Abstract
C60 fullerene is a promising material due to its unique physiochemical properties. However, previous studies have reported that colloidal aggregates of C60 (nC60) produce toxicity in fish and human cell cultures. The preparation method of the nC60 raises questions as to whether the observed effects stem from the fullerenes themselves or from the organic solvents used during the preparation of the suspensions. In this paper we set out to elucidate the mechanism by which tetrahydrofuran (THF) treatment to enhance the preparation of nC60 leads to cytotoxicity in a mouse macrophage cell line. Our results demonstrate that THF/nC60 but not fullerol or aqueous nC60 generates cellular toxicity through a pathway that involves increased intracellular flux and mitochondrial perturbation in RAW 264.7 cells. Interestingly, the supernatant of the THF/nC60 suspension rather than the colloidal fullerene aggregates mimics the cytotoxic effects due to the presence of γ-butyrolactone and formic acid. Thus, the role of nC60 in the cellular responses is likely not due to the direct effect of the nC60 material surface on the cells but related to the conversion of THF into a toxic by-product during the preparation of the suspension.
Keywords: Fullerene, nC60, THF, nanoparticles, toxicology
INTRODUCTION
The quantities of nanomaterials produced per year are large and increasing rapidly. Among them, the fullerene is being manufactured on a large-scale and is a promising material due to its high electron affinity and reactivity with nucleophiles. Fullerenes may serve as photosensitizers, producing reactive oxygen species (ROS) (1), but are also widely reported to be anti-oxidants or radical sponges (2). These properties have been explored for possible medical applications such as DNA cleavage (3), tumor destruction (4), antiviral action (5).
Observations such as DNA cleavage and tumor destruction also suggest that, with adequate exposure, these nanoparticles could have deleterious consequences on biological processes in humans and the environment. Unfortunately, little is known about their hazard potential. Two previous studies concluded that colloidal aggregates of C60 (nC60) produced oxidative damage in the brains of largemouth bass (6) and relatively high toxicity in tissue culture experiments, using human cells (7). A mechanism of ROS production and subsequent attack of C60 on cell membrane lipids was invoked to explain these results. However, the preparation of the nC60 raises questions as to whether the observed effects stem from the fullerenes themselves or from the organic solvents used in preparing the suspension. We have previously shown that the colloidal aggregates of fullerenes used in these studies, THF/nC60, likely contained significant quantities of the residual solvent, tetrahydrofuran (THF) (8). Another study (9) compared toxicity of colloidal C60 prepared with and without polar solvents and concluded that suspensions prepared without using polar organic solvents not only lack toxicity in rodents but they also protected the livers of the exposed animals in a dose-dependent manner against free-radical damage. Another study in larval zebra fish (10) attributed the toxicity of THF/nC60 to THF decomposition products rather than the colloidal fullerene aggregates. This is also in keeping with the demonstration by (11) that γ-irradiation rendered THF/nC60 innoxious, actually converting the suspension to a cytoprotective agent. It has also recently been reported (12) that the formation of THF-peroxide from THF/nC60 may react with indigo dye leading to oxidative stress in Escherichia coli. However, this previous work does not exclude possible toxicity of nC60 itself and recommends prudence when interpreting toxicity data.
Against this background, the goal of the present study was to assess the biological impact of THF/C60 aggregates from the perspective of possible cytotoxic products being generated by the fullerene/solvent or THF/solvent interaction. We compare THF/nC60 aggregates with fullerol and water-made nC60 called aq/nC60 in a mouse macrophage cell line, which represents a cell type targeted by nanoparticles at the point of entry into the body of a number of mammalian species. We found that fullerene particles alone were not toxic under the metrics of cytotoxicity employed in this study and that the apparent toxicity of THF/nC60 resides in the soluble components in the particle suspensions. THF itself at the concentrations used to prepare THF/nC60 was not toxic, but two THF degradation by-products identified in solution could produce the toxicity of the THF/nC60 suspension. These data show that the toxicity observed may be misinterpreted as originating from nC60. Indirect mechanisms of toxicity are valid from a perspective of the necessity to treat these materials with solvents and other suspending media and we provide a practical approach to discern between nanoparticle toxicity and the potential toxicity of the medium into which the nanoparticles are transferred.
MATERIALS AND METHODS
Experimental design
Our aim was to analyze whether the nC60 or the synthesis residues (THF and its degradation by-products) was contributing to the toxic effect previously observed with the THF/nC60. We conducted a three-steps experiment. First, the toxicity of THF/nC60 suspension was assessed and compared to the toxicity of aq/nC60. Second, toxicity assays were performed on the supernatant of the THF/nC60 stock suspension (liquid phase) and the centrifugate/dialysate resuspended in water (solid phase). All the toxicity associated with the THF/nC60 suspension comes from this supernatant; its chemical composition was analyzed. Third, the toxicity of the main components of this supernatant (γ-butyrolactone and formic acid) was assessed compare to the toxicity of the THF alone.
Preparation of C60 aggregates
Fullerols (C60(OH)18) (MER©, USA) were introduced to water using a sonication bath and filtered on 0.2 μm membrane. For the aq/nC60, C60 powder (MER©) was crushed, introduced to water, and sonicated during 15h under vacuum. For the THF/ nC60, C60 powder (MER©) were prepared following the method outlined by (13) and altered by (14).
The concentration of nC60 in the aq/nC60 and fullerols suspensions was determined by Total Organic Carbon TOC (Shimadzu 5050A, USA) and adjusted to 20 mg/L. As carbon compounds related to THF are present in the THF/nC60 suspension, its concentration was determined UV-vis spectrophotometry (Hitachi U2810, USA) (see Supporting information).
Gas chromatography-mass spectrometry GC/MS (Shimadzu QP5050A, USA) was used to identify organic residues present in the THF/nC60 suspension according to (15, 16). Acquisition was done in a selected ion-monitoring (SIM) mode (detector voltage: 1.2 kV, solvent cut time: 3 min). The concentration of formic acid was determined by ionic chromatography IC (Dionex DX120, USA) equipped with a column pack (Ionpack AS14A, Dionex), an AG14 guard column, an auto-sampler (JASCO 851-AS). The concentration of γ-butyrolactone was determined using a gas chromatography-flame ionization detector GC/FID (17A, Shimadzu, USA). The flame ionization process was performed with a DBI-30W J&W Scientific fused silica capillary column (see Supporting Information).
Physico-chemical characterization
Particle size distribution was carried out via dynamic light scattering (DLS) using a CGS 3 (ALV-GmbH, Germany) equipped with a helium-neon laser (633.4 nm). Suspensions were analyzed at 25°C in 5 mm diameter cells with the photomultiplier setting to a scattering angle of 90°. The zeta potential was determined using a Zetasizer Nano ZS (Malvern, UK) equipped with a 633-nm helium-neon laser. The size and zeta potential measurements were performed at 25°C, without addition of salt, and at pH 6.6 ± 0.4 for the three fullerene-based nanoparticles. The measurements of aggregation states in culture media were performed after centrifugation of the Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, USA) at 50 000 rpm during 30 minutes.
The superoxide anions were targeted and measured via the reduction of XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) (17, 18) when combined with superoxide dismutase (SOD) as a control for non-superoxide reductions (19). The reduction of XTT induces an increase in optical density at 470 nm used to quantify the amount of superoxide present. 5 μM of each type of nanoparticles was tested for superoxide production in the presence of 5.6 mM NADH (see Supporting Information).
Cell culture and treatment
RAW 264.7 cells were cultured in a 5% CO2 in DMEM containing 5% Fetal Bovine Serum (Atlanta Biologicals, USA), 5000 U/ml penicillin, 500 mg/L streptomycin, and 2 mM L-glutamine (Invitrogen, USA). Cells were cultured in 48-well plates at a density of 105 cells/well and exposed to nanoparticles in 0.4 mL of medium at 37°C for the indicated time periods. All the nanoparticles solutions were prepared fresh from stock solutions. The fullerene dose used in this study (20 mg/L) is within the range of amounts used in past studies to demonstrate fullerene toxicity (between 10 and 50 mg/L for (7)).
Cellular staining with fluorescent probes and flow cytometry
Cells were stained with the fluorescent dyes diluted in DMEM. The following dye combinations were added for the indicated time periods at 37 °C in the dark: (i) 47.5 mg/L propidium iodide (PI) (Sigma, USA) for 15min in 200 μL DMEM to assess cell death; (ii) 2.5 μM dichlorofluorescein diacetate (DCF) for 15min to assess H2O2 production; (iii) 5 μM Fluo-4 for 30min to assess cytoplasmic free calcium; (iv) 4 μM Rhod-2 for 30min to assess the mitochondrial free calcium (Molecular Probes, USA). Flow cytometry was performed using a FACScan (Becton Dickinson, USA) equipped with a single 488 nm argon laser. DCF and Fluo-4 stained cells are analyzed on the FL-1 channel. Rhod-2 stained cells are analyzed on the FL-2 channel. Data are expressed as fold increase in mean fluorescence intensity normalized to control cells. PI stained cells are analyzed on channel FL-2. Data are expressed as percent positive cells, which are a distinct population from PI negative cells. An unpaired Student’s t-test was used to assess the statistical significance.
RESULTS
Physico-chemical behavior of fullerene-based nanoparticles in the nutritive medium
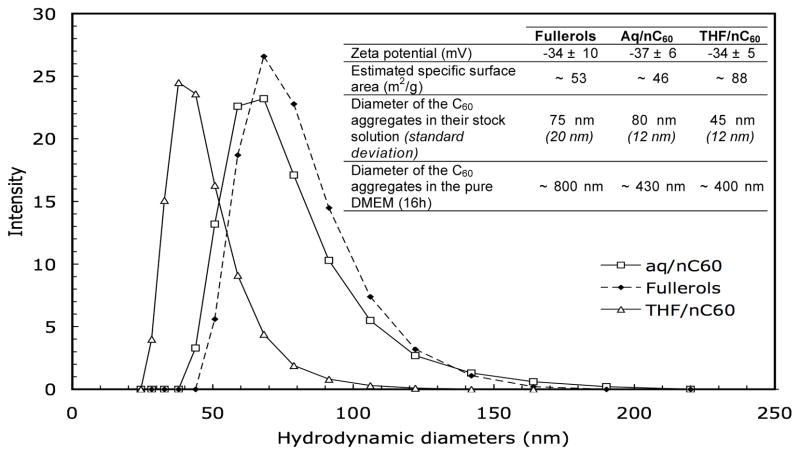
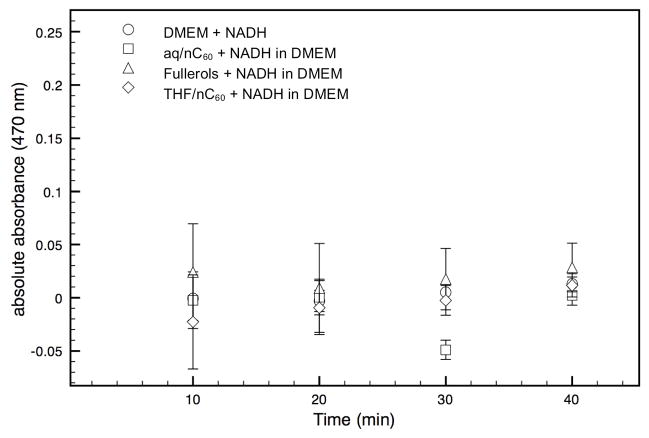
The three C60 aggregates have similar zeta potential values of −35.5±1.5 mV. This leads to relatively stable C60 suspensions with mean hydrodynamic diameters of 45–80 nm (Figure 1) and a specific surface area estimated between 46–88 m2/g. When these stock solutions are diluted in DMEM for 16h, rapid aggregation occurs. The THF/nC60 and aq/nC60 suspensions had smaller mean aggregate hydrodynamic diameters of 400–450 nm compare to the ~800 nm of the fullerols. Colloidal destabilization is mainly related to the neutral pH, the elevated ionic strength of the DMEM, and the presence of serum. Because previous work reported that the toxicity of nC60 was related to the production of ROS by the fullerenes, we followed superoxide generation by THF/nC60, fullerol and aq/nC60. No significant difference in abiotic generation of superoxide was seen between the control (DMEM with fetal bovine serum), the three fullerene-based nanoparticles in the dark (Figure 2). Thus, neither form of nC60 produced significant quantities of ROS, while fullerol produces singlet oxygen under UV light, particularly when an appropriate electron donor such as NADH is present (20). These results demonstrate that abiotic ROS production cannot account for THF/nC60 toxicity.
Figure 1.
Distribution of the hydrodynamic diameters of the aq/C60, THF/nC60 and fullerols in the their initial stock suspensions. Addition to DMEM resulted in rapid aggregation (inset).
Figure 2.
Reactive Oxygen Species generation by the C60 aggregates (5 μM) in complemented DMEM. NADH (5.6 mM) is present as an electron donor. Low absorbance of XTT indicates a lack of significant superoxide activity in these suspensions. Error bars represent 95% confidence interval where n=3 for each point.
Assessment of cellular induction of H2O2 production by fullerene-based nanoparticles
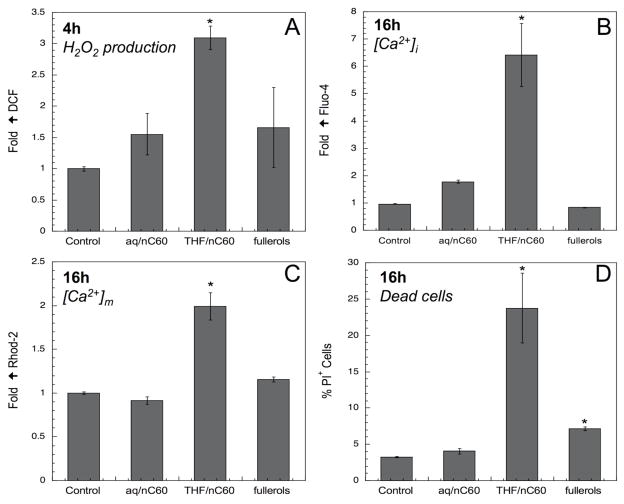
It is possible that nanoparticles that are incapable of abiotic ROS production may promote cells to produce ROS as a result of a functionalized surface, e.g. cationic attachment (21). ROS production (H2O2) in response to exposure to C60 aggregates was assessed in a macrophages cell line through the use of the fluorescent dye DCF (Figure 3A). While in the presence of aq/nC60 and fullerol there were insignificant (p>0.05) increases in the fluorescence intensity, THF/nC60 suspensions induced a small but significant increase after 4h. This H2O2 production is unlikely from fullerene origin and suggests that the THF/nC60 suspension might contain non-particulate toxic components that could be responsible for cellular ROS production.
Figure 3.
H2O2 production in RAW 264.7 in presence of aq/nC60, THF/nC60 and fullerol (A). Effect of nanoparticles on intracellular [Ca2+]i (B) and mitochondrial [Ca2+]m (C) calcium levels. Changes in cellular toxicity (PI uptake) during fullerene-based nanoparticles exposure (D). *:p<0.05.
Cellular and mitochondrial calcium levels and cellular cytotoxicity
Nanoparticle-induced ROS production is capable of generating cellular oxidant injury. An increase in the intracellular calcium concentration or [Ca2+]i constitutes a major cellular response to oxidative stress. Ca2+ can be derived from numerous sources, including cellular organelles such as mitochondria and the endoplasmic reticulum (22). Cellular staining with Fluo-4 showed a 6.5-fold increase in [Ca2+]i in response to THF/nC60, whereas no increase was observed with aq/nC60 or fullerols (Figure 3B). This rise in [Ca2+]i can trigger additional cellular responses, including Ca2+ sequestration by mitochondria. This serves to buffer rapid increases in [Ca2+]i up to a threshold, beyond which the Ca2+ triggers opening of the mitochondrial permeability transition pore. Mitochondrial Ca2+ levels, [Ca2+]m, were assessed by cellular staining with Rhod-2 (Figure 3C). THF/nC60 exposure induced a significant (2-fold) increase in Rhod-2 fluorescence and in cytotoxicity as determined by propidium iodide (PI) staining (Figure 3D). In contrast, aq/nC60 and fullerol did not produce a significant increase in [Ca2+]m while fullerol had a small but significant effect on nuclear PI uptake (Figure 3D). These data demonstrate that THF/nC60 suspensions induce an increase in mitochondrial Ca2+ levels that was associated with a cytotoxic outcome. Since THF/nC60 is incapable of spontaneous ROS production (Figure 2), the increase in DCF fluorescence is likely represents a cellular response to a chemical component in the suspension.
Comparative toxicity of the nanoparticulate and supernatant phases of the THF/nC60 suspension
The role played by possible chemical impurities present in the solution following the synthesis of THF/nC60 was assessed by comparing the toxicity of the solution phase after the removal of the nC60 to the toxicity of the whole THF/nC60 suspension. To remove the C60 aggregates, the stock solutions of aq/nC60, fullerol and THF/nC60 were either centrifuged at 16,000 rpm or dialyzed across a membrane with a cut-off of 4 nm. Cytotoxic assays were performed on the whole suspension, supernatant, dialysate and the solid phase C60 aggregates. No significant toxicity was observed for the as-synthesized aq/nC60 and fullerol suspension as well as the centrifuged pellet resuspended in water nor their supernatants (Figure 4). This differs from the almost 100% toxicity of the whole THF/nC60 suspension (LS) as well as its centrifuged supernatant (L). In contrast, the resuspended THF/nC60 pellet was devoid of toxicity after resuspension and addition to the cell culture (Figure 4). These data are in favor of a toxic component in the supernatant. This point was further illustrated by analyzing the THF/nC60 dialysate, which generated the same level of toxicity as the centrifugation supernatant (Figure 4). This dialysate was proven to be devoid of particles since no characteristic fullerene peak was observed by UV-vis spectroscopy (see Supporting information, Figure S1). These results confirm that the toxic component does not migrate with the THF/nC60 aggregates and is likely is due to residual THF or its by-products.
Figure 4.
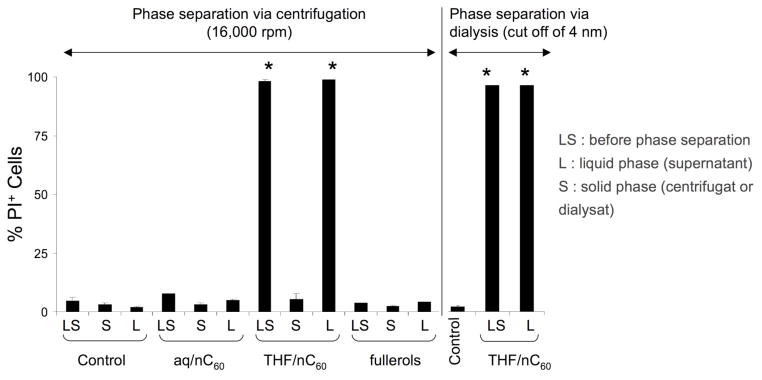
Comparative toxicity (PI) of the supernatant (liquid phase containing the dissolved residue from the synthesis) and the solid phase containing C60 aggregates. The phase separation is performed by centrifugation at 16,000 rpm for all particles. Dialysis was performed with cut-off of 4 nm dialysis tubes for THF/nC60. The toxicity observed in presence of THF/nC60 comes from the supernatant.
Identification of the toxic chemical components in the THF/nC60 supernatant
An estimated 26 ± 2 % (20 mg/L) of the TOC content of the THF/nC60 stock suspension is attributed to the nC60 with 74 ± 2 % (56 mg/L) of the signal coming from dissolved carbon compounds. No THF is detected in the THF/nC60 stock solution by GC/FID. A search for suspected by-products of THF degradation focused on succinic acid, acetic acid, γ-butyrolactone, glycolic acid, and formic acid since these are the possible intermediates for the photocatalytic degradation of THF (23). GC/MS detected the presence formic acid (6% of the TOC or 8 mg/L) and the γ-butyrolactone (22% of the TOC or 22 mg/L) in the THF/nC60 (see Supporting information, Figure S2).
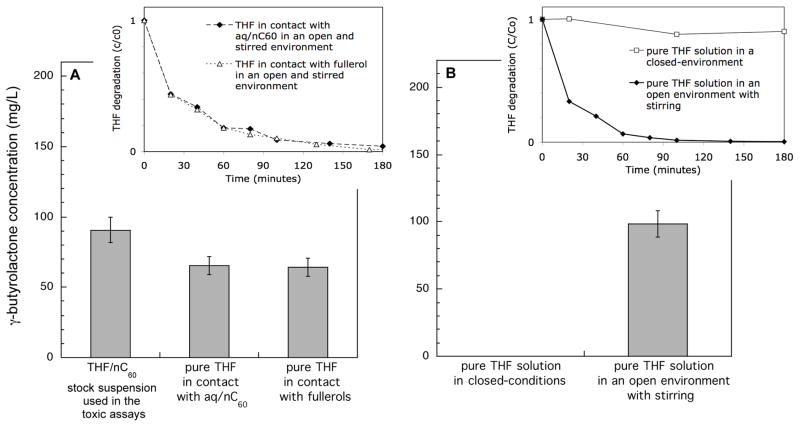
The kinetic of the THF degradation were studied using fresh suspensions made with aq/nC60 and fullerol to which THF were added. After 3h in presence of nC60, THF was no longer detectable and γ-butyrolactone appeared in the suspensions (Figure 5A). But this THF degradation does not required the presence of nC60. Indeed, this reaction occurs when a pure solution of THF (without nC60) is maintained (during < 2h) in an open, stirred-environment that promotes contact with oxygen (Figure 5B).
Figure 5.
THF degradation (insets) and γ-butyrolactone appearance after 180 minutes (main graphs) in the dark. (A) In presence of nC60 (aq/nC60 or fullerols). The suspension are stirred in an open environment. (B) In absence on nC60 in closed-conditions or in an open environment.
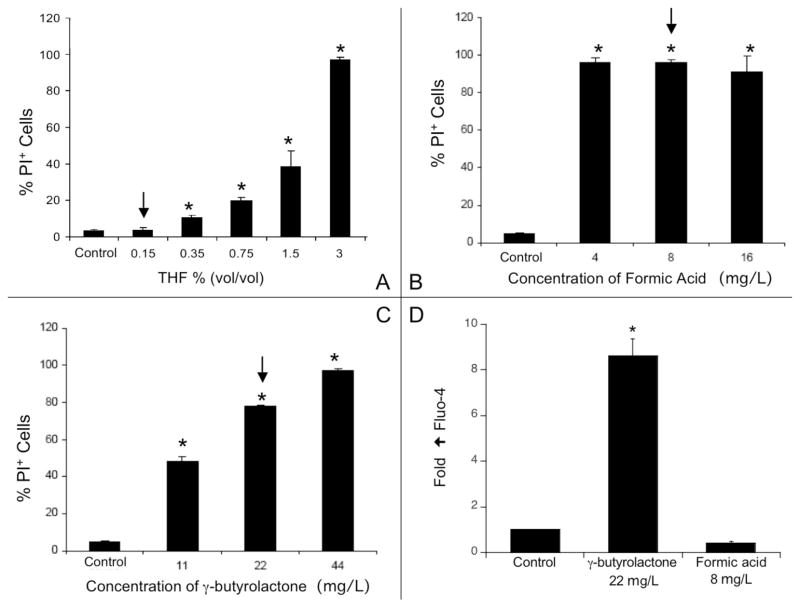
Cellular toxicity was assessed in the presence of pure and fresh THF, γ-butyrolactone or formic acid. A solution containing 0.15vol. THF in DMEM did not induce toxicity (Figure 6A). This is ~15 times higher than the total carbon concentration in the THF/nC60 stock suspension. THF alone showed evidence of toxicity only when concentrations exceed 0.35vol. Similar toxicity assays performed on the two by-products confirmed that they are highly toxic at the amounts present in the THF/nC60 stock suspension (Figure 6B and C). γ-butyrolactone but not the formic acid stimulated an increase [Ca2+]i (Figure 6D). These results show that at least two THF by-products can explain the cellular toxicity induced by THF/nC60 suspension.
Figure 6.
(A) Toxicity (PI) of a pure THF solution towards RAW 264.7 cells. The arrow indicates a concentration in THF ~15 times larger than the total organic carbon concentration of the THF/nC60 stock suspension. (B) Toxicity (PI) of formic acid and (C) γ-butyrolactone. The arrows indicate the concentration measured in the THF/nC60 stock suspension. (D) Effects of formic acid and γ-butyrolactone on intracellular [Ca2+]i calcium levels. *: p<0.05.
DISCUSSION
A significant finding is that the cellular toxicity observed for the THF/nC60 resides primarily in the supernatant of the nC60 suspensions. We arrive at this finding based on results obtained after separating the fullerene aggregates from the supernatant by either centrifugation or dialysis. THF/nC60-induced cytotoxicity appears to be is mediated by the oxygenation of THF into at least two toxic by-products (γ-butyrolactone and formic acid) in amounts sufficient to induce cell death. Among these breakdown products, γ-butyrolactone was capable of inducing intracellular Ca2+ release and an increase in mitochondrial Ca2+ that contribute to cellular toxicity by mitochondrial perturbation. One mechanism to explain the degradation of THF is that it is initially oxygenated to γ-butyrolactone, in turn degraded to several compounds such as the observed formic acid as proposed by (23):
![]()
(12) also observed the formation γ-butyrolactone in the THF/nC60 suspension. Our results complete this study since this toxic compound comes from the oxygenation of the THF rather than its interaction with the nC60. This is reinforced by (10) showing that the by-products of THF oxidation could be responsible of the toxicity of THF/nC60 suspensions in larval zebra fish. In contrast to our study, (10) did not observe formic acid as a by-product in their suspensions. However their THF/nC60 were prepared in the dark under an N2 atmosphere while our THF/nC60 were prepared under ambient light and O2.
Earlier studies on nC60 toxicity attributed abiotic ROS production as the source of injury (7). However the lack of ROS production by aq/nC60 and THF/nC60 suspensions under abiotic conditions calls these results into question and is further bolstered by the finding that fullerol, which was capable of abiotic ROS production, was devoid of toxicity. The small but significant amount of H2O2 production that we observed with the THF/nC60 suspension is likely from cellular origin and could reflect mitochondrial, either by the toxic THF by-products or as a concomitant of cell death. Thus, instead of a fullerene-induced oxidative stress mechanism, in our experimental conditions most of the toxicity resides in the use of THF, which leads to toxic breakdown products as described above.
This study resolves much of the controversy surrounding THF/nC60 toxicity in highlighting the toxic effect that THF produces in eukaryotic cell cultures. This is distinct from a lower-level toxicity observed for aq/nC60 in microbial cell cultures and in some human cell cultures (7). Thus, the true toxicity of C60 is certainly far less than previously reported and in no case can conclusions be drawn on the toxicity of nC60 based on studies using THF/nC60. The methodology proposed in this study deals with short-term assays on cultured cells, but can be applied to different organisms, or other solvents. For instance, previous studies (24, 25) have used DMSO to dispersed nC60 and have observed that DMSO/nC60 induces changes in the gene expression in zebrafish embryos. Even if the DMSO does not induce toxicity at the concentrations studied (24) the same recommendation of prudence in interpreting data from studies with THF/nC60 applies to the DMSO/nC60 studies given the possibility of toxic DMSO by-products (26–28).
Supplementary Material
Acknowledgments
Funding for this study was provided by the NSF Cooperative Agreement Number EF0830093, Center for Environmental Implications of Nanotechnology (CEINT), NSF-funded University of California Center for the Environmental Impact of Nanotechnology (UCCEIN), the US Public Health Service Grants U19AI070453, RO1ES016746, and RO1ES015498, the US EPA STAR award (RD83241301) to the Southern California Particle Center, the UC Lead Campus for Nanotoxicology Training and Research (UCTSR&TP), as well as the US EPA (EPAG2006STARF1Toxicology). We thank Shihong Lin, Yao Xiao, David Jassby and Brett Fair.
Footnotes
SUPPORTING INFORMATION AVAILABLE
UV-vis, GC-MS data and experimental details. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Anderson JL, An Y-Z, Rubin Y, Foote CS. Photophysical Characterization and Singlet Oxygen Yield of a Dihydrofullerene. J Am Chem Soc. 1994;116(21):9763, 9764. [Google Scholar]
- 2.Dugan LL, Gabrielsen JK, Yu SP, Lin T-S, Choi DW. Buckminsterfullerenol Free Radical Scavengers Reduce Excitotoxic and Apoptotic Death of Cultured Cortical Neurons. Neurobiology of Disease. 1996;3(2):129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 3.Samal S, Geckeler KE. DNA-Cleavage by Fullerene-Based Synzymes. Macromolecular Bioscience. 2001;1(8):329–331. [Google Scholar]
- 4.Tabata Y, Murakami Y, Ikada Y. Antitumor Effect of Poly(Ethylene Glycol)-Modified Fullerene. Fullerenes, Nanotubes and Carbon Nanostructures. 1997;5(5):989–1007. [Google Scholar]
- 5.Kasermann F, Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral Research. 1997;34(1):65–70. doi: 10.1016/s0166-3542(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 6.Oberdörster E. Manufactured nanomaterials (fullerene, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perpect. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. The differential cytotoxicity of water soluble fullerenes. Nano Lett. 2004;4:1881–1887. [Google Scholar]
- 8.Brant J, Lecoanet H, Hotze M, Wiesner M. Comparison of Electrokinetic Properties of Colloidal Fullerenes (n-C60) Formed Using Two Procedures. Environ Sci Technol. 2005;39(17):6343–6351. doi: 10.1021/es050090d. [DOI] [PubMed] [Google Scholar]
- 9.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60] Fullerene is a Powerful Antioxidant in Vivo with No Acute or Subacute Toxicity. Nano Lett. 2005;5(12):2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 10.Henry TB, Menn FN, Fleming JT, Wilgus J, Compton RN, Sayler GS. Attributing Effects of Aqueous C60 Nano-Aggregates to Tetrahydrofuran Decomposition Products in Larval Zebrafish by Assessment of Gene Expression. Environ Health Perspect. 2007;115(7):1059–1065. doi: 10.1289/ehp.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isakovic A, Markovic Z, Nikolic N, Todorovic-Markovic B, Vranjes-Djuric S, Harhaji L, Raicevic N, Romcevic N, Vasiljevic-Radovic D, Dramicanin M, Trajkovi V. Inactivation of nanocrystalline C60 cytotoxicity by γ-irradiation. Biomaterials. 2006;27(29):5049–5058. doi: 10.1016/j.biomaterials.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Cho M, Fortner JD, Lee J, Huang C-H, Hughes JB, Kim J-H. Delineating Oxidative Processes of Aqueous C60 Preparations: Role of THF Peroxide. Environmental Science & Technology. 2009;43(1):108–113. doi: 10.1021/es8019066. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi S, Alargova RG, Tsujii K. Stable dispersions of fullerenes, C-60 and C-70, in water: Preparation and characterization. Langmuir. 2001;17:6013–6017. [Google Scholar]
- 14.Fortner JD, Lyon DY, Sayes CM, Boyd AM, Falkner JC, Hotze EM, Alemany LB, Tao YJ, Guo W, Ausman KD, Colvin VL, Hughes JB. C-60 in water: Nanocrystal formation and microbial response. Environmental Science & Technology. 2005;39:4307–4316. doi: 10.1021/es048099n. [DOI] [PubMed] [Google Scholar]
- 15.Del Barrio MA, Pengzu Zhou JH, Cauchon N. Simultaneous determination of formic acid and formaldehyde in pharmaceutical excipients using headspace GC/MS. Journal of Pharmaceutical and Biomedical Analysis. 2006;41:738–743. doi: 10.1016/j.jpba.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Paoli GD. A Rapid GC-MS Determination of Gamma-Hydroxybutyrate in Saliva. Journal of Analytical Toxicology. 2007;32:298–302. doi: 10.1093/jat/32.4.298. [DOI] [PubMed] [Google Scholar]
- 17.Ukeda H, Maeda S, Ishii T, Sawamura M. Spectrophotometric Assay for Superoxide Dismutase Based on Tetrazolium Salt 3′-{1-[(Phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulfonic Acid Hydrate Reduction by Xanthine-Xanthine Oxidase. Analytical Biochemistry. 1997;251(2):206–209. doi: 10.1006/abio.1997.2273. [DOI] [PubMed] [Google Scholar]
- 18.Bartosz G. Use of spectroscopic probes for detection of reactive oxygen species. Clinica Chimica Acta. 2006;368(1–2):53–76. doi: 10.1016/j.cca.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 19.McCord JM, Fridovic I. Utility of Superoxide Dismutase in Studying Free Radical Reactions 2. Mechanism of Mediation of Cytochrome-C Reduction by a Variety of Electron Carriers. Journal of Biological Chemistry. 1970;245(6):1374–1378. [PubMed] [Google Scholar]
- 20.Pickering KD, Wiesner MR. Fullerol-Sensitized Production of Reactive Oxygen Species in Aqueous Solution. Environmental Science & Technology. 2005;39(5):1359–1365. doi: 10.1021/es048940x. [DOI] [PubMed] [Google Scholar]
- 21.Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano. 2008 doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006;6(8):1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 23.Mehrvar M, Anderson WA, Moo-Young M. Photocatalytic degradation of aqueous tetrahydrofuran, 1,4-dioxane, and their mixture with TiO2. International Journal of Photoenergy. 2000;2(2):67–80. [Google Scholar]
- 24.Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45(9):1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usenko CY, Harper SL, Tanguay RL. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicology and Applied Pharmacology. 2008;229(1):44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Lee C, Yoon J. Kinetics and mechanisms of DMSO (dimethylsulfoxide) degradation by UV/H2O2 process. Water Research. 2004;38(10):2579–2588. doi: 10.1016/j.watres.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 27.Meinelt T, Pietrock M, Burnison K, Steinberg C. Formaldehyde toxicity is altered by calcium and organic matter. Journal of Applied Ichthyology. 2005;21(2):121–124. [Google Scholar]
- 28.Kocan RM, Landolt ML, Sabo KM. In vitro toxicity of eight mutagens/carcinogens for three fish ceil lines. Bulletin of Environmental Contamination and Toxicology. 1979;23(1):269–274. doi: 10.1007/BF01769954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.