Abstract
For sexually and directly transmitted infectious diseases, social connections influence transmission because they determine contact between individuals. For pathogens that are indirectly transmitted by arthropod vectors, the movement of the vectors is thought to diminish any potential role of social connections. Results from a recent study of mosquito-borne dengue virus (DENV), however, indicate that human movement alone can explain significant spatial variation in urban transmission rates. Because movement patterns are structured by social ties, this result suggests that social proximity may be a good predictor of infection risk for DENV and other pathogens transmitted by the mosquito Aedes aegypti. Here we investigated the effect of socially structured movement on DENV transmission using a spatially explicit, agent-based transmission model. When individual movements overlap to a high degree within social groups we were able to recreate infection patterns similar to those detected in dengue-endemic, northeastern Peru. Our results are consistent with the hypothesis that social proximity drives fine-scale heterogeneity in DENV transmission rates, a result that was robust to the influence of mosquito dispersal. This heterogeneity in transmission caused by socially structured movements appeared to be hidden by the diffusive effect of mosquito dispersal in aggregated infection dynamics, which implies heterogeneity could be present and active in real dengue systems without being easily noticed. Accounting for socially determined, overlapping human movements could substantially improve the efficiency and efficacy of dengue surveillance and disease prevention programs as well as result in more accurate estimates of important epidemiological quantities, such as R0.
Keywords: Infectious disease, Spatial epidemiology, Social networks, Agent-based model
Introduction
Social connections between people play an important role in the spatial spread of many infectious diseases because individuals familiar with each other are more likely to come into contact within the narrow spatial and temporal dimensions often needed for pathogen transmission to occur. Exactly how social connections shape the spatial spread of a pathogen depends, in part, on the mode of transmission. For example, spatial propagation of sexually transmitted diseases (STDs) is achieved almost exclusively through social connections. When observed geographically, STD infections might appear to be randomly distributed across a city due to the small number of links needed to connect any two randomly chosen individuals (Watts and Strogatz, 1998). Alternatively, John Snow’s famous ‘ghost map’ of the 1854 outbreak of indirectly transmitted cholera in London showed that infections were so geographically localized he was able to identify the exact source of exposure (Snow, 1855). Social connections in the case of this water-borne disease thus played a relatively minor role in its spread. Mosquito-borne pathogens, like malaria and dengue, have historically been treated more like cholera than an STD because of the presence and impact of the mosquito in the transmission cycle (Johnson, 2008; Bousema et al., 2012; Schærström, 1996). The standard view is that each infected human host is a spatially fixed pathogen source, with subsequent infections localized to that source on the scale of mosquito dispersal. When mosquito dispersal occurs on the same spatial scale as human movement, which is a reasonable assumption for malaria, it is difficult to test this concept and parse apart the relative roles of mosquito and human movement in fine-scale, local transmission. For dengue virus (DENV), whose primary mosquito vector, Aedes aegypti, is known to be relatively sedentary and prone to disperse only short distances (e.g., <100 m, (Gubler and Kuno, 1997; Harrington et al., 2005; Getis et al., 2003)), recent empirical evidence (Stoddard et al., 2013) strongly suggests that human movement drives fine-scale, spatial transmission. Thus there is the potential that, similar to sexually and directly transmitted diseases (May, 2006), social connections will influence the spatiotemporal dynamics of dengue because members of social groups will often visit the same places where mosquitoes reside.
Dengue is a mosquito-borne disease common in the tropics and sub-tropics that is caused by infection with any of four distinct, but closely related, virus serotypes that are transmitted primarily by A. aegypti. A. aegypti lives in and around human dwellings and bites during the day. Adults disperse by flying typically short distances and may have difficulty navigating through urban landscapes (Harrington et al., 2005; Hemme et al., 2010; Scott et al., 2000a,b; Getis et al., 2003). Because of these features, patterns of human movement—especially in the large urban populations where dengue is prevalent—play a potentially large role in virus spread and persistence (Stoddard et al., 2009; Wen et al., 2012; Padmanabha et al., 2012; Teurlai et al., 2012; Mondini et al., 2009; Barmak et al., 2011; Vazquez-Prokopec et al., 2010). Variation in human movements patterns, however, are almost never incorporated in mathematical models of vector-borne diseases (Reiner et al., 2013). The vast majority of these models assume a ‘well-mixed’ human population, where each individual is equally likely to encounter every other individual and every mosquito. In reality, individuals vary considerably in the frequency, distance, and nature of their movements (González et al., 2008; Song et al., 2010; Vazquez-Prokopec et al., 2013), in ways that have implications for transmission (Perkins et al., 2013). Results from a recent study in Iquitos, Peru (Stoddard et al., 2013) indicate that individuals infected with DENV, when compared to uninfected controls, experienced greater virus exposure across locations they visited recently, regardless of the geographical distance from their home (i.e., kilometers). The percent of homes recently visited by a DENV infected person (i.e., an index case, using the vernacular of contact tracing (Ahrens and Pigeot, 2005)) where at least one concurrent DENV-infected individual lived (40%) was significantly higher than for homes visited by an uninfected, control individual (15%). The increased rate of infection was not correlated with distance from the index individuals’ home, precluding the possibility that mosquito movement explained the observed infection patterns. Because there is an estimated 15–17 day delay between primary and secondary DENV infections due to intrinsic and extrinsic incubation periods (Aldstadt et al., 2012), other concurrently observed infections must have occurred around the same time as the DENV infected index person that initiated the contact cluster investigation. Thus, people who lived in houses connected by the movements of an infected individual shared an elevated risk of DENV infection with the index. The relative size of this elevated risk, when compared to neighborhood-wide infection rates, was too large to be explained due to coincidental infections across multiple locations within the neighborhood or city.
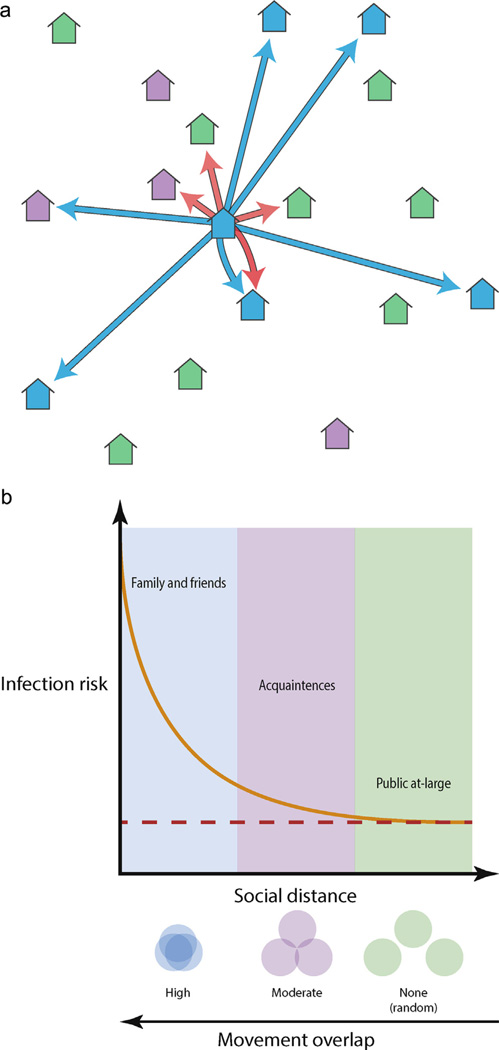
An explanation for why infection risk is elevated in households visited by a DENV infected individual concerns social, not geographic, proximity (Fig. 1). The houses a person spends time in tended to be their own and those of friends and family (Fig. 1a). By extension, the people living in those places were socially connected. Thus, we expect members of social groups to overlap in their movements, frequently visiting many of the same places; e.g., they go to the house of a grandmother, uncle or friend or those people come to their home. Individual risk of infection in a given house would then correlate with social proximity to the residents (Fig. 1b). The observation of multiple concurrent infections across connected houses (Stoddard et al., 2013) could then arise via two, non-exclusive, processes: (1) approximately two weeks before the index became ill, an infective individual visited and infected mosquitoes in many of the same houses the DENV infected index person subsequently spent time in and/or (2) individuals living in houses used by the index person congregated recently at and were infected in the same house where the index was infected. On a population level, social connections would thus structure patterns of individual human movement and DENV spread.
Fig. 1.
The social proximity hypothesis. (a) As an illustration, we place 18 houses into 3 social groups: members of the same social group as the central home (blue houses), homes of acquaintances (i.e. infrequently visited homes, purple houses) and houses not visited by members of the central home (green houses). Blue arrows indicate houses visited in a given time interval by residents of the central home. Red arrows indicate houses within the dispersal range of mosquitoes living within the central home. (b) Given a household with an infectious individual mosquito, the people at greatest risk of infection there are family and friends (i.e., same social group). People more socially distant from the household, i.e., acquaintances, are less likely to visited the infested house and so their risk of infection is smaller. Thus, as social distance between people increases, the probability they will overlap somewhere mosquitoes are present diminishes. This reduces the risk of infection until it equals the risk due entirely to an infectious mosquito traversing the distance between the two houses (dashed red line).
The importance of socially structured movement for pathogen transmission has been well studied for directly transmitted diseases (Salathé and Jones, 2010; Salathé et al., 2010; Danon et al., 2009; Keeling et al., 2010), but not for indirectly transmitted, mosquito-borne diseases. An important distinction of the latter is that both mosquito and human movement will contribute to pathogen transmission at fine spatial and temporal scales. Mosquitoes may disperse to houses that are geographically close (Fig. 1a). Conversely, people are most likely to visit houses that are socially close, which can be nearby or far away (Fig. 1a). Though the data from Iquitos are consistent with the social proximity hypothesis for DENV spread (Fig. 1), we do not know how much movements do overlap nor to what extent social connections actually influence transmission dynamics. Here we tested the feasibility of the social proximity hypothesis with the following three assumptions in mind. First, socially driven, overlapping human movement is necessary to recreate observed DENV infection patterns. Second, diffusive mosquito movement weakens the impact of structured social movement. Third, the level of structured movement required to overcome diffusive mosquito movement and recreate observed infection patterns is not so strong that it is epidemiologically impossible given the rates of known ongoing, endemic transmission. To assess these assumptions of the social proximity hypothesis we constructed a spatially explicit, agent-based DENV transmission model. By varying the extent to which individual movements are defined by social connections or restricted to the households of their social group and tuning the level of mosquito dispersal, we investigated the consequences of overlapping human movement on DENV transmission dynamics across fine and aggregated spatial scales and the influence of mosquito movement on the impact of structured human movements.
Methods
Our base agent-based model (ABM) simplified several components of DENV transmission dynamics to focus on how the social structuring of human movement affects virus transmission (denoted Scenario 1, see Appendix 1 for a detailed description of the model). Of primary importance to our objectives is the extent of movement overlap among different individuals, specifically, how much time do different individuals spend in the same houses, be it their own or someone else’s. We started by simulating a grid of 400 houses with six residents each, to be consistent with the average household size in Iquitos (Stoddard et al., 2013). We then randomly assigned houses into social groups and, for every member of every house, we dictated how many of the houses they visited came from within their social group and how many came at random from the rest of the available houses (i.e., outside their social group). We set the number of houses within each social group at 6 and the number of houses each individual could visit (excluding their own) at 5, which is within the range of values observed in Iquitos (Stoddard et al., 2013; Vazquez-Prokopec et al., 2013).
We considered two additional simulation scenarios in which we altered our assumptions on household size and human movement (see SI). In Scenario 2, instead of using the mean number of residents for each home from previous studies, we used the fitted negative binomial distribution (μ = 6.2, θ = 9.07, (Stoddard et al., 2013)). By drawing the household size of each home from this distribution we allowed some homes to be sparsely populated while others were crowded, matching observations from Iquitos. In Scenario 3, based on data from the initial cluster study (Stoddard et al., 2013), we noted that 15% of the population said that they had not left their home for any substantial period of time during the outbreak. Keeping 85% of the population’s movements as in the original scenario, in Scenario 3 we forced 15% of the population to never leave their home.
We defined the number of homes an individual visits (excluding their own) within their social group as κ. At larger values of κ, we considered movements to be more socially structured because individuals would spend their time largely at houses within their social group (e.g., at κ = 5 social groups are completely disconnected). By varying κ from 0 to 5, we thus assessed the relative consequences of different levels of structure in movement patterns for virus transmission (illustrated for κ = 0, 3 and 5 in Fig. S1). We also considered the effect of mosquito movement in our models by varying the extent of dispersal among adjacent houses. Note that in the absence of mosquito dispersal and at κ = 5, transmission quickly died out within infested groups because the virus could not move from one group to another and susceptible individuals are rapidly eliminated. Due to the briefness of the outbreaks, we did not consider scenarios where the visits outside the social group changed throughout the outbreak, though over longer endemic transmission, these types of changes in movement patterns may be important.
We considered a third alteration of our base approach that questioned the importance of having mosquito movements limited to the nearest neighbors (see SI for full description). Based on results from a study of mosquito populations in Iquitos, that adult mosquitoes clustered at distances as far as 30 m from a house ((Getis et al., 2003)), we modified the basic model with mosquitoes moving to the nearest neighbors and two houses away from the location of the mosquito’s blood meal. We refer to this as Scenario 4. Finally, combining all the variations away from the base model together into a single set of simulations, Scenario 5 allowed for heterogeneous household size (Scenario 2), heterogeneous human movement (Scenario 3) and extended mosquito movement (Scenario 4).
The two important statistics from the Iquitos contact cluster study (Stoddard et al., 2013) that instigated this investigation were the infestation rates in the homes visited by an individual with an acute DENV infection versus the homes visited by an individual who had fever, but not DENV (as defined in Stoddard et al. (2013), infestation rate was the percent of homes an individual visited that had at least one active DENV infection). We tracked these quantities in our simulation studies (See Appendix 1), which we formally defined as: (1) the infestation rate of homes visited by a DENV+individual (denoted η+) and (2) the infestation rate of homes visited by an individual with fever but not DENV (denoted η−). Our goal was to identify if and when socially structured movements in our simulations would generate the variation in transmission rates that was observed in the Iquitos field study; i.e., η+ = 40% and η− = 15% (15% is quite a high background rate of infestation and is likely due to the study occurring during the first 2 years of a novel serotype’s invasion into Iquitos).
To account for variation, we sampled different clusters within the population several times throughout periods when transmission was occurring (for complete details, see Appendix A.1.10). Due to a lack of seasonal forcing, when there was no overlap (κ = 0), transmission tended to end after more than 95% of the population was exposed. In Iquitos, at the end of the study the percent of the population that was susceptible was approximately 25% (Stoddard et al., 2013). Consequently, we sampled the population every time the percent susceptible dropped by 5%, but stopped once the population dropped to 25% susceptible. For each simulation, we computed statistics based on the similarity of simulation results to field data from Iquitos (denoted η̂+ and η̂−). By running simulations 300 times for every combination of κ and level of mosquito movement, and taking the average of η̂+ and η̂−, we created a set of quantities for each scenario: η̅+ and η̅−.
Results
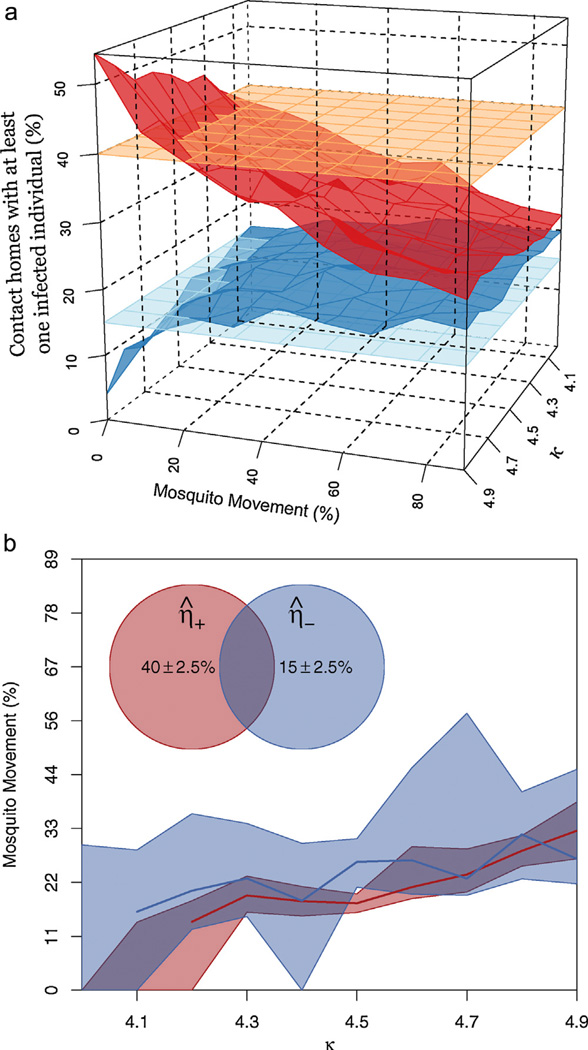
When κ = 0 there was no structure to human movement patterns (i.e., people moved randomly) and thus, as expected, there was no discernible difference between η̅+ and η̅−; both were equal to 25%. Our hypothesis predicts that as κ increases, the difference between η̅+ and η̅− should grow, and that ultimately there should be a κ such that η̅+ = 40% and η̅− = 15%. For κ ≤ 3 there was a minimal difference between η̅+ and η̅−. For κ = 4, the target values were achieved, but only in unrealistic scenarios without mosquito movement (Fig. 2a, with mosquito movement at 0% and individuals who moved among houses as explained below at 100%).
Fig. 2.
Pattern matching (η̅+ and η̅−) of simulation results. (a) Left panel: Values of η̅+ (top, red surface, the percent of homes in a DENV positive cluster that contained at least one concurrently infectious person) and η̅− (bottom, dark blue surface, the percent of homes in a DENV negative cluster that contained at least one concurrently infectious person) across different values of mosquito movement and κ (the number of homes and individual visits, excluding their own, that come from their social group). The empirical values observed in Iquitos are given by the orange and light blue surfaces for the DENV positive and DENV negative clusters, respectively. (b) Right panel: The dark red and dark blue lines indicate where the simulated surfaces intersect with the empirically observed values for DENV positive and DENV negative clusters respectively. To incorporate some uncertainty in the empirically observed values, we found what values of mosquito movement and κ corresponded with simulated values “close” to those observed in Iquitos (where close is within 2.5%). The purple shaded region corresponds to combination of mosquito movement and κ that closely approximated the empirical patterns found in Iquitos.
Mosquito movement predictably diminished the relative effect of the strength of structure in human movement and for any level of κ, increased mosquito movement forced η̅+ and η̅− closer together. To allow for mosquito dispersal, but still test our first assumption that overlapping human movement is necessary to recreate observed dengue transmission patterns, we were forced to alter the movement algorithms to allow for a stronger level of overlap without needing to set κ = 5. The latter resulted in each social group effectively becoming an island. We therefore allowed a percentage of the members in a social group to limit the locations they visited to their social group. The remaining group members had one of the houses they visited come from homes outside of their social group, thus allowing κ to take intermediate values between 4 and 5. If κ = 4.5 then 50% of all people visited only homes within their social group, and 50% visited one home outside their social group.
For values of κ between 4 and 5 by increments of 0.1, resulting values of η̅+ and η̅− are summarized by the two surfaces in Fig. 2a. For larger values of κ, a correspondingly higher level of mosquito movement resulted in η̅+ and η̅− values similar to empirical results. Including a small amount of uncertainty in the empirical estimates of η̅+ and η̅− (Fig. 2b), we found that a small range of mosquito movement values—for any value of κ—resulted in outcomes that were similar to those observed empirically. A large amount of mosquito movement eliminated any substantial differences in η̅+ and η̅− values. Our results, therefore, may appear to be more sensitive to mosquito movement than human movement structure as we defined it. It should be noted, however, that Fig. 2a and b only displays a limited range of κ, because when κ was less than 4, η̅+ and η̅− were both between the surfaces representing the empirical infection patterns observed in Iquitos. As κ approached 5, any level of reality in the simulations was lost and η̅+ approached 1. In those cases almost every simulation, independent of the amount of mosquito movement, resulted in the transmission dying out due to localized virus fade out driven by rapidly increasing herd immunity within infested social groups.
Our results indicate that socially structured movements can strongly influence η̅+ and η̅− and that mosquito movement weakens this influence consistent with our first two assumptions. The strength of the structure required to achieve this result, however, was high and bordered on levels that would not allow transmission to persist. As such, our third assumption may not have been supported. The observed structure may have left a strong and noticeable signature on the spatially aggregated time-series of new infections. For example, if the group structure was such that infections essentially jumped, in sequence, from one social group to the next, transmission would not reach the level of an outbreak. Instead, there would be a constant low level of transmission; a pattern that was not observed in Iquitos.
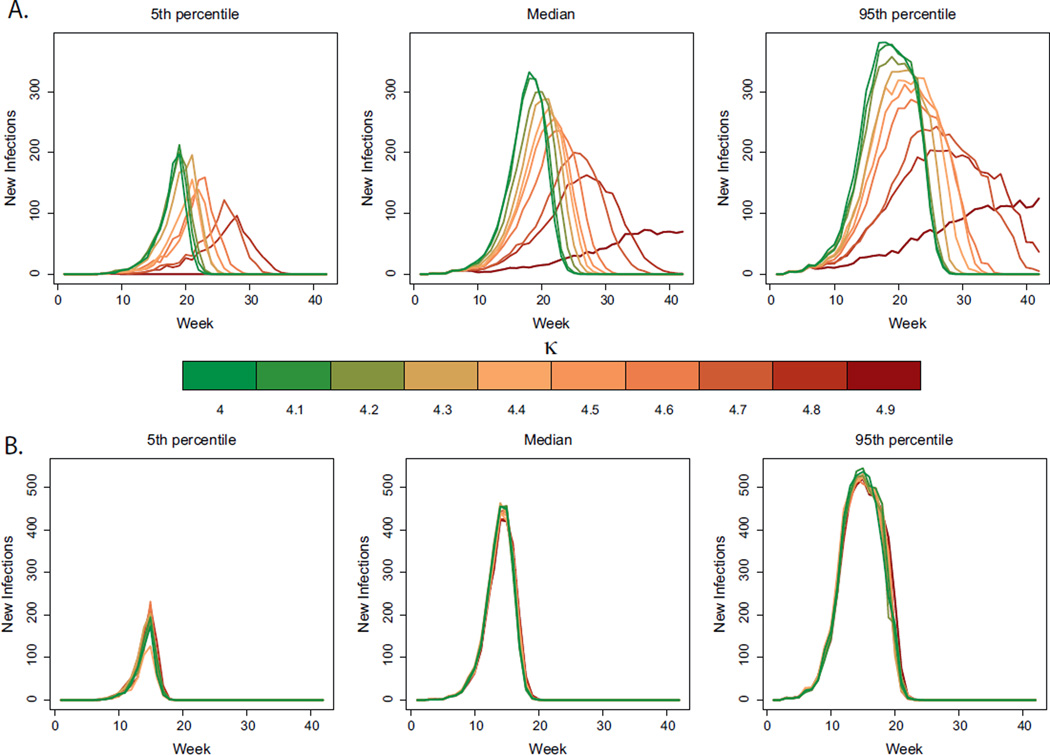
To determine the effect of structure in human movement on infection frequency through time, in Fig. 3 we plotted the median DENV incidence curves for the simulations in Fig. 2 based on a 0% mosquito movement (Fig 3a) and 44% risk of mosquito movement to adjacent houses (Fig. 3b). When no mosquitoes moved, there were clear signatures of structured human movement. In particular, when κ was high (e.g. κ = 4.9), outbreaks were delayed and of a smaller overall size. These signatures of structured human movement disappeared, however, when mosquito movement was incorporated (Fig. 3b). In contrast to the effect on η̅+ and η̅−, socially structured movements had no discernible effect on the timing and magnitude of peaks in DENV incidence in all plausible scenarios; i.e., those where the mosquito was allowed to move (Figs. 3b and S2b–i). Thus, a posteriori, if one tracks infections only at the aggregated, city level, the heterogeneity in transmission driven by socially structured movements is undetectable, constituting a “hidden heterogeneity.”
Fig. 3.
Hidden heterogeneity. (a) 5th percentile (left panel), median (middle panel) and 95th percentile (right panel) for weekly incidence curves of DENV for different simulation scenarios with no mosquito movement. The percent of people that visited a home outside their social group varies from 10% (dark red, corresponding to κ = 4.9) to 100% (dark green, corresponding to κ = 4). (b) 5th percentile (left panel), median (middle panel) and 95th percentile (right panel) for weekly incidence curves of DENV for different simulation scenarios (holding mosquito movement constant at 44%). The percent of people that visited a home outside their social group varies from 10% (dark red, corresponding to κ = 4.9) to 100% (dark green, corresponding to κ = 4).
We repeated the same analyses for Scenarios 2–5 (for complete details, see Appendix A.2). In each case, the same patterns emerged. Namely, the level of structured human movement required was high and the empirical patterns were only recreated in scenarios where there was a small amount of mosquito movement. When mosquitoes dispersed more frequently, the household sizes varied, or when there was limited social movement, there was no discernible difference between the infestation rates of houses visited by DENV+individuals when compared to houses visited by febrile controls (Figs. S3, S5, S7 and S9). Further, in each scenario, when the incidence data was aggregated on a week time-scale, there was a clear influence of human movement structure on the epidemic curve when mosquitoes were not allowed to move. As mosquito movement was incorporated in realistic ways the aggregated epidemic curves became insensitive to how much human movement was socially structured, although the spatial heterogeneity in transmission rates remained high, i.e., a “hidden heterogeneity” (Figs. S4, S6, S8 and S10).
Discussion
We conducted 5 separate simulation experiments designed to explore how and when overlapping human movements could drive fine-scale heterogeneity in mosquito-borne DENV transmission. Our base scenario highlights that the amount of overlap in movements within social groups must be significant to generate the same levels of heterogeneity in transmission rates as those observed in Iquitos, Peru. Importantly, mosquito movement predictably tends to diffuse the effect, necessitating more overlap to recreate the patterns. Subsequent simulation experiments incorporating more detail with respect to household size, individual movement patterns and mosquito movement all recapitulate the main results of the original experiment, strengthening our conclusions that socially driven, overlapping movement likely plays an important role in structuring the spread of DENV through a human population. This indicates that dengue exhibits properties of directly transmitted diseases, in that social proximity drives infection risk. In contrast to other infectious diseases, the presence of a diffusive mosquito vector, even one with minimal dispersal tendencies, strongly influences spatially aggregated transmission dynamics, hiding the signature of structured human movement.
Social structure, such as overlapping human movement within subsets of the population, has been extensively investigated in directly transmitted pathogen systems, such as influenza (Watts and Strogatz, 1998; Eubank et al., 2004; Keeling, 1999; Keeling and Eames, 2005; Mossong et al., 2008). When contacts interact with each other directly and there is no distinction between a contact visiting and a contact being visited (i.e., no directionality to interactions, indicating a symmetric contact network), the concept of communities is analogous to overlapping social movement (Radicchi et al., 2004; Girvan and Newman, 2002). Communities are subsets of people who have more connections with other members of their community than with the outside world.
The strength of communities within a population can affect transmission dynamics (Salathé and Jones, 2010; Salathé et al., 2010; Keeling et al., 2010) and, thus, optimization of control strategies (Salathé and Jones, 2010). While it may be impractical to implement in practice over large populations, knowledge of a DENV infected person’s social group (via contact tracing), could improve retrospective control efforts such as targeted indoor insecticide spraying. At the very least, the presence and importance of socially structured human movement in DENV transmission helps explain the poor performance of some retrospective control efforts, like targeted insecticide spraying only in the immediate vicinity (≤100 m) of the home of people with clinically apparent DENV infections (Scott and Morrison, 2010).
Based on our results, there are at least four reasons to expect that prospective control efforts will be strongly affected by socially structured human movement. First, testing the effect size of control efforts will be confounded by overlapping movements. For example, control strategies are often initiated in one neighborhood, while another neighborhood is kept uncontrolled for the purposes of comparison (Wolbers et al., 2012). If, however, social groups in the population significantly bridge the two neighborhoods (social groups based on host-to-host interaction, not spatial associations), then the results of the study would be compromised by contamination of treatment and control groups. Second, a common difficulty in assessing the effect of a control effort is that it is seldom clear if the observed effect is due to the treatment or due to some unaccounted for confounding variable or the random chance that DENV transmission occurred in one area, but not another. In contexts where DENV transmission dynamics are dominated by social proximity, relative to geographic proximity, treated and untreated groups should be based on social grouping. By placing both groups in the same general geographic area, one can control for geographic risk. Third, by combining knowledge of the strength of the social structure of human movement with entomological surveys, a more accurate understanding of individual time at risk versus time under protection could be made. This would be important new information for calculating a more precise (and continuous) quantification of the effect size of the control effort. Fourth, as availability of a licensed dengue vaccine approaches (Sabchareon et al., 2012), the incorporation of social groups into the models used to compute vaccine delivery strategies will support more efficient vaccination campaigns. This concept has already been reported for socially structured human movement and directly transmitted infectious diseases (Salathé and Jones, 2010).
Our agent-based modeling approach has strengths and weaknesses. On one hand, linking fine-scale spatiotemporal heterogeneity to underlying processes is beyond the capabilities of simple compartmental models. For us, the need to track the similarity and dissimilarity of movements of residents of the same homes and the same neighborhoods necessitated an approach that was based on the individual. On the other hand, agent-based models can quickly become complex as more and more realistic processes and parameters are incorporated. Because we were primarily interested in the potential impact of overlapping social movement on transmission dynamics, we incorporated more complexity regarding movement while retaining simplicity in other aspects of the model. Our model did not incorporate fully realistic mosquito-borne disease ecology. Nor did it allow for heterogeneity in larval development site abundance and we did not incorporate seasonality. Dengue virus transmission is extremely complex and fully recreating all aspects, even on the scale of a 400-house neighborhood, is computationally prohibitive. By using agent-based models, however, we were able to incorporate complex human movement structure into a dengue model that we feel adequately treats the other components of transmission in order to assess the plausibility of our hypothesis.
Our results indicate that when even a small amount of mosquito movement is incorporated into transmission simulations, the impact of structured human movement becomes hidden in aggregated transmission dynamics. This emphasizes an important difference between directly transmitted diseases and mosquito-borne dengue. Structured human movement clearly affects the overall timing and size of an outbreak when a pathogen is only moved around a city by people (Fig. 3a). In partial agreement with previous standard views that mosquito movement diminished the role of human movement on transmission, even when there is strongly structured human movement (Fig. 3b, κ = 4.9), the overall size and timing of an outbreak is essentially identical to scenarios with less structured movement. As is evident from Fig. 2, however, when data are collected on the correct spatio-temporal scale (i.e., by looking within the homes an infectious person visits), mosquito movement cannot destroy the relationships generated by socially driven movement as measured by household infestation rates. Our results indicate that although social structure in human movement can have a significant effect on DENV transmission, its effect is likely a “hidden heterogeneity.” This implies that socially structured human movement is having an important, but often unnoticed, effect on DENV transmission not just in Iquitos, but also in many DENV transmission contexts around the world.
Recent empirical data indicate that it is likely that there is overlap in human movement that underlies DENV transmission dynamics (Stoddard et al., 2013). That data cannot, however, be used to deduce the strength of overlap, nor the strength or size of social groups that give rise to patterns of overlap. It is possible that certain social groups are more likely to support DENV transmission and amplification than others. Dengue prevention programs could target (or at least be sure not to miss) the most socially and epidemiologically important groups with a vaccine and/or vector control. Given the importance of both overlapping human movement and mosquito movement, we need to develop an optimal control strategy that considers both types of movement. It will be important for intervention strategies to optimize the distribution of interventions, which will require knowledge of the underlying structure of human movement that supports virus transmission and spread. Our simulation study is a starting point intended to test the feasibility of the social proximity hypothesis. We did not prove that social movement is a significant driver of DENV transmission, merely that it is consistent with a systematic assessment of DENV transmission dynamics and that it has the potential for important epidemiologic impacts. Studies from an epidemiological, entomological and social science point of view are now needed to ascertain in greater detail how social structure of human movement is quantitatively related to the risk of DENV infection in light of inherent variation between different social groups.
Supplementary Material
Acknowledgments
This work was supported by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directory, Department of Homeland Security, and Fogarty International Center, National Institutes of Health. T.W.S. acknowledges funding from the Bill & Melinda Gates Foundation (OPP52250), the Innovative Vector Control Consortium, and the NIH (R01-AI069341, R01-AI091980, and R01-GM08322). We would like to thank Alex Perkins, David Smith and Sandra Olkowski for insightful comments that greatly improved the manuscript and the figures.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.epidem.2013.12.003.
References
- Ahrens W, Pigeot I. Handbook of Epidemiology. Springer; 2005. [Google Scholar]
- Aldstadt J, Yoon IK, Tannitisupawong D, Jarman R, Thomas S, Gibbons R, Uppapong A, Iamsirithaworn S, Rothman A, Scott T, Endy T. Space–time analysis of hospitalised dengue patients in rural Thailand reveals important temporal intervals in the pattern of dengue virus transmission. Tropical Medicine and International Health: TM and IH. 2012;17:1076–1085. doi: 10.1111/j.1365-3156.2012.03040.x. http://dx.doi.org/10.1111/j.1365-3156.2012.03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmak D, Dorso C, Otero M, Solari H. Dengue epidemics and human mobility. Physical Review E. 2011;84 doi: 10.1103/PhysRevE.84.011901. http://dx.doi.org/10.1103/PhysRevE.84.011901. [DOI] [PubMed] [Google Scholar]
- Bousema T, Griffin J, Sauerwein R, Smith D, Churcher T, Takken W, Ghani A, Drakeley C, Gosling R. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Medicine. 2012;9 doi: 10.1371/journal.pmed.1001165. http://dx.doi.org/10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon L, House T, Keeling MJ. The role of routine versus random movements on the spread of disease in Great Britain. Epidemics. 2009;1 doi: 10.1016/j.epidem.2009.11.002. http://dx.doi.org/10.1016/j.epidem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubank S, Guclu H, Kumar V, Marathe M, Srinivasan A, Toroczkai Z, Wang N. Modelling disease outbreaks in realistic urban social networks. Nature. 2004;429:180–184. doi: 10.1038/nature02541. http://dx.doi.org/10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue Aedes aegypti, in Iquitos, Peru. The American Journal of Tropical Medicine and Hygiene. 2003;69:494–505. [PubMed] [Google Scholar]
- Girvan M, Newman M. Community structure in social and biological networks. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7821–7826. doi: 10.1073/pnas.122653799. http://dx.doi.org/10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M, Hidalgo C, Barabási AL. Understanding individual human mobility patterns. Nature. 2008;453:779–782. doi: 10.1038/nature06958. http://dx.doi.org/10.1038/nature06958. [DOI] [PubMed] [Google Scholar]
- Gubler D, Kuno G. Dengue and dengue hemorrhagic fever. New York: Cab International Wallingford; 1997. [Google Scholar]
- Harrington L, Scott T, Lerdthusnee K, Coleman R, Costero A, Clark G, Jones J, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman J. Dispersal of the dengue vector Aedes aegypti within and between rural communities. The American Journal of Tropical Medicine and Hygiene. 2005;72:209–220. [PubMed] [Google Scholar]
- Hemme R, Thomas C, Chadee D, Severson D. Influence of urban landscapes on population dynamics in a short-distance migrant mosquito: evidence for the dengue vector Aedes aegypti. PLoS Neglected Tropical Diseases. 2010;4 doi: 10.1371/journal.pntd.0000634. http://dx.doi.org/10.1371/journal.pntd.0000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GD. Prospective spatial prediction of infectious disease: experience of New York State (USA) with West Nile Virus and proposed directions for improved surveillance. Environmental and Ecological Statistics. 2008;15 http://dx.doi.org/10.1007/s10651-007-0057-5. [Google Scholar]
- Keeling M. The effects of local spatial structure on epidemiological invasions. Proceedings of the Royal Society B. 1999;266:859–867. doi: 10.1098/rspb.1999.0716. http://dx.doi.org/10.1098/rspb.1999.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M, Eames K. Networks and epidemic models. Journal of the Royal Society, Interface. 2005;2:295–307. doi: 10.1098/rsif.2005.0051. http://dx.doi.org/10.1098/rsif.2005.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M, Danon L, Vernon M, House T. Individual identity and movement networks for disease metapopulations. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8866–8870. doi: 10.1073/pnas.1000416107. http://dx.doi.org/10.1073/pnas.1000416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. Network structure and the biology of populations. Trends in Ecology & Evolution. 2006;21:394–399. doi: 10.1016/j.tree.2006.03.013. http://dx.doi.org/10.1016/j.tree.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mondini A, de Moraes Bronzoni R, Nunes S, Chiaravalloti Neto F, Massad E, Alonso W, Lzzaro E, Ferraz A, de Andrade Zanotto P, Nogueira M. Spatio-temporal tracking and phylodynamics of an urban dengue 3 outbreak in São Paulo, Brazil. PLoS Neglected Tropical Diseases. 2009;3 doi: 10.1371/journal.pntd.0000448. http://dx.doi.org/10.1371/journal.pntd.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba G, Wallinga J, Heijne J, Sadkowska-Todys M, Rosinska M, Edmunds W. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Medicine. 2008;5 doi: 10.1371/journal.pmed.0050074. http://dx.doi.org/10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha H, Durham D, Correa F, Diuk-Wasser M, Galvani A. The interactive roles of Aedes aegypti super-production and human density in dengue transmission. PLoS Neglected Tropical Diseases. 2012;6 doi: 10.1371/journal.pntd.0001799. http://dx.doi.org/10.1371/journal.pntd.0001799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins TA, Scott TW, Le Menach A, Smith DL. Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLOS Computational Biology. 2013 doi: 10.1371/journal.pcbi.1003327. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicchi F, Castellano C, Cecconi F, Loreto V, Parisi D. Defining and identifying communities in networks. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2658–2663. doi: 10.1073/pnas.0400054101. http://dx.doi.org/10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner R, Jr, Perkins T, Barker C, Niu T, Chaves L, Ellis A, George D, Le Menach A, Pulliam J, Bisanzio D, Buckee C, Chiyaka C, Cummings D, Garcia A, Gatton M, Gething P, Hartley D, Johnston G, Klein E, Michael E, Lindsay S, Lloyd A, Pigott D, Reisen W, Ruktanonchai N, Singh B, Tatem A, Kitron U, Hay S, Scott T, Smith D. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. Journal of the Royal Society, Interface. 2013;10 doi: 10.1098/rsif.2012.0921. http://dx.doi.org/10.1098/rsif.2012.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel T, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. The Lancet. 2012 doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- Salathé M, Jones J. Dynamics and control of diseases in networks with community structure. PLoS Computational Biology. 2010;6 doi: 10.1371/journal.pcbi.1000736. http://dx.doi.org/10.1371/journal.pcbi.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathé M, Kazandjieva M, Lee JW, Levis P, Feldman MW, Jones JH. A high-resolution human contact network for infectious disease transmission. Proceedings of the National Academy of Sciences of the United States of America. 2010;107 doi: 10.1073/pnas.1009094108. http://dx.doi.org/10.1073/pnas.1009094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schærström A. A Time Geographical Approach in Medical Geography. Lund University Press; 1996. Pathogenic Paths? [Google Scholar]
- Scott T, Morrison A. Vector dynamics and transmission of dengue virus: implications for dengue surveillance and prevention strategies: vector dynamics and dengue prevention. Current Topics in Microbiology and Immunology. 2010;338:115–128. doi: 10.1007/978-3-642-02215-9_9. [DOI] [PubMed] [Google Scholar]
- Scott T, Amerasinghe P, Morrison A, Lorenz L, Clark G, Strickman D, Kittayapong P, Edman J. Longitudinal studies of Aedes aegypti (diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. Journal of Medical Entomology. 2000a;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Scott T, Morrison A, Lorenz L, Clark G, Strickman D, Kittayapong P, Zhou H, Edman J. Longitudinal studies of Aedes aegypti (diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. Journal of Medical Entomology. 2000b;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Snow J. On the Mode of Communication of Cholera. John Churchill; 1855. [Google Scholar]
- Song C, Koren T, Wang P, Barabási AL. Modelling the scaling properties of human mobility. Nature Physics. 2010;6 http://dx.doi.org/10.1038/nphys1760. [Google Scholar]
- Stoddard S, Morrison A, Vazquez-Prokopec G, Paz Soldan V, Kochel T, Kitron U, Elder J, Scott T. The role of human movement in the transmission of vector-borne pathogens. PLoS Neglected Tropical Diseases. 2009;3 doi: 10.1371/journal.pntd.0000481. http://dx.doi.org/10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard S, Forshey B, Morrison A, Paz-Soldan V, Vazquez-Prokopec G, Astete H, Reiner R, Vilcarromero S, Elder J, Halsey E, Kochel T, Kitron U, Scott T. House-to-house human movement drives dengue virus transmission. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:994–999. doi: 10.1073/pnas.1213349110. http://dx.doi.org/10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teurlai M, Huy R, Cazelles B, Duboz R, Baehr C, Vong S. Can human movements explain heterogeneous propagation of dengue fever in Cambodia? PLoS Neglected Tropical Diseases. 2012;6 doi: 10.1371/journal.pntd.0001957. http://dx.doi.org/10.1371/journal.pntd.0001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec G, Kitron U, Montgomery B, Horne P, Ritchie S. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Neglected Tropical Diseases. 2010;4 doi: 10.1371/journal.pntd.0000920. http://dx.doi.org/10.1371/journal.pntd.0000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Bisanzio D, Stoddard ST, Paz-Soldan V, Morrison AC, Elder JP, Ramirez-Paredes J, Halsey ES, Kochel TJ, Scott TW, et al. Using GPS technology to quantify human mobility, dynamic contacts and infectious disease dynamics in a resource-poor urban environment. PLOS ONE. 2013;8:e58802. doi: 10.1371/journal.pone.0058802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D, Strogatz S. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. http://dx.doi.org/10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wen TH, Lin MH, Fang CT. Population movement and vector-borne disease transmission: differentiating spatial–temporal diffusion patterns of commuting and noncommuting dengue cases. Annals of the Association of American Geographers. 2012;102 http://dx.doi.org/10.1080/00045608.2012.671130. [Google Scholar]
- Wolbers M, Kleinschmidt I, Simmons C, Donnelly C. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Neglected Tropical Diseases. 2012;6 doi: 10.1371/journal.pntd.0001937. http://dx.doi.org/10.1371/journal.pntd.0001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.