Abstract
Background
Breast cancer occurs rarely in men. To the best of our knowledge, there are no population-based estimates of the incidence of HER2-neu-positive breast cancer or of the distribution of breast cancer subtypes among male patients. We explored breast tumor subtype distribution by race/ethnicity among men in the large, ethnically diverse population of California.
Methods
We included male breast cancer patients diagnosed with invasive breast cancer between 2005-2009 with known ER, PR and HER2-neu status reported to the California Cancer Registry. Among the patients with hormone receptor (HR)-positive tumors, survival probabilities between groups were compared using log-rank tests.
Results
Six-hundred and six patients were included. Median age at diagnosis was 68 years. Four hundred and ninety four (81.5%) patients had HR+ tumors, defined as ER+ and/or PR+ and HER2-negative. Ninety (14.9%) had HER2-neu-positive, and 22 (3.6%) had triple receptor-negative tumors (TN). Among HR+ patients, Non-Hispanic Blacks and Hispanics were more likely to have PR negative tumors compared to Non-Hispanic Whites. There was a borderline statistically significant difference in survival according to tumor subtype (p=0.088). Differences in survival according to race/ethnicity were seen among all patients (p=0.087) and among those with HR+ tumors (p=0.0170), with Non-Hispanic Blacks having poorer outcomes.
Conclusions
In this large, representative cohort of male breast cancer patients, the distribution of tumor subtypes was different from that reported for females and varied by race/ethnicity. Non-Hispanic Blacks were more likely to have triple receptor-negative tumors and more likely to have ER+/PR- tumors than white men.
BACKGROUND
Male breast cancer is an uncommon disease, and it accounts for only 0.6-1% of all breast cancer cases 1. In 2011, it is estimated that a total of 2140 males will be diagnosed with breast cancer in the United States 2. The etiology of male breast cancer is unclear, but hormonal levels and testicular abnormalities play a role in the development of this disease. BRCA2 mutations have been shown to confer a significant risk of breast cancer in men. Other recognized risk factors include radiation exposure, family history of breast cancer, Klinefelter syndrome and different benign breast conditions 3, 4.
Prior reports suggest that cancers of the male breast are more likely than female breast cancers to have a ductal histology and significantly more likely to express hormone receptors, even after adjustment for tumor stage, grade, and patient age 5, 6. Early reports had suggested equivalent or even higher rates of Her2-neu overexpression in male than female breast cancer 4, 7-9. However, those studies were small and were performed before improved standardization of assay methodology and may have overestimated Her2-neu overexpression 3, 10.
As knowledge about the biology of breast cancer improves, it has become clear that breast cancers should be classified according to tumor subtype. The categorization of breast cancers into hormone positive, Her2-neu positive and triple receptor-negative tumors has important implications in the prognosis and in the management of breast cancer patients. Among female patients with breast cancer there are strong racial/ethnic differences in the tumor subtype distribution 11. In this population-based study we explored the distribution of the different breast cancer tumor subtypes according to race/ethnicity among male breast cancer patients identified through the California Cancer Registry (CCR).
METHODS
Study Population and Variables
We used data from the California Cancer Registry (CCR), a population-based registry that collects data from all the cancer cases diagnosed in California since 1988. By state law all cancer cases are reported to the CCR from hospitals and any other facility providing care or therapy to cancer patients residing in California 12; it is estimated that case ascertainment is 99% complete 13. Cancer patient information including demographic characteristics (age, race/ethnicity), tumor characteristics and treatment information were abstracted from the medical record by trained tumor registrars. The CCR collected data on estrogen receptor (ER) and progesterone receptor (PR) since 1990 and started collecting HER2-neu data since 1999; however, for HER2-neu reporting, uptake was not immediate. Issues associated with collection and reporting of HER2-neu status have been previously described 14. Because HER2-neu was more likely to be present in the medical record and thereby reported to the CCR for patients diagnosed after 2005, we limited our assessment to cases diagnosed in this year or later.
For the present study, all male breast cancer (International Classification of Disease-Oncology codes [ICD-O-3] C50.0-C50.9) patients diagnosed with a first primary invasive breast cancer between 2005 and 2009 were identified (n=829). Patients diagnosed on death certificate or autopsy only (n=4) and those with missing or invalid follow up data were excluded (n=1); additionally only patients with complete information on ER, PR and HER2-neu status were included (resulting n=606). Patients excluded from our study for unknown receptor status did not differ significantly from those included with respect to age or race/ethnicity. No differences were seen in the distribution according to stage (all p>0.05), however patients excluded from the study had a higher proportion of unknown stage (p<.001). In addition, patients diagnosed in 2005, were more likely to be excluded than patients who were diagnosed in subsequent years (p<0.020)
We used the American Joint Committee on Cancer (AJCC) staging system (6th edition). Tumor histology was classified as ductal (ICD-O-3 morphology code 8500, 8501, 8502, 8503, 8504, 8507, 8708, 8523), lobular (ICD-O-3 morphology code 8520, 8521) and papillary/mixed/other (morphology code 8550, 8260, 8522, 8524). Breast cancer subtypes were defined as: Hormone receptor-positive (HR) (ER-positive and/or PR-positive and HER2-neu-negative), HER2-neu-positive (HER2-neu-positive, regardless or ER and PR) and triple -negative (TN) (HER2-neu-negative and ER-negative and PR-negative). Race/ethnicity was categorized into four mutually exclusive groups: Non-Hispanic White, Non-Hispanic Black, Hispanic and Asian/Pacific Islander (API)/other.
Data analysis
Descriptive statistics were used to evaluate the characteristics of the patient population. Differences between groups were assessed using chi square test and Fisher's exact test. For each patient, information on vital status was obtained from the CCR. The CCR regularly updates vital status information through hospital follow-up and linkages with state and national databases and agencies, such as the National Death Index, Social Security Administration, Centers for Medicaid and Medicare Services, Office of Statewide Health Planning and Development, CalVoter, Indian Health Service, National Change of Address, Department of Motor Vehicles, birth certificate linkages, and state vital statistics. Seventy-four percent of our cases had confirmed vital status as of December 31st, 2010, and 88% had confirmed vital status within 3 months of that date. Survival time was measured in months since diagnosis. Kaplan-Meier analysis was used to calculate probability of survival. Cases were censored if they were alive as of December 31, 2010. The median follow-up time was 33.7 months (range 2.7-71.9 months). Cases with complete follow-up did not differ significantly on age, race, stage, tumor subtype, year of diagnosis, or socioeconomic status from cases without current follow-up (chi square p>.05).
Survival analysis according to tumor subtype and race/ethnicity was performed, and differences between groups were compared using the log-rank test. A sub analysis of survival by race/ethnicity was also performed limited to patients with HR-positive tumors. A Cox proportional hazard analysis was performed, including race/ethnicity, stage and age in the model. Tumor subtype had a significant interaction with time and violated the proportional hazards assumption; therefore we stratified the final model by subtype. Statistical analyses were performed using SAS version 9.3 software (SAS institute Inc., Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria).
De-identified data from the CCR was used for all the analyses; however, in order to preserve confidentiality, in all the tables, cells with numbers < 5 were suppressed. The IRB of the Cancer Prevention Institute of California approved this analysis, while that of the University of Texas MD Anderson Cancer Center provided an exemption.
RESULTS
A total of 829 male patients with primary invasive breast cancer were identified between 2005 and 2009. Of them, 606 (73.2%) had available information on ER, PR and HER2 status. Among the 606 patients included in this study, the median age at diagnosis was 68 years old. The majority of the patients were Non-Hispanic White (71%, n=431), 7.4% (n=45) were Non-Hispanic Black, 11.1% (n=67) were Hispanic, and 10.4% (n=63) were API/other/unknown. Four hundred and ninety four (81.5%, 95%CI 78.4%-84.6%) of the patients had HR-positive tumors, ninety (14.9%, 95%CI 12.0%-17.7%) had HER2-positive and 22 (3.6%, 95%CI 2.1%-5.1%) had TN tumors. Table-1 shows patient characteristics according to tumor subtype. There was a statistically significant difference in the distribution of tumor subtype according to age (p=0.020) with younger patients having more HER2-positive disease. Among Non-Hispanic Whites, 82.8% (95%CI 79.3%-86.4%) of the patients had HR-positive tumors, 14.6% (95%CI 11.3%-18.0%) had HER2-positive and 2.6%(95%CI 1.1%-4.0%) had TN breast cancer. In contrast, the distribution among Non-Hispanic Blacks according to tumor subtype was 73.3% (95%CI 60.4%-86.3%); 17.8% (95%CI 6.6%-29.0%) and 8.9% (95%CI 0.6%-17.2%) and for Hispanics was 77.6% (95%CI 67.6%-87.6%), 16.4% (95%CI 7.6-27.5%) and 6.0% (95%CI 0.3%-11.6%) for HR-positive, HER2-neu-positive and TN tumors, respectively.
Table 1.
Patient characteristics according to tumor subtype
| Tumor Subtype | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | HR+ | Her 2+ | Triple negative | |||||
| N | N | Row % | N | Row % | N | Row % | ||
| 606 | 494 | 81.5% | 90 | 14.9% | 22 | 3.6% | ||
| Age at diagnosis | p=0.0204 | |||||||
| <50 | 60 | 40 | 66.7% | 17 | 28.3% | <5 * | 5.0% | |
| 50-69 | 268 | 223 | 83.2% | 38 | 14.2% | 7 | 2.6% | |
| 70+ | 278 | 231 | 83.1% | 35 | 12.6% | 12 | 4.3% | |
| Race | p=0.2274 | |||||||
| Non-Hispanic White | 431 | 357 | 82.8% | 63 | 14.6% | 11 | 2.6% | |
| Non-Hispanic Black | 45 | 33 | 73.3% | 8 | 17.8% | <5 * | 8.9% | |
| Hispanic | 67 | 52 | 77.6% | 11 | 16.4% | <5 * | 6.0% | |
| API/other/unknown | 63 | 52 | 82.5% | 8 | 12.7% | <5 * | 4.8% | |
| Year of diagnosis | ||||||||
| 2005 | 87 | 66 | 75.9% | 17 | 19.5% | <5 * | 4.6% | |
| 2006 | 133 | 102 | 76.7% | 22 | 16.5% | 9 | 6.8% | |
| 2007 | 114 | 94 | 82.5% | 16 | 14.0% | <5 * | 3.5% | |
| 2008 | 133 | 110 | 82.7% | 20 | 15.0% | <5 * | 2.3% | |
| 2009 | 139 | 122 | 87.8% | 15 | 10.8% | <5 * | 1.4% | |
| Socioeconomic status | p=0.4822 | |||||||
| 1 | 70 | 57 | 81.4% | 8 | 11.4% | 5 | 7.1% | |
| 2 | 97 | 77 | 79.4% | 19 | 19.6% | <5 * | 1.0% | |
| 3 | 125 | 101 | 80.8% | 19 | 15.2% | 5 | 4.0% | |
| 4 | 119 | 95 | 79.8% | 19 | 16.0% | 5 | 4.2% | |
| 5 | 195 | 164 | 84.1% | 25 | 12.8% | 6 | 3.1% | |
| Tumor histology | p=0.6915 | |||||||
| ductal | 513 | 419 | 81.7% | 77 | 15.0% | 17 | 3.3% | |
| lobular | 11 | 9 | 81.8% | <5 * | 18.2% | 0 | 0 | |
| papillary/mixed/other | 82 | 66 | 80.5% | 11 | 13.4% | 5 | 6.1% | |
| Grade | p<.0001 | |||||||
| Grade I | 83 | 71 | 85.5% | 9 | 10.8% | <5 * | 3.6% | |
| Grade II | 315 | 271 | 86.0% | 42 | 13.3% | <5 * | 0.6% | |
| Grade III/ IV | 183 | 133 | 72.7% | 34 | 18.6% | 16 | 8.7% | |
| Unknown | 25 | 19 | 76.0% | 5 | 20.0% | <5 * | 4.0% | |
| Stage at diagnosis | p=0.2277 | |||||||
| I | 195 | 167 | 85.6% | 21 | 10.8% | 7 | 3.6% | |
| II | 248 | 203 | 81.9% | 36 | 14.5% | 9 | 3.6% | |
| III | 101 | 80 | 79.2% | 18 | 17.8% | <5 * | 3.0% | |
| IV/ unknown | 62 | 44 | 71.0% | 15 | 24.2% | <5 * | 4.8% | |
| Surgery | p=0.0002 | |||||||
| No surgery | 32 | 22 | 68.8% | 8 | 25.0% | <5 * | 6.3% | |
| Mastectomy | 495 | 413 | 83.4% | 73 | 14.7% | 9 | 1.8% | |
| Breast conservation | 79 | 59 | 74.7% | 9 | 11.4% | 11 | 13.9% | |
| Radiation therapy | p=0.6657 | |||||||
| no/unknown | 441 | 356 | 80.7% | 69 | 15.6% | 16 | 3.6% | |
| yes | 165 | 138 | 83.6% | 21 | 12.7% | 6 | 3.6% | |
| Vital status as of Dec. 31, 2010 | p=0.0770 | |||||||
| Dead | 117 | 89 | 76.1% | 20 | 17.1% | 8 | 6.8% | |
| Alive | 489 | 405 | 82.8% | 70 | 14.3% | 14 | 2.9% | |
Cell counts <5 have been suppressed to protect patient confidentiality
HR-positive patients represented the most common breast cancer subtype in our patient population (n=494), so we evaluated ER and PR status separately. Table-2 shows the different combinations of ER and PR status among HR-positive patients according to race/ethnicity. We observed that Hispanics and Non-Hispanic Blacks were more likely to have ER-positive and PR-negative tumors compared to Non-Hispanic Whites (17.3% (95%CI 7.0%-27.6%), 15.2% and 4.5% (95% CI 2.3-6.6%), respectively p<0.001).
Table 2.
Tumor receptor status combinations according to race for HR+ and Her2- patients
| Race | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Non-Hispanic White | Non-Hispanic Black | Hispanic | API/other/unknown | |||||
| N | N | Column % | N | Column % | N | Column % | N | Column % | |
| HR status | |||||||||
| ER+PR+ | 456 | 336 | (94.1%) | 27 | (81.8%) | 42 | (80.8%) | 51 | (98.1%) |
| ER+PR- | 31 | 16 | (4.5%) | 5 | (15.2%) | 9 | (17.3%) | <5 * | (1.9%) |
| ER-PR+ | 7 | 5 | (1.4%) | <5 * | (3.0%) | <5 * | (1.9%) | 0 | 0 |
| All | 494 | 357 | (100.0%) | 33 | (100.0%) | 52 | (100.0%) | 52 | (100.0%) |
P=<0.001
Cell counts <5 have been suppressed to protect patient confidentiality
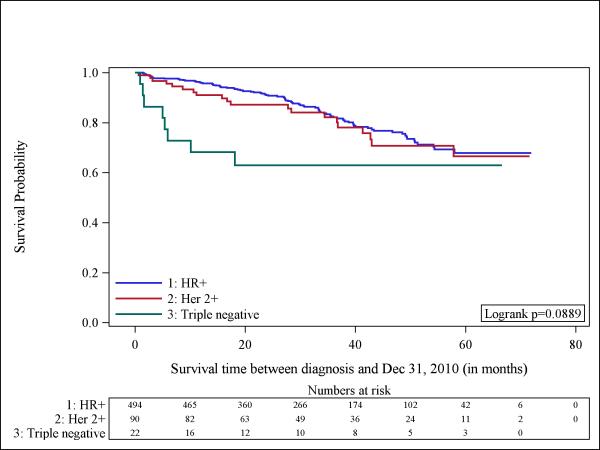
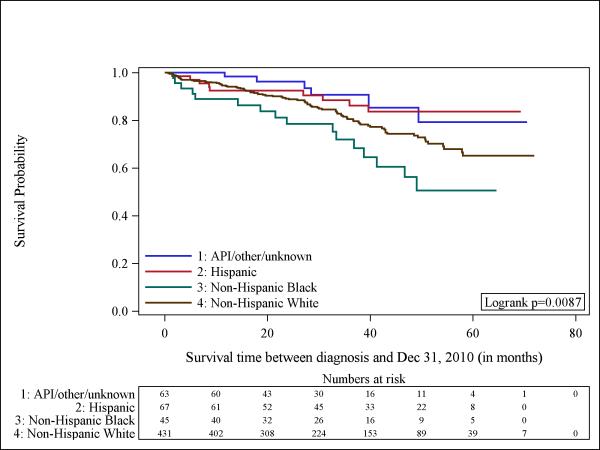
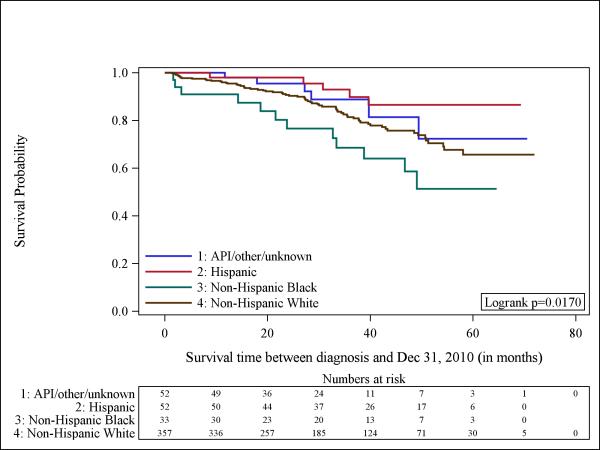
Although we had small numbers of cases and deaths to study (total number of events= 117), we next evaluated whether overall survival varied by tumor subtype. Figure-1 shows a Kaplan-Meier curve that suggests worse survival among patients with TN tumors, although the log-rank-test was borderline significant (p=0.088). Figure-2 shows significant differences in survival by race/ethnicity among all the participants (n=606) with Non-Hispanic Blacks being the group with the worst outcomes (p=0.008). The observed differences could be explained by the different distribution of tumor subtype according to race. For that reason, we aimed to explore the survival of patients according to each tumor subtype by race/ethnicity. Unfortunately, given the small number of patients we could not examine separate influences for HER2-neu-positive and TN tumors. In Figure-3 we show the Kaplan Meier curve according to race/ethnicity for patients with HR-positive tumors (n=494), demonstrating significant survival differences according to race/ethnicity (p=0.017). Among this group of patients, Non-Hispanic Blacks had the worst survival.
Fig 1.
Kaplan-Meier curves illustrate the probability of survival among men with breast cancer according to tumor subtype (n = 606). Hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2) and triple negative.
Fig 2.
Kaplan-Meier curves illustrate the probably of survival among men with breast cancer according to race/ethnicity. API indicates Asian/Pacific Islander (n = 606).
Fig 3.
Kaplan-Meier curves illustrate the probably of survival among men with breast cancer according to race/ethnicity for those with (hormone receptor)-positive tumors (n = 494). API indicates Asian/Pacific Islander.
After adjusting for subtype, stage and age and using Non-Hispanic Whites as a reference category, we observed no significant differences in survival according to race/ethnicity (Hispanics HR: 0.78 95% CI 0.38-1.58; Non-Hispanic Blacks HR: 1.32; 95% CI 0.75-2.33 and API/other HR: 0.631 95% CI 0.27-1.46), suggesting that the effect of race/ethnicity on survival was attenuated by stage.
DISCUSSION
To the best of our knowledge, this is the first and largest population-based study evaluating tumor subtype distributions in male breast cancer patients. The majority of the male breast cancer patients in our study (81.5%) had HR-positive tumors. We observed that a clinically significant proportion of patients have HER2-positive tumors (14.9%), and a low proportion have TN tumors (3.6%). In a large study including more than 60,000 females using data from the CCR, 63.8% of the patients had HR-positive, 22.8% had HER2-neu-positive and 13.4% had TN tumors 15, suggesting that male patients have lower rates of HER-2 positive and triple negative tumors than their female counterparts. It has been well described that male patients with breast cancer are more likely than their female counterparts to have HR-positive tumors 3, 5, 6, 16. It is estimated that the incidence of ER and PR– positive tumors is 82% and 75% among males and 69% and 52% among females4. The data on HER2 overexpression is inconsistent in the literature; older studies reported similar or higher rates of HER2-neu overexpression between males and females 3, 4, 7-9, with pooled data suggesting a 35% rate of overexpression in males. In a study published by Bloom et al 10, 58 male breast cancer specimens were evaluated. HER2-neu overexpression was identified in only one case (1.7%) and amplification was not seen. Importantly, this study included a subset of patients (n=26) that had been considered Her2-neu overexpressed in a previous report 9 and none of them was found to have overexpression at the time of the reevaluation. The small number of patients and the differences in techniques used for HER2-neu determination may underlie the overestimation of the previously reported rates of HER2-neu expression among male breast cancer patients 3. In our study, 14.9% of the patients were categorized as HER2-neu-positive. Our results are similar to the 17% rate that Muir and colleagues reported 16 and to the 15% rate of HER2 amplification that the European Institute of Oncology described 17; however, these are small studies including only 59 and 27 patients, respectively. The rates of TN tumors in male breast cancer patients are not clearly described and the frequency remains largely unknown. We observed a 3.8% rate of TN tumors, a rate that is substantially lower than what has been reported in female patients. A recent study by Arslan et al described a 5.9% rate of TN patients in a Turkish population of male breast cancer patients (n=118) 18.
Among female patients with breast cancer, the categorization of breast tumors into different subtypes has important prognostic implications. After adjusting for clinical and demographic characteristics, patients with HR-positive tumors have better outcomes compared with patients with HER2-neu-positive and TN tumors. It is not clear if breast cancer subtypes are associated with the same prognostic factors among males. A Mayo Clinic study reported no association between HER2-neu overexpression and survival in univariate analysis among a cohort of 76 male breast cancer patients 8. More recently Arslan et al (n=118), observed a non statistically significant difference in 5-year overall survival among male patients with HER2-neu-positive compared to patients with HER2-neu-normal tumors (85 months vs 144 months, p=0.30) 18. In our study we observed that patients with TN tumors tend to have worse survival compared to patients with HR positive or HER2-neu-positive tumors; this difference had borderline statistical significance (p=0.088). It is important to point out that our patient population included patients that were treated after the approval of trastuzumab-based chemotherapy; therefore, is possible that the patients with HER2-neu-positive tumors received this treatment, likely improving their outcomes when compared with patients with TN tumors. Our results suggest that among male breast cancer patients, tumor subtype does hold prognostic significance. However, the small number of patients with TN and HER2-neu-positive tumors, the short follow-up, and the fact that we could not perform any multivariable analysis to adjust for clinically relevant characteristics, warrant careful interpretation.
Among female patients with breast cancer, clear racial/ethnic differences exist in the distribution of tumor subtypes 11, 15. Non-Hispanic Blacks have higher rates of TN or basal-like tumors and are less likely to have tumors that are ER-positive and PR-positive. In our study, a similar pattern seems to be present among male patients with breast cancer, although this difference was not statistically significant (p=0.227). The high frequency of tumors associated with poor prognosis is one theory promulgated to explain, at least in part, the poor outcomes seen in Non-Hispanic Black female patients compared to other ethnic/racial groups. Among female breast cancer patients with HR-positive tumors, worse outcomes have been reported in Non-Hispanic Blacks compared to Non-Hispanic Whites 19, 20. In our study, among male breast cancer patients with HR-positive tumors (n=494), Non-Hispanic Blacks had worse survival compared to the other racial/ethnic groups (p=0.017).
When we explored separately the distribution PR status among patients with ER-positive tumors, significant differences were observed (p<0.001). Only 4.25% of Non-Hispanic Whites had PR-negative tumors, compared with 15.26% and 17.3% among Non-Hispanic Blacks and Hispanics, respectively. In a large study from the CCR evaluating 1796 male patients with breast cancer from 1988-2000 21, a similar observation was described. Among patients with known PR status (n=862), Non-Hispanic Whites, Hispanic and Asians had PR-positive tumors (81%, 90% and 79.6%, respectively) more frequently than Non-Hispanic Blacks (64.1%). We consider this to be an important observation that can help understand the poor outcomes seen among Non-Hispanic Blacks. In female patients, ER+/PR- tumors have been associated with tamoxifen resistance and a more aggressive phenotype compared to ER+/PR+ tumors22-24. Female patients older than 60 years old with ER+/PR- tumors have worse outcomes compared with patients in the same age category but with ER+/PR+ tumors25.
In an exploratory analysis we aimed to eliminate the potential effect of ER+/PR-tumors by limiting the survival analysis according to race/ethnicity to patients with ER+/PR+ tumors exclusively. Non-Hispanic Blacks had worse survival (p=0.023). Unfortunately, this does not help explain the clinical behavior of Hispanic patients who despite also having high rates of ER+/PR- tumors have better survival compared to Non-Hispanic Blacks.
Our study had several important limitations. Despite including one of the largest and most diverse populations of male breast cancer patients in the U.S., the overall number of patients with HER2-positive and TN tumors was small. Also, our necessarily short follow-up and the small number of events limited our statistical analyses and we were only able to build a multivariate model to evaluate outcomes adjusting for a limited number of baseline characteristics. Limitations inherent to population-based studies such as potential errors in reporting or differences in laboratory techniques to assign the tumor marker status cannot be quantified. The status of the biomarkers (ER, PR and HER2) was obtained from the CCR but it was originated in different local pathology laboratories, therefore no information on quality control is available and no central biomarker review was possible. Also, the categories that we used in this manuscript to categorize tumor subtypes are derived exclusively from data on ER, PR and HER2; no information was available on molecular profiles. Our cohort included only patients with known ER, PR and HER2 status. Patients included in the study had similar characteristics than those excluded, however those excluded were more likely to be diagnosed in 2005. A sensitivity analysis (data not shown) including only patients diagnosed from 2006-2009 (n=519) show similar results.
To our knowledge, this is the first population-based study reporting tumor subtypes among male breast cancer patients. The majority of the patients had HR-positive tumors; however, HER2-neu overexpression was seen in 14.9% of the patients and 3.6% of the patients had TN tumors; this observation has potential prognostic and treatment implications. Among male patients with breast cancer, differences in tumor subtype exist among different racial/ethnic groups. Non-Hispanic Blacks were numerically more likely to have triple receptor-negative tumors and more likely to have ER+/PR- tumors than Non-Hispanic Black patients.
Financial support/Acknowledgments
This study was supported by the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) program under contract HHSN261201000040C awarded to the Cancer Prevention Institute of California (CPIC), DHHS - NIH - National Cancer Institute: 2P30 CA016672 (PP-RP6) Cancer Center Support grant to MD Anderson Cancer Center.
Dr. Sharon H. Giordano is supported by ACS (RSGI-09-149-01-CPHS) and CPRIT (RP101207-P02).
Other information: The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the SEER Program under contract HHSN261201000040C awarded to CPIC (formerly the Northern California Cancer Center), contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute; and R01-ES015552 from the NIEHS and R01-CA121052 from NCI. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors thank Scarlett Gomez for her contributions to this study.
Corrections to Figure Captions for NIHMS #417913 Male Breast Cancer According to Tumor Subtype and Race
Footnotes
The authors have no financial disclosures.
No authors report any financial conflict of interest.
REFERENCES
- 1.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51–7. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137(8):678–87. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114–22. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77–86. doi: 10.1023/B:BREA.0000010701.08825.2d. [DOI] [PubMed] [Google Scholar]
- 6.Stalsberg H, Thomas DB, Rosenblatt KA, et al. Histologic types and hormone receptors in breast cancer in men: a population-based study in 282 United States men. Cancer Causes Control. 1993;4(2):143–51. doi: 10.1007/BF00053155. [DOI] [PubMed] [Google Scholar]
- 7.Leach IH, Ellis IO, Elston CW. c-erb-B-2 expression in male breast carcinoma. J Clin Pathol. 1992;45(10):942. doi: 10.1136/jcp.45.10.942-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayson D, Erlichman C, Suman VJ, et al. Molecular markers in male breast carcinoma. Cancer. 1998;83(9):1947–55. doi: 10.1002/(sici)1097-0142(19981101)83:9<1947::aid-cncr10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Gattuso PRVGL Castelli MJ, Wick RM. Prognostic Factors for Carcinoma of the Male Breast. Int J Surg Pathol. 1995;2(3):199–206. [Google Scholar]
- 10.Bloom KJ, Govil H, Gattuso P, Reddy V, Francescatti D. Status of HER-2 in male and female breast carcinoma. Am J Surg. 2001;182(4):389–92. doi: 10.1016/s0002-9610(01)00733-4. [DOI] [PubMed] [Google Scholar]
- 11.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 12.Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001;19(2):343–53. doi: 10.1200/JCO.2001.19.2.343. [DOI] [PubMed] [Google Scholar]
- 13.Telli ML, Chang ET, Kurian AW, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127(2):471–8. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer KR BM, Creech C, Schlang NC, Caggiano V. Data quality assesment of HER2 in the Sacramento region of the California Cancer Registry. Journal of Registry Management. 2007;34:4–7. [Google Scholar]
- 15.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15(6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 16.Muir D, Kanthan R, Kanthan SC. Male versus female breast cancers. A population-based comparative immunohistochemical analysis. Arch Pathol Lab Med. 2003;127(1):36–41. doi: 10.5858/2003-127-36-MVFB. [DOI] [PubMed] [Google Scholar]
- 17.Curigliano G, Colleoni M, Renne G, et al. Recognizing features that are dissimilar in male and female breast cancer: expression of p21Waf1 and p27Kip1 using an immunohistochemical assay. Ann Oncol. 2002;13(6):895–902. doi: 10.1093/annonc/mdf166. [DOI] [PubMed] [Google Scholar]
- 18.Arslan UY, Oksuzoglu B, Ozdemir N, et al. Outcome of non-metastatic male breast cancer: 118 patients. Med Oncol. 2011 doi: 10.1007/s12032-011-9978-9. [DOI] [PubMed] [Google Scholar]
- 19.Chavez-Macgregor M, Litton J, Chen H, et al. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116(17):4168–77. doi: 10.1002/cncr.25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparano JW,W, Stears V, et al. Black race is associated with a worse outcome in patients with hormone receptor positive, Her2-normal breast cancer treated with chemohormonal therapy. Cancer Res. 2010;69:494s. [Google Scholar]
- 21.O'Malley C, Shema S, White E, Glaser S. Incidence of male breast cancer in california, 1988-2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat. 2005;93(2):145–50. doi: 10.1007/s10549-005-4517-z. [DOI] [PubMed] [Google Scholar]
- 22.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–61. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 23.Neven P, Pochet N, Drijkoningen M, et al. Progesterone receptor in estrogen receptor-positive breast cancer: the association between HER-2 and lymph node involvement is age related. J Clin Oncol. 2006;24(16):2595. doi: 10.1200/JCO.2005.05.1334. author reply 95-7. [DOI] [PubMed] [Google Scholar]
- 24.Thakkar JP, Mehta DG. A review of an unfavorable subset of breast cancer: estrogen receptor positive progesterone receptor negative. Oncologist. 2011;16(3):276–85. doi: 10.1634/theoncologist.2010-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu KD, Liu GY, Di GH, et al. Progesterone receptor status provides predictive value for adjuvant endocrine therapy in older estrogen receptor-positive breast cancer patients. Breast. 2007;16(3):307–15. doi: 10.1016/j.breast.2006.12.011. [DOI] [PubMed] [Google Scholar]