of subject 3's liver lesions and the results obtained are shown in Tables 2, 3. The proposed MR-based motion correction technique significantly increased TBR as compared to no motion correction, with improvements in the range of 21.6%–44.6% for MC-OP-OSEM vs OP-OSEM. Likewise, apparent tumors sizes were reduced by 12.5%–29.4% when the motion correction was employed. PSF modeling yielded little improvement when no motion correction was applied, with enhancements of −3.0% to 17.8% for TBR and 0.1%–1.8% FWHM reduction for PSF-OP-OSEM vs OP-OSEM. The effects of PSF modeling were more significant when correcting for motion at the same time: 4.8%–33.4% for TBR and 4.65–10.7% FWHM reduction for MC-PSF-OP-OSEM vs MC-OP-OSEM. As compared to OP-OSEM, MC-PSF-OP-OSEM yielded a 27.5%–93.0% increase for TBR and 21.8%–34.2% FWHM reduction.
Table 2.
Lesion contrasts (TBR) in subject 3.
| OP-OSEM | OP-OSEM (gated) | PSF-OP-OSEM | MC-OP-OSEM | PSF-MC-OP-OSEM | |
|---|---|---|---|---|---|
| Lesion 1 | 8.26 | 13.2 | 9.74 | 11.96 | 15.96 |
| Lesion 2 | 9.34 | 12.88 | 9.10 | 11.36 | 11.91 |
| Lesion 3 | 8.52 | 12.8 | 9.74 | 12.35 | 13.42 |
Table 3.
Lesions FWHM (mm) in subject 3.
| OP-OSEM | OP-OSEM (gated) | PSF-OP-OSEM | MC-OP-OSEM | PSF-MC-OP-OSEM | |
|---|---|---|---|---|---|
| Lesion 1 | 10.81 | 9.08 | 10.81 | 9.46 | 8.44 |
| Lesion 2 | 19.21 | 12.74 | 19.26 | 13.56 | 12.64 |
| Lesion 3 | 16.03 | 10.86 | 16.31 | 11.53 | 11 |
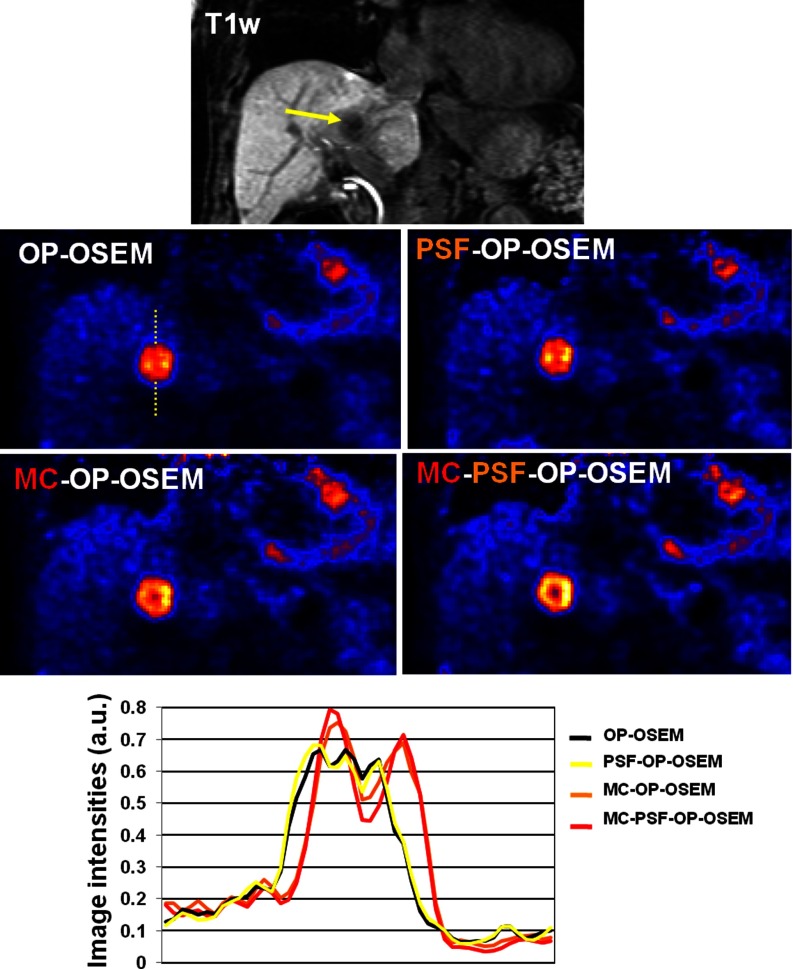
The proposed methods improved delineation of complex tumors structures with heterogeneous FDG uptake. Figure 7 shows a PET coronal slice from subject 3 reconstructed using the four reconstruction different methods, together with the corresponding T1-weighted coronal MRI slice (top row). The chosen slice intersects one liver lesion which has a complex structure with areas of higher and lower uptake due to its necrotic core. Without motion correction, the heterogeneous FDG uptake distribution is hardly visible. As can be seen, motion correction largely improved delineations of these structures by enhancing metabolically active tumor walls and reducing intensities in the core of the tumor. Modeling the PSF in addition to the motion correction further improved intensity recovery of the metabolically active portion of the tumor, as illustrated by the line profiles going through the lesion (Fig. 7, bottom).
Figure 7.
Same PET coronal slice from subject 3 obtained using different reconstruction methods. The lesion visible on this slice has heterogeneous FDG uptake. Line profiles (bottom row) show the advantage of the proposed methods as compared to conventional reconstructions.
DISCUSSION
This study demonstrates that significant improvement in PET-MR oncologic scans can be obtained by incorporating PSF modeling and MR-based respiratory motion fields within the PET iterative reconstruction process. This approach may allow more effective utilization of the PET-MR scanner in tumor imaging studies, in both the lower abdomen, with moving lesions, and other parts of the body unaffected by motion.
The PSF of Biograph mMR scanner was modeled by exponential functions with offsets intended to model the longer tails of the PSF in this system.29 These kernels match more closely to the point source sinogram data and exhibit better recovered point source intensity as compared to Gaussian kernels. The radial asymmetry was intended to model intercrystal scattering effects, increasing toward the edges of the FOV. Some hyper-resolution artifacts (“edge artifacts”), frequently observed in PSF reconstructions, were observed in the ACR phantom reconstructions. These artifacts are due to an overestimation of PSF blurring effects and can be alleviated by deconvolving numerical broadening effects from the measured PSF kernel.29 However, since this will decrease the impact of PSF modeling, reduced detectability of small, low-intensity tumors may be expected, hampering the overall benefit of PSF reconstructions for tumor imaging studies.
The ACR phantom data analysis showed that the proposed PSF reconstruction improves contrast recovery (13.3%–35.0%) and limits image noise propagation as compared to OP-OSEM. These results were confirmed in oncologic patient studies with static lesions, where improvements in lesion contrast in the range of 19.7%–109% were observed. As expected, the smallest lesions benefited the most from PSF modeling, especially the one located in the patient's arm, due to its location at the extremities of the FOV. These results confirm the importance of PSF modeling for detection and assessment of small residual lesions, especially following treatment.
A dedicated MRI acquisition sequence (NAV-TrueFISP) was developed to track respiratory motion. This sequence uses MR navigators to track the lung/liver interface (or diaphragm) allowing to rebin both PET and MR data into specific respiratory phases. Because the navigators directly track internal motion of organs, they are more accurate gating information than external devices that track the motion indirectly (e.g., respiratory bellows).37, 38 In addition, bellows signal recorded by the wireless physiological measurement unit may undergo substantial distortion from fast MRI gradient switches, degrading the accuracy of the respiratory phase information. Seven amplitude-based respiratory frames were considered in this study, in agreement with the recommended number of respiratory frames.39 Using a lower number of frames by authorizing larger respiratory motion amplitude in each frame would result in increased intragate motion blurring and hampered motion correction accuracy.
The NAV-TrueFISP sequence is somewhat similar to the one presented in Ref. 16, except that we used True-FISP contrast to measure respiratory motion. TrueFISP has the advantage of brightly displaying liver vasculature, which provides anatomical landmarks within the homogeneous liver tissue for robust registration performance. In the current implementation of the NAV-TrueFISP sequence, the acquisition of each slice is repeated multiple times to cover all respiratory positions for each slice. Thus, a lot of acquired data is redundant and only 25% of the acquired TrueFISP data were kept to build the seven-phase respiratory motion model. In future work, we plan to use real-time respiratory phase feedback from the navigators or respiratory bellows to prospectively acquire MR data by filling a table of slice locations and respiratory phases. This will allow faster and more efficient construction of the motion model, leaving more time for other clinical MR protocols to be performed.
The total acquisition time for the NAV-TrueFISP protocol was less than 3 min in subject 3's liver scan. This protocol can be performed at anytime during the PET-MR examination and interleaved with other clinical MR sequences. Assuming no large voluntary patient motion, the NAV-TrueFISP derived motion model can be used to correct PET events for motion throughout the entire PET-MR acquisition. Binning of PET events into respiratory phases can be performed using bellows, list-mode driven gating of PET data40 or MR navigators inserted into clinical MR sequences.
The results obtained in the liver patient study show that the proposed PET reconstruction algorithm, modeling both MR-based motion fields and PSF, can significantly enhance the quality of PET-MR imaging studies in the lower abdomen. Respiratory motion blurring was visibly removed from the images without rejecting any event, hence preserving the complete statistics of the data. The use of motion-dependent attenuation correction factors for each acquired line of response also improved image quantitative accuracy by reducing emission/attenuation spatial inconsistencies. Significant improvements in semiquantitative metrics (e.g., 54.4%–98.1% for TBR and 21.8%–34.2% tumor apparent size reduction in subject 3) were obtained using our approach compared to conventional OP-OSEM reconstruction. The largest improvement was observed in subject 3's smallest and uppermost liver lesion experiencing the most motion. Our methods also improved the delineation of the lesion's necrotic core that was otherwise invisible, as shown in Fig. 7. Due to their complex structure, these tumors are particularly vulnerable to degrading effects from motion and PVEs, and high resolution is necessary to image them. This can be achieved by using the proposed PET-MR approach. Moreover, the results obtained show that the impact of PSF modeling on lesions’ TBR and apparent sizes was more important when correcting for motion at the same time. This demonstrates the importance of motion correction to fully benefit from resolution recovery provided by PSF modeling in PET-MR of the lower abdomen.
The attenuation effects of the MR surface coils, which can cause substantial underestimation of local radiotracer concentration and artifacts in the vicinity of the coil,41, 42 were neglected in this study. However, the comparison between reconstruction methods and the conclusions drawn in this work remain valid since the same bias affects all reconstructed volumes. Surface coils attenuation effects could be corrected using the technique suggested by Paulus and colleagues.42
CONCLUSION
This work demonstrates that modeling PSF and MR-based tissue deformations in the iterative PET reconstruction process significantly improves oncologic PET-MR imaging studies. The results obtained suggest that PET-MR acquisition with the proposed methods can significantly enhance the performance of tumors diagnosis and staging as compared to conventional methods. This approach may facilitate more thorough implementation of the scanner's unique properties in oncologic studies of both static and moving areas of interest.
ACKNOWLEDGMENTS
This work was supported in part by NIH R21-EB012326 and R01-CA165221. The authors would like to thank Dr. Ciprian Catana and Dr. Alexander Guimaraes for their help with the abdominal study, Dr. Xuping Zhu for her help preparing the point sources, and Grae Arabasz and Shirley Hsu for their help during the acquisitions.
References
- Daou D., “Respiratory motion handling is mandatory to accomplish the high-resolution PET destiny,” Eur. J. Nucl. Med. Mol. Imaging 35, 1961–1970 (2008). 10.1007/s00259-008-0931-x [DOI] [PubMed] [Google Scholar]
- Vedam S., Kini V., Keall P., Ramakrishnan V., Mostafavi H., and Mohan R., “Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker,” Med. Phys. 30, 505–513 (2003). 10.1118/1.1558675 [DOI] [PubMed] [Google Scholar]
- Papathanassiou D., Becker S. P., Amir R., Menéroux B. T., and Liehn J.-C., “Respiratory motion artefact in the liver dome on FDG PET/CT: Comparison of attenuation correction with CT and a caesium external source,” Eur. J. Nucl. Med. Mol. Imaging 32, 1422–1428 (2005). 10.1007/s00259-005-1868-y [DOI] [PubMed] [Google Scholar]
- Sureshbabu W. and Mawlawi O., “PET/CT imaging artifacts,” J. Nucl. Med. Technol. 33, 156–161 (2005). [PubMed] [Google Scholar]
- Liu C., L. A.PierceII, Alessio A. M., and Kinahan P. E., “The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging,” Phys. Med. Biol. 54, 7345–7362 (2009). 10.1088/0031-9155/54/24/007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio A. M., Kohlmyer S., Branch K., Chen G., Caldwell J., and Kinahan P., “Cine CT for attenuation correction in cardiac PET/CT,” J. Nucl. Med. 48, 794–801 (2007). 10.2967/jnumed.106.035717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M. M., Cohade C., Nakamoto Y., Marshall L. T., Leal J. P., and Wahl R. L., “Clinically significant inaccurate localization of lesions with PET/CT: Frequency in 300 patients,” J. Nucl. Med. 44, 240–243 (2003). [PubMed] [Google Scholar]
- Nehmeh S. A., Erdi Y. E., Ling C. C., Rosenzweig K. E., Schoder H., Larson S. M., Macapinlac H. A., Squire O. D., and Humm J. L., “Effect of respiratory gating on quantifying PET images of lung cancer,” J. Nucl. Med. 43, 876–881 (2002). [PubMed] [Google Scholar]
- Boucher L., Rodrigue S., Lecomte R., and Bénard F. O., “Respiratory gating for 3-dimensional PET of the thorax: Feasibility and initial results,” J. Nucl. Med. 45, 214–219 (2004). [PubMed] [Google Scholar]
- Dawood M., Buther F., Jiang X., and Schafers K. P., “Respiratory motion correction in 3-D PET data with advanced optical flow algorithms,” IEEE Trans. Med. Imaging 27, 1164–1175 (2008). 10.1109/TMI.2008.918321 [DOI] [PubMed] [Google Scholar]
- Lamare F., Carbayo M. L., Cresson T., Kontaxakis G., Santos A., Rest C. C. Le, Reader A., and Visvikis D., “List-mode-based reconstruction for respiratory motion correction in PET using non-rigid body transformations,” Phys. Med. Biol. 52, 5187–5204 (2007). 10.1088/0031-9155/52/17/006 [DOI] [PubMed] [Google Scholar]
- Qiao F., Pan T., J. W.ClarkJr., and Mawlawi O. R., “A motion-incorporated reconstruction method for gated PET studies,” Phys. Med. Biol. 51, 3769–3783 (2006). 10.1088/0031-9155/51/15/012 [DOI] [PubMed] [Google Scholar]
- Guerin B., Cho S., Chun S., Zhu X., Alpert N., Fakhri G. El, Reese T., and Catana C., “Nonrigid PET motion compensation in the lower abdomen using simultaneous tagged-MRI and PET imaging,” Med. Phys. 38, 3025–3038 (2011). 10.1118/1.3589136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S. Y., Reese T. G., Ouyang J., Guerin B., Catana C., Zhu X., Alpert N. M., and Fakhri G. El, “MRI-based nonrigid motion correction in simultaneous PET/MRI,” J. Nucl. Med. 53, 1284–1291 (2012). 10.2967/jnumed.111.092353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petibon Y., Ouyang J., Zhu X., Huang C., Reese T., Chun S., Li Q., and El Fakhri G., “Cardiac motion compensation and resolution modeling in simultaneous PET-MR: A cardiac lesion detection study,” Phys. Med. Biol. 58, 2085–2102 (2013). 10.1088/0031-9155/58/7/2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würslin C., Schmidt H., Martirosian P., Brendle C., Boss A., Schwenzer N. F., and Stegger L., “Respiratory motion correction in oncologic PET using T1-weighted MR imaging on a simultaneous whole-body PET/MR system,” J. Nucl. Med. 54, 464–471 (2013). 10.2967/jnumed.112.105296 [DOI] [PubMed] [Google Scholar]
- King A., Tsoumpas C., Buerger C., Schulz V., Marsden P., and Schaeffter T., “Real-time respiratory motion correction for simultaneous PET-MR using an MR-derived motion model,” IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 (IEEE, New York, 2011), pp. 3589–3594.
- King A. P., Buerger C., Tsoumpas C., Marsden P., and Schaeffter T., “Thoracic respiratory motion estimation from MRI using a statistical model and a 2-D image navigator,” Med. Image Anal. 16, 252–264 (2012). 10.1016/j.media.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Polycarpou I., Tsoumpas C., and Marsden P., “Analysis and comparison of two methods for motion correction in PET imaging,” Med. Phys. 39, 6474–6483 (2012). 10.1118/1.4754586 [DOI] [PubMed] [Google Scholar]
- Asma E., Manjeshwar R., and Thielemans K., “Theoretical comparison of motion correction techniques for PET image reconstruction,” IEEE Nuclear Science Symposium Conference Record, 2006 (IEEE, New York, 2006), Vol. 3, pp. 1762–1767.
- Geworski L., Knoop B. O., de Cabrejas M. L., Knapp W. H., and Munz D. L., “Recovery correction for quantitation in emission tomography: A feasibility study,” Eur. J. Nucl. Med. 27, 161–169 (2000). 10.1007/s002590050022 [DOI] [PubMed] [Google Scholar]
- Soret M., Bacharach S. L., and Buvat I., “Partial-volume effect in PET tumor imaging,” J. Nucl. Med. 48, 932–945 (2007). 10.2967/jnumed.106.035774 [DOI] [PubMed] [Google Scholar]
- Alessio A. M., Kinahan P. E., and Lewellen T. K., “Modeling and incorporation of system response functions in 3-D whole body PET,” IEEE Trans. Med. Imaging 25, 828–837 (2006). 10.1109/TMI.2006.873222 [DOI] [PubMed] [Google Scholar]
- Strul D., Slates R., Dahlbom M., Cherry S. R., and Marsden P., “An improved analytical detector response function model for multilayer small-diameter PET scanners,” Phys. Med. Biol. 48, 979–994 (2003). 10.1088/0031-9155/48/8/302 [DOI] [PubMed] [Google Scholar]
- Cloquet C., Sureau F., Defrise M., Van Simaeys G. T., Trotta N., and Goldman S., “Non-Gaussian space-variant resolution modelling for list-mode reconstruction,” Phys. Med. Biol. 55, 5045–5066 (2010). 10.1088/0031-9155/55/17/011 [DOI] [PubMed] [Google Scholar]
- Alessio A. M., Stearns C. W., Tong S., Ross S. G., Kohlmyer S., Ganin A., and Kinahan P. E., “Application and evaluation of a measured spatially variant system model for PET image reconstruction,” IEEE Trans. Med. Imaging 29, 938–949 (2010). 10.1109/TMI.2010.2040188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panin V. Y., Kehren F., Michel C., and Casey M., “Fully 3-D PET reconstruction with system matrix derived from point source measurements,” IEEE Trans. Med. Imaging 25, 907–921 (2006). 10.1109/TMI.2006.876171 [DOI] [PubMed] [Google Scholar]
- Sureau F. C., Reader A. J., Comtat C., Leroy C., Ribeiro M.-J., Buvat I., and Trébossen R., “Impact of image-space resolution modeling for studies with the high-resolution research tomograph,” J. Nucl. Med. 49, 1000–1008 (2008). 10.2967/jnumed.107.045351 [DOI] [PubMed] [Google Scholar]
- Watson C. C., “Measurement of the physical PSF for an integrated PET/MR using targeted positron beams,” IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2012 (IEEE, New York, 2012), pp. 2089–2095.
- Drzezga A., Souvatzoglou M., Eiber M., Beer A. J., Fürst S., Martinez-Möller A., Nekolla S. G., Ziegler S., Ganter C., and Rummeny E. J., “First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses,” J. Nucl. Med. 53, 845–855 (2012). 10.2967/jnumed.111.098608 [DOI] [PubMed] [Google Scholar]
- Delso G., Furst S., Jakoby B. R., Ladebeck R., Ganter C., Nekolla S. G., Schwaiger M., and Ziegler S. I., “Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner,” J. Nucl. Med. 52, 1914–1922 (2011). 10.2967/jnumed.111.092726 [DOI] [PubMed] [Google Scholar]
- Chun S. Y. and Fessler J. A., “A simple regularizer for B-spline nonrigid image registration that encourages local invertibility,” IEEE J. Sel. Top. Signal Process. 3, 159–169 (2009). 10.1109/JSTSP.2008.2011116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma-Carbayo M. J., Derbyshire J. A., Sampath S., Santos A., Desco M., and McVeigh E. R., “Unsupervised estimation of myocardial displacement from tagged MR sequences using nonrigid registration,” Magn. Reson. Med. 59, 181–189 (2008). 10.1002/mrm.21444 [DOI] [PubMed] [Google Scholar]
- Chun S. and Fessler J., “Noise properties of motion-compensated tomographic image reconstruction methods,” IEEE Trans. Med. Imaging 32, 141–152 (2013). 10.1109/TMI.2012.2206604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C., “New, faster, image-based scatter correction for 3D PET,” IEEE Trans. Nucl. Sci. 47, 1587–1594 (2000). 10.1109/23.873020 [DOI] [Google Scholar]
- Pratx G., Chinn G., Olcott P. D., and Levin C. S., “Fast, accurate and shift-varying line projections for iterative reconstruction using the GPU,” IEEE Trans. Med. Imaging 28, 435–445 (2009). 10.1109/TMI.2008.2006518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M., Khasgiwala V., Savord B., Chen M., Chuang M., Edelman R., and Manning W., “Comparison of respiratory suppression methods and navigator locations for MR coronary angiography,” AJR, Am. J. Roentgenol. 168, 1369–1375 (1997). 10.2214/ajr.168.5.9129447 [DOI] [PubMed] [Google Scholar]
- Kim B. S., Kim J. H., Choi G. M., Kim S. H., Park J. K., Song B.-C., and Kang W., “Comparison of three free-breathing T2-weighted MRI sequences in the evaluation of focal liver lesions,” Am. J. Roentgenol. 190, W19–W27 (2008). 10.2214/AJR.07.2043 [DOI] [PubMed] [Google Scholar]
- Dawood M., Büther F., Stegger L., Jiang X., Schober O., Schäfers M., and Schäfers K. P., “Optimal number of respiratory gates in positron emission tomography: A cardiac patient study,” Med. Phys. 36, 1775–1784 (2009). 10.1118/1.3112422 [DOI] [PubMed] [Google Scholar]
- Büther F., Dawood M., Stegger L., Wübbeling F., Schafers M., Schober O., and Schafers K. P., “List mode-driven cardiac and respiratory gating in PET,” J. Nucl. Med. 50, 674–681 (2009). 10.2967/jnumed.108.059204 [DOI] [PubMed] [Google Scholar]
- MacDonald L. R., Kohlmyer S., Liu C., Lewellen T. K., and Kinahan P. E., “Effects of MR surface coils on PET quantification,” Med. Phys. 38, 2948–2956 (2011). 10.1118/1.3583697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus D. H., Braun H., Aklan B., and Quick H. H., “Simultaneous PET/MR imaging: MR-based attenuation correction of local radiofrequency surface coils,” Med. Phys. 39, 4306–4315 (2012). 10.1118/1.4729716 [DOI] [PubMed] [Google Scholar]